Abstract
Purpose
Carbon ion radiotherapy (CIRT) is an emerging radiotherapy modality with potential advantages over conventional photon-based therapy, including exhibiting a Bragg peak and greater relative biological effectiveness, leading to a higher degree of cell kill. Currently, 13 centers are treating with CIRT, although there are no centers in the United States. We aimed to estimate the number of patients eligible for a CIRT center in the United States.
Materials and Methods
Using the National Cancer Database, we analyzed the incidence of cancers frequently treated with CIRT internationally (glioblastoma, hepatocellular carcinoma, cholangiocarcinoma, locally advanced pancreatic cancer, non–small cell lung cancer, localized prostate cancer, soft tissue sarcomas, and specific head and neck cancers) diagnosed in the United States in 2015. The percentage and number of patients likely benefiting from CIRT was estimated with inclusion criteria from clinical trials and retrospective studies, and that ratio was applied to 2019 cancer statistics. An adaption correction rate was applied to estimate the potential number of patients treated with CIRT. Given the high dependency on prostate and lung cancers and the uncertain adoption of CIRT in those diseases, the data were then reanalyzed excluding those diagnoses.
Results
Of the 1 127 455 new cases of cancer diagnosed in the United States in 2015, there were 213 073 patients (18.9%) eligible for treatment with CIRT based on inclusion criteria. When applying this rate and the adaption correction rate to the 2019 incidence data, an estimated 89 946 patients (42.2% of those fitting inclusion criteria) are eligible for CIRT. Excluding prostate and lung cancers, there were an estimated 8922 patients (10% of those eligible for CIRT) eligible for CIRT. The number of patients eligible for CIRT is estimated to increase by 25% to 27.7% by 2025.
Conclusion
Our analysis suggests a need for CIRT in the United States in 2019, with the number of patients possibly eligible to receive CIRT expected to increase during the coming 5 to 10 years.
Keywords: carbon, hadron, US, heavy
Introduction
Cancer remains one of the most significant health issues in the United States, where cancer-related mortality is the second leading cause of death [1]. The American Cancer Society (Washington, DC) estimates 1 762 450 new cancer diagnoses and 606 880 cancer deaths projected in the United States in 2019 [1, 2]. Radiation therapy remains an important treatment modality, with approximately one third of all cancer patients receiving radiation as first-line treatment and one half receiving radiation during the course of their treatment [3, 4]. Given the widespread use of radiation therapy in the treatment of cancers, multiple techniques have been developed to improve the therapeutic ratio by decreasing dose to healthy tissues and thus reducing toxicities.
Carbon ion radiotherapy (CIRT) is one of these evolving radiotherapy modalities that have several potential advantages over conventional photon-based radiotherapy. Carbon ions have greater linear energy transfer and relative biologic effectiveness compared with megavoltage photons, creating more double-strand breaks and DNA destruction (“cluster damage”), leading to a greater degree of cell kill. Additionally, CIRT has a lower oxygen enhancement ratio and is thus more effective in hypoxic environments. In addition, CIRT exhibits the physical advantage of a Bragg peak, where there is little dose deposited proximal and distal to the target, with the greatest amount of energy released at a certain depth [5, 6]. Together, the radiobiologic and physical properties of CIRT may help achieve the goal of reducing the dose to healthy tissues, although clinical data remain limited.
Currently, there are 13 centers in 5 countries (Japan, Germany, Austria, China, and Italy) treating patients with CIRT, with more under development [5, 7, 8]. Currently, there are no centers operating with CIRT in the United States, although there has been increased interest in recent years. The University of Texas Southwestern recently opened a phase III trial randomizing patients to CIRT (NCT03536182 [9]), and Albert Einstein College of Medicine, in collaboration with the Shanghai Proton and Heavy Ion Center, recently completed accrual for a phase I trial investigating CIRT (NCT03403049 [10]). The precise role and the need for CIRT in the United States remain controversial, particularly within the context of the larger debate in the United States regarding the cost of health care [11].
There have been multiple studies investigating the need of CIRT and heavy particle therapy in other countries, including in Japan, Korea, Italy, and Iran [12–15]. Furthermore, Pommier et al [16] estimated patient recruitment for a carbon ion center using both geographic and oncologic variables. Currently, there are no studies, to our knowledge, investigating the number of potential patients who may be eligible for CIRT in the United States.
Materials and Methods
This analysis was performed using National Cancer Database (NCDB), a joint project of the Commission on Cancer of the American College of Surgeons (Chicago, Illinois) and the American Cancer Society. The NCDB includes approximately 70% of all newly diagnosed patients with cancer in the United States and Puerto Rico from American College of Surgeons–certified cancer programs.
To estimate the number of patients eligible for CIRT in the United States, the incidence of the most frequently treated cancers with CIRT were determined for the year 2015, the most recent year available in the NCDB. The number of patients eligible for CIRT was then estimated for each disease site, based on inclusion criteria from previously published protocols and retrospective studies from Germany and Japan. The following disease sites were included: glioblastoma (based on the CLEOPATRA protocol, using CIRT as a boost [17]), hepatocellular carcinoma (PROMETHEUS-01 protocol/NCT01167374 [18]), cholangiocarcinoma (J-CROS study [19]), locally advanced pancreatic cancer (PHOENIX and CIPHER/NCT03536182 studies [9]), early and locally advanced non–small cell lung cancer (multiple retrospective studies [20–23]), localized prostate cancer (multiple retrospective studies [23–26]), soft tissue sarcomas (multiple retrospective studies [27, 28]) and head and neck cancers, including salivary gland cancers (COSMIC protocol/NCT01154270 [29]), sinonasal cancers (NCT01220752 and retrospective studies [30]) and nasopharyngeal cancers (multiple retrospective studies [31, 32]). Further histologic classification (such as adenoid cystic carcinoma or mucosal melanoma) of the general disease sites was possible given the limitations of the various databases used (Table 1).
Table 1.
Inclusion criteria based on previous carbon ion radiotherapy studies.
|
Disease site |
Study, source |
Inclusion criteria |
| Glioblastoma | CLEOPATRA study; Combs et al, 2010 [17] | • Unifocal, supratentorial glioblastoma, • Treatment with temozolomide, • Previous photon irradiation, • Age older than 18 y |
| Hepatocellular carcinoma | PROMETHEUS-01 study; Combs et al, 2011 [18] | • Hepatocellular carcinoma, • No extrahepatic spread, • Age older than 18 y |
| Cholangiocarcinoma | J-CROS study; Kasuya et al, 2019 [19] | • Intrahepatic or perihilar cholangiocarcinoma, • Treated with chemotherapy, • No surgery allowed |
| Locally advanced pancreatic cancer | PHOENIX [9], CIPHER/NCT03536182 study; UTSWMC, 2018 | • Locally advanced or unresectable pancreatic cancer, • No evidence of distant metastases, • No previous radiation, • Age older than 18 y |
| Non–small cell lung cancer | Miyamoto et al, 2003 [20], Miyamoto et al, 2007 [21], Yamamoto et al, 2017 [22], Hayashi et al, 2019 [23] | • Stage I non-small cell lung cancer, • Locally advanced non-small cell lung cancer, • Squamous cell, adenocarcinoma, or large cell of the lung, • Inoperable or refusing surgery |
| Localized prostate cancer | Nomiya et al, 2016 [24], Zhang et al, 2019 [25], Kasuya et al, 2017 [26] | • Prostate adenocarcinoma, • T1-3N0, • M0 disease |
| Soft tissue sarcoma | Sugahara et al, 2012 [27], Kamada et al. 2002 [28] | • Localized sarcomas, • Extremity, pelvis, or spine location, • No history of previous radiation, • Inoperable |
| Salivary gland | COSMIC (NCT01154270) Jensen et al, 2010 [29] | • Inoperable tumor, • Grade 2/3, • R1 or R2 resection, • T3 or T4, • Perineural invasion |
| Sinonasal cancers | Koto et al, 2014 [30] | • Definitive carbon ion therapy, • Medically inoperable, • Age 15-79, • No previous radiation |
| Nasopharyngeal cancers | Akbaba et al. 2019 [31, 32] | • Locally advanced nasopharyngeal tumors, • Paranasal sinus tumors were excluded |
Abbreviation: UTSWMC, University of Texas Southwest Medical Center.
The proportion of patients eligible for CIRT, based on the year 2015, was then applied to the current estimated 2019 cancer incidence based on the American Cancer Society estimates [1, 2] to determine the number of patients diagnosed in 2019 who could benefit from CIRT. An adaptation-rate correction, based on Japanese and Korean treatment data, was then applied to each disease site to better estimate the number of patients likely to receive CIRT [12, 13, 33]. For example, in Korea, an estimated 10% of patients with salivary gland cancers who are eligible for CIRT will be treated with that modality. There are an estimated 4154 patients who met the eligibility criteria for CIRT in the United States in 2019, before the application of the adaption correction, with an estimated 415 patients estimated to receive CIRT after the correction of 10%. Because prostate and lung cancers are the most common disease sites in the United States and the expected use of CIRT in those diseases in the United States is unknown, the analysis was repeated omitting those disease sites to provide a lower estimate of patients with diseases benefiting the most from CIRT.
As development and commissioning of a CIRT center in the United States will take years before treating the first patients, the number of patients eligible for CIRT was estimated with data from Pan et al [34], who estimated the number of patients receiving radiotherapy as a primary treatment in 2025. The estimation of increased use was then applied to the estimated number of patients benefiting from CIRT in 2019 to derive the 2025 statistics.
Results
There were approximately 1 127 455 patients diagnosed with cancer in the United States in 2015. Of the disease sites selected for analysis, there were 332 672 cases (29.5%). Approximately 213 073 patients (64.0% of those with selected disease sites; 18.9% of all patients diagnosed in 2015) were eligible for CIRT based on the inclusion criteria in 2015. When applying the above ratio of eligible patients (18.9%) to the 1.7 million patients estimated to be diagnosed with cancer in 2019, approximation 359 243 newly diagnosed cancer patients were eligible to receive CIRT in 2019 based on the inclusion criteria (Table 2; Figure 1). After applying the disease-site specific correction for adaption rates based on previous analysis, and described in table 3, [13], the estimated number of patients treated with CIRT is 89 946 (Table 3). The three disease sites with the largest potential population were the prostate (61 849 patients; 35.4%), non–small cell lung cancer (18 174 patients; 20.2%), and hepatocellular carcinoma (3734 patients; 4.2%).
Table 2.
Estimated number of patients potentially eligible from carbon ion radiotherapy in 2015 and 2019.
|
Disease site |
2015 cases in the United States, No. |
Patients eligible for CIRT in 2015, No. (%) |
Estimated 2019 cases [1, 2], No. |
Estimated patients eligible for CIRT in 2019, No. (%) |
| Glioblastoma | 19 840 | 4335 (21.8) | 23 820 | 5205 (21.9) |
| Head and neck | 6606 | 5177 (78.4) | 5300 | 4154 (78.4) |
| Hepatocellular carcinoma | 18 735 | 13 872 (74.0) | 42 030 | 31 120 (74.0) |
| Cholangiocarcinoma | 5368 | 1753 (32.7) | 12 360 | 4036 (32.7) |
| Pancreas | 35 188 | 17 786 (50.5) | 56 770 | 28 695 (50.5) |
| Non–small cell lung cancer | 127 272 | 72 417 (56.9) | 228 150 | 129 816 (56.9) |
| Prostate | 104 072 | 85 710 (82.4) | 174 650 | 143 836 (82.4) |
| Soft tissue sarcomas | 8985 | 6846 (76.2) | 16 250 | 12 381 (76.2) |
The number of 2015 cases was estimated based on our analysis of the NCDB. The number of patients eligible in 2015 was based on inclusion criteria using trials for Asia and Europe. The estimated number of eligible patients in 2019 was based on taking the percentage of patients eligible in 2015 and applying that to the 2015 data. Please see the text for details regarding the specific calculation.
Figure 1.
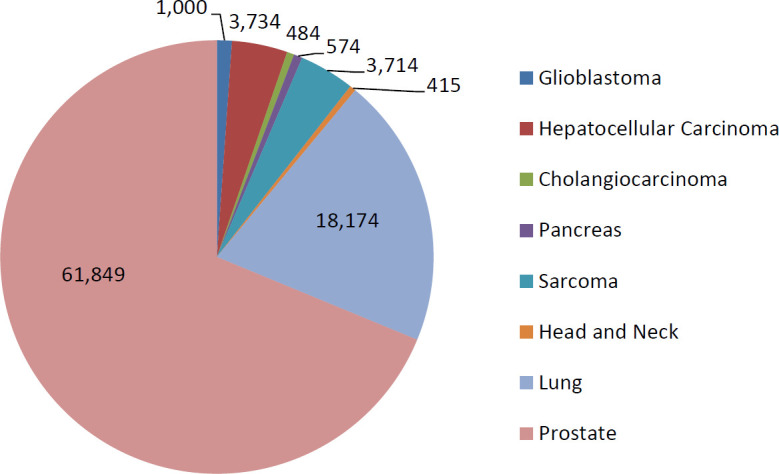
Estimated total number of patients potentially eligible for carbon ion radiotherapy in 2019 by disease site.
Table 3.
Estimation of adaptation rates of carbon ion radiotherapy in the United States.
|
Disease site |
Estimated patients eligible for CIRT in 2019, No. |
GHMC adaption rate [12, 13] |
GHMC estimation |
NIRS adaption rate [13, 33] |
NIRS estimation |
Korean adaptation rate [13] |
Korean estimation |
US estimation for 2019 |
| Glioblastoma | 5205 | — | — | — | — | — | — | 1041a |
| Head and neck | 4154 | 0.38 | 1578 | 0.10 | 415 | 0.10 | 415 | 415 |
| Hepatocellular carcinoma | 31 120 | 0.25 | 7780 | 0.12 | 3734 | 0.12 | 3734 | 3734 |
| Cholangiocarcinoma | 4036 | 0.25 | 1109 | 0.12 | 484 | 0.12 | 484 | 484 |
| Pancreas | 28 695 | — | — | 0.02 | 574 | 0.02 | 574 | 574 |
| Non–small cell lung cancer | 129 816 | 0.10 | 12 982 | 0.14 | 18 174 | 0.14 | 18 174 | 18 174 |
| Prostate | 143 836 | 0.15 | 21 575 | 0.43 | 61 849 | 0.43 | 61 849 | 61 849 |
| Soft tissue sarcomas | 12 381 | 0.80 | 9905 | — | — | 0.30 | 3714 | 3714 |
Abbreviations: CIRT, carbon-ion radiotherapy; GHMC, Gunma University Heavy Ion Medical Center; NIRS, National Institute of Radiological Sciences.
Based on an estimate of 0.20 adaptation rate.
After excluding the patients with prostate cancer (104 072 and 143 836 cases in 2015 and 2019, respectively) and lung cancer (127 272 and 129 816 in 2015 and 2019, respectively), given the high incidence and alternative effective treatment options available, there were approximately 44 592 patients in 2015 and 81 438 patients in 2019 estimated to be eligible for treatment with CIRT before applying the adaptation rate. After applying the adaptation rates, approximately 8922 patients were eligible for CIRT in 2019 (Figure 2). Most patients remaining had hepatocellular carcinoma, followed by soft tissue sarcoma.
Figure 2.
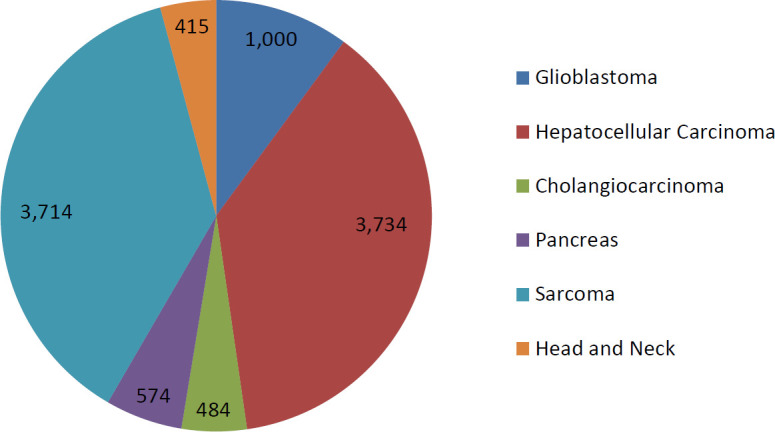
Estimated number of patients potentially eligible for carbon ion radiotherapy in 2019 by disease site, excluding prostate and lung cancers.
The number of patients potentially eligible for CIRT is expected to continue to grow for a total number of 114 823 patients in 2025 and 11 883 when excluding prostate and lung cancer, based on disease-specific growth estimates. The results are summarized in Table 4. For example, in 2019, there are an estimated 4306 patients with locally advanced pancreatic cancer who meet eligibility criteria for CIRT and 706 after the adaptation calculation. Based on an estimated 23% growth of eligible pancreas patients, and applying the same adaption correction, there will be an estimated 706 patients who eligible for CIRT in 2025.
Table 4.
Estimated potential number of patients eligible for carbon ion radiotherapy in the United States in 2025.
|
Disease site |
Estimated patients eligible for CIRT in 2019, No. |
Estimated percentage of growth [by Pan et al ], % |
Estimated patients eligible for CIRT in 2025, No. |
US estimation for 2025, No. |
| Glioblastoma | 5205 | 13 | 5881 | 1176 |
| Head and neck | 4154 | 18 | 4901 | 490 |
| Hepatocellular carcinoma | 31 120 | 22 | 37 967 | 4556 |
| Cholangiocarcinoma | 4036 | 18 | 4763 | 572 |
| Pancreas | 28 695 | 23 | 35 295 | 706 |
| Non–small cell lung cancer | 129 816 | 24 | 160 972 | 22 536 |
| Prostate | 143 836 | 30 | 186 986 | 80 404 |
| Soft tissue sarcomas | 12 381 | 18 | 14 610 | 4383 |
Abbreviation: CIRT, carbon-ion radiotherapy.
Discussion
Based on our analysis, approximately 9000 patients were eligible for CIRT in 2019, with that estimate increasing to approximately 90 000 with the inclusion of prostate and non–small cell lung cancers. Not surprisingly, prostate and non–small cell lung cancer comprise most of the patients, although traditionally less-common and difficult-to-treat diseases, such as nasopharyngeal/sinonasal tumors, cholangiocarcinoma, and locally advanced pancreatic cancers, may derive a particular benefit from CIRT when given either as definitive treatment alone or as a boost and comprise a large subset of potential patients.
The numbers of patients that are eligible for CIRT are similar to the numbers previously reported as estimates from other countries. In our study, approximately 5.1% (89 946 of 1 762 450) of all patients (0.5% when excluding prostate and lung) of all patients diagnosed with cancer in the United States in 2019 are eligible for CIRT. The National Center for Oncological Hadron Therapy (Pavia, Italy) found that 3694 patients, comprising 6% of newly diagnosed cancer patients in Italy, were eligible for CIRT [14]. Similarly, Mayer et al [35] reported that 5.6% of all patients newly diagnosed with cancer, approximately 2044 patients per year, are eligible for hadron therapy [35]. In Japan, Tsujii [36] reported that approximately 15% of patients receiving radiation are eligible for charged-particle radiotherapy. Another study performed by Gunma University Heavy Ion Medical Center found that approximately 2% of patients newly diagnosed with a variety of cancers, approximately 1527 patients, were eligible for CIRT in Gumna and the neighboring prefectures [12, 13]. In a multistep probabilistic spatial model, taking into consideration various geographic and oncologic factors, Pommier et al [16] estimated the recruitment for CIRT for a center in France. Assuming the presence of 2 CIRT facilities, the estimated number of patients per year was 1771 when restricted to France with 1466 additional patients when expanded to the United Kingdom, Spain, and Portugal. Notably, many of these comparative studies included disease sites, such as prostate and lung, based on regional treatment patterns.
Given our estimate of approximately 9000 patients eligible for CIRT per year in the United States, it becomes important to consider the number of centers needed to meet a potentially high demand. Pommier et al [16] noted a reference value of approximately 888 patients per year for a single center in France when only recruiting those patients from France. Based on the estimate of approximately 900 patients per year from Pommier et al and assuming that every patient recruited is treated, the US has a patient population (approximately 9000 patients eligible per our data) to support 10 CIRT centers. It is necessary that those centers be geographically diverse to allow access for the greatest number of patients because traveling in the US generally takes more time than through a country in Europe. Furthermore, by developing randomized protocols in the United States, we can more accurately estimate the number of centers needed, and if the technique provides a cost-effective treatment modality in the US payment model.
One of the greatest barriers to adoption of CIRT in the United States is the high cost of development and operation of CIRT facilities. Peeters et al [37] estimates the capital cost of constructing a combined particle-therapy center to be approximately 138.6 million (approximately $164 million as of September 2020) with high operational costs. Furthermore, the National Institute of Radiological Sciences (Chiba, Japan) estimates approximately 1826 million yen per year (approximately $17 million) in operating costs and depreciation, with a personnel cost of $150 million yen ($1.4 million) [38]. The overall construction and operating costs remain a limiting factor in the United States, especially given the ever-changing reimbursement landscape [11]. Given the relatively high cost of particle therapy compared with photon irradiation, developing appropriate reimbursement remains a potential pitfall to the adoption of CIRT in the United States. As a specialty, radiation oncology has seen a decrease in reimbursements over the past decade because freestanding clinics experienced Medicare cuts of approximately 20% between 2009 and 2014 [39]. To compound that problem, the prior authorization process will likely lead to denials of a large percentage of patients eligible for CIRT, with 32% of adult patients being denied proton therapy in 1 study [40].
Additionally, the Centers for Medicare and Medicaid Services Innovation Centers developed a new radiation-oncology alternative payment model that emphasizes value-based payments as opposed to volume-based payments [39]. This will likely lead to the adoption of more cost-effective and hypofractionated techniques, which may further limit the adoption of heavy ion therapy in the United States.
Although the initial and treatment costs of CIRT remain high, there are several articles suggesting the cost-effectiveness of CIRT in certain disease sites, including skull-base chordomas, in international systems [41–43]. Because the US health care system has a unique system of reimbursement, the cost effectiveness of this expensive treatment remains unknown, although the data from other treatment centers can likely be extrapolated to the US. The exact cost effectiveness of CIRT in the United States will likely be a complex interaction of disease site specificity, endpoint selection, geographic location of the treating center, and current reimbursement model, which can all only be fully understood upon treatment of the initial cohort of patients with CIRT.
There are several other factors that are barriers to adoption of CIRT in the United States. To address some of these limitations, Lazar et al [44] coined the mnemonic RESIDUE to help discuss the challenges associated with adoption of CIRT. Briefly, they suggested the following additional factors need to be addressed: radiobiologic knowledge to address uncertainty in fraction sizes and dose; operational and financial expenses; size, weight, and cost of accelerators and gantries; integrating technology to advance delivery of treatment; defining the patient population who may benefit most; determining the uncertainties of effective dose and range at the end of the Bragg peak; and evidence of cost effectiveness [44]. Currently, there is considerable uncertainty with dosimetric planning, with 3 different models for calculating relative biologic effectiveness available, each yielding a different dose and fractionation scheme [45]. In addition, CIRT exhibits a fragmentation tail, which creates greater dose uncertainty distal to the Bragg peak compared with proton irradiation [46]. Additionally, most centers treating with CIRT use fixed-beam arrangements because rotating gantries are not widely available [5, 7, 8]. There is also a greater degree of motion uncertainty when treating with CIRT compared with photon or proton-based irradiation [47]. Together, these limitations will need to be addressed before CIRT becomes more widely used in the United States.
Another current barrier to adoption in the United States is the lack of current randomized data to suggest a benefit of CIRT, although the development of a CIRT center in the United States has the potential to further develop new protocols and to advance preclinical research. Currently, early clinical prospective and retrospective studies have suggested both the safety and the efficacy of CIRT for a variety of disease sites, although there remains few phase III protocols [48–60]. Based on those early studies, multiple protocols are currently enrolling patients internationally [61]. Developing US-based protocols can further establish the role of CIRT in our heterogeneous patient population and can fully elicit whether the benefits of CIRT remain a cost-efficient option. Centers developing randomized studies do involve a substantial amount of risk that CIRT is neither safe nor more effective, despite having a significantly higher cost than more-conventional treatments. Additionally, treating with CIRT in the United States will allow further development of CIRT in combination with new treatment modalities, including immunotherapy, FLASH radiotherapy, and other advanced modalities; many of which are already under investigation [11]. Additional prospective data are needed to further evaluate and define the potential number of patients eligible for, the potential adoption of, and the potential clinical benefit of, CIRT in the US population.
Despite these estimates, our study has several notable limitations. First, the data were collected from the NCDB, with the standard limitations of using a large, centralized database. The NCDB reportedly includes up to 70% of all patients with cancer in the United States, although there are a significant number of patients that are omitted who may be eligible for CIRT. Additionally, in an effort to anticipate the potential need in 2019 and 2025, data were obtained from other sources, and precise estimations using those data sources were unable to be obtained. For instance, the 2019 incidence data reported only on “lung and bronchus” cancers, including both non–small cell and small cell lung cancers. Although including those other disease sites leads to an overestimation, including the 2019 data provides a more up-to-date estimate of the number of patients eligible based on the current cancer incidence. One of the greatest areas of benefit for CIRT is in the setting of oligometastatic disease, although the number of patients presenting with oligometastatic disease is not readily available using the large databases. Second, our estimations likely overestimate the use of CIRT because we used Japanese and Korean adaptation rates. Our study depends heavily on the adaption rates and use patterns of Japan, in which a larger proportion of patients with a given disease are near carbon facilities. For instance, in Japan, the adaption rate for sarcoma is approximately 80%, a number that is potentially unattainable in the US population, given distance of people from treatment centers. Furthermore, those geographic limitations greatly reduce the number of patients potentially treated with heavy ion therapy, although that is difficult to quantify. Furthermore, our study also estimates the rates of CIRT in 2025 based on growth rates from the year 2019, which may overestimate the cancer burden in 2025. Most patients eligible for CIRT have either localized prostate cancer or early stage non–small cell lung cancer, and many of those patients are likely to receive other forms of effective, definitive treatment. Third, our study was also unable to include patients requiring retreatment with radiation for recurrent disease, which is one of the populations that may derive the greatest benefit from CIRT. Despite those limitations, our study provides estimates of the number of patients that can benefit from CIRT. In addition, we do not consider other potential benefits of carbon therapy, outside the scope of the Japanese and Korean research.
Conclusions
Our study estimated the number of patients eligible for carbon ion radiotherapy in the United States to be 8922 to 89 946 patients, comprising approximately 5% of newly diagnosed cancers in 2019. This estimate will likely continue to grow, with an estimated 11 883 to 114 823 patients eligible for carbon ion radiotherapy in 2025, based on inclusion criteria.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: Bradford S. Hoppe, MD, MPH, and Anita Mahajan, MD, are Associate Editors of the International Journal of Particle Therapy. Daniel M. Trifiletti, MD, has received clinical trial funding from Novocure and publishing fees from Springer, outside of the submitted work. All authors contributed to the writing, editing, and final approval of this article. The remaining authors have no conflicts of interest to disclose.
Funding: The authors have no funding to disclose.
Ethical Approval: As a Surveillance, Epidemiology, and End Results (SEER, National Cancer Institute, Bethesda, MD) analysis, this study was determined to be exempt from institutional review board approval.
References
- 1. .Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2. .Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER cancer statistics review 1975–2016. 2019 https://seer.cancer.gov/csr/1975_2016/ Published. . Updated April 9, 2020. Accessed November 15, 2019.
- 3. .Royce TJ, Qureshi MM, Truong MT. Radiotherapy utilization and fractionation patterns during the first course of cancer treatment in the United States from 2004 to 2014. J Am Coll Radiol. 2018;15:1558–64. doi: 10.1016/j.jacr.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 4. .Jaffray DA, Gospodarowicz MK. Radiation therapy for cancer. In: Gelband H, Jha P, Sankaranarayanan R, Horton , editors. Cancer Disease Control Priorities. Vol. 3. Washington, DC: International Bank for Reconstruction and Development and World Bank; 2015. pp. 239–48. Vol. 3rd ed. [PubMed] [Google Scholar]
- 5. .Rackwitz T, Debus J. Clinical applications of proton and carbon ion therapy. Semin Oncol. 2019;46:226–32. doi: 10.1053/j.seminoncol.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 6. .Mohamad O, Sishc BJ, Saha J, Pompos A, Rahimi A, Story MD, Davis AJ, Kim DWN. Carbon ion radiotherapy: a review of clinical experiences and preclinical research, with an emphasis on DNA damage/repair. Cancers (Basel) 2017;9:66. doi: 10.3390/cancers9060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. .Kamada T. Twenty years of carbon ion radiation therapy at the national institute of radiological sciences: accomplishments and prospects. Int J Part Ther. 2016;2:459–63. doi: 10.14338/IJPT-15-00030.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. .Particle Therapy Co-Operative Group. Particle therapy facilities in clinical operation. 2019 https://www.ptcog.ch/index.php/facilities-in-operation Published. Updated July 2020. Accessed November 15, 2019.
- 9. .University of Texas Southwestern Medical Center. Trial of carbon ion versus photon radiotherapy for locally advanced, unresectable pancreatic cancer (CIPHER) 2018 https://clinicaltrials.gov/ct2/show/NCT03536182 Published May 24. . Updated February 24, 2020. Accessed November 15, 2019.
- 10. .Combs SE, Habermehl D, Kieser M, Dreher C, Werner J, Haselmann R, Jakel O, Jager D, Buchler MW, Debus J. Phase I study evaluating the treatment of patients with locally advanced pancreatic cancer with carbon ion radiotherapy: the PHOENIX-01 trial. BMC Cancer. 2013;13:419. doi: 10.1186/1471-2407-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. .Blakely EA, Faddegon B, Tinkle C, Bloch C, Dominello M, Griffin RJ, Joiner MC, Burmeister J. Three discipline collaborative radiation therapy (3DCRT) special debate: The United States needs at least one carbon ion facility. J Appl Clin Med Phys. 2019;20:6–13. doi: 10.1002/acm2.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. .Ohno T, Nakano T, Kanai T, Yamada S. Carbon ion radiotherapy at Gunma University: currently indicated cancer and estimation of need. Del McDaniel F, Doyle BL, editors. 21st AIP International Conference Proceedings Application of Accelerators in Research and Industry. 2011;1336:391–6. In. (Vol. 1336; August 8–13, 2010; Fort Worth, TX) [Google Scholar]
- 13. .Cho I, Seo YS, Jung WG, Kim MS. Estimation of the medical need for carbon-ion radiotherapy in Korea. J Radiat Res. 2018;59:588–92. doi: 10.1093/jrr/rry046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. .Krengli M, Orecchia R. Medical aspects of the National Centre For Oncological Hadrontherapy (CNAO-Centro Nazionale Adroterapia Oncologica) in Italy. Radiother Oncol. 2004;73(suppl 2):S21–3. doi: 10.1016/s0167-8140(04)80007-0. [DOI] [PubMed] [Google Scholar]
- 15. .Hosseini MA, Mohamadianpanah M, Zare-Bandeamiri M, Mosleh-Shirazi MA. A preliminary study on the estimation of the number of cancer patients eligible for hadron therapy in Iran and Fars Province. Iran J Med Sci. 2018;43:313–7. [PMC free article] [PubMed] [Google Scholar]
- 16. .Pommier P, Lievens Y, Feschet F, Borras JM, Baron MH, Shtiliyanova A, Pijls-Johannesma M. Simulating demand for innovative radiotherapies: an illustrative model based on carbon ion and proton radiotherapy. Radiother Oncol. 2010;96:243–9. doi: 10.1016/j.radonc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 17. .Combs SE, Kieser M, Rieken S, Habermehl D, Jakel O, Haberer T, Nikoghosyan A, Haselmann R, Unterberg A, Wick W, Debus J. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: the CLEOPATRA trial. BMC Cancer. 2010;10:478. doi: 10.1186/1471-2407-10-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. .Combs SE, Habermehl D, Ganten T, Schmidt J, Edler L, Burkholder I, Jakel O, Haberer T, Debus J. Phase I study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: the PROMETHEUS-01 trial. BMC Cancer. 2011;11:67. doi: 10.1186/1471-2407-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. .Kasuya G, Terashima K, Shibuya K, Toyama S, Ebner DK, Tsuji H, Okimoto T, Ohno T, Shioyama Y, Nakano T, Kamada T. Japan Carbon-Ion Radiation Oncology Study Group. Carbon-ion radiotherapy for cholangiocarcinoma: a multi-institutional study by and the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Oncotarget. 2019;10:4369–79. doi: 10.18632/oncotarget.27028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. .Miyamoto T, Yamamoto N, Nishimura H, Koto M, Tsujii H, Mizoe JE, Kamada T, Kato H, Yamada S, Morita S, Yoshikawa K, Kandatsu S, Fujisawa T. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2003;66:127–40. doi: 10.1016/s0167-8140(02)00367-5. [DOI] [PubMed] [Google Scholar]
- 21. .Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, Hirasawa N, Sugawara T, Yamamoto N, Koto M, Ezawa H, Kadono K, Tsujii H, Mizoe JE, Yoshikawa K, Kandatsu S, Fujisawa T. Working Group for Lung Cancer. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2:916–26. doi: 10.1097/JTO.0b013e3181560a68. [DOI] [PubMed] [Google Scholar]
- 22. .Yamamoto N, Miyamoto T, Nakajima M, Karube M, Hayashi K, Tsuji H, Tsujii H, Kamada T, Fujisawa T. A dose escalation clinical trial of single-fraction carbon ion radiotherapy for peripheral stage I non-small cell lung cancer. J Thorac Oncol. 2017;12:673–80. doi: 10.1016/j.jtho.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 23. .Hayashi K, Yamamoto N, Nakajima M, Nomoto A, Tsuji H, Ogawa K, Kamada T. Clinical outcomes of carbon-ion radiotherapy for locally advanced non-small-cell lung cancer. Cancer Sci. 2019;110:734–41. doi: 10.1111/cas.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. .Nomiya T, Tsuji H, Kawamura H, Ohno T, Toyama S, Shioyama Y, Nakayama Y, Nemoto K, Tsujii H, Kamada T. A multi-institutional analysis of prospective studies of carbon ion radiotherapy for prostate cancer: a report from the Japan Carbon ion Radiation Oncology Study Group (J-CROS) Radiother Oncol. 2016;121:288–93. doi: 10.1016/j.radonc.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 25. .Zhang Y, Li P, Yu Q, Wu S, Chen X, Zhang Q, Fu S. Preliminary exploration of clinical factors affecting acute toxicity and quality of life after carbon ion therapy for prostate cancer. Radiat Oncol. 2019;14:94. doi: 10.1186/s13014-019-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. .Kasuya G, Ishikawa H, Tsuji H, Haruyama Y, Kobashi G, Ebner DK, Akakura K, Suzuki H, Ichikawa T, Shimazaki J, Makishima H, Nomiya T, Kamada T, Tsujii H. Working Group for Genitourinary Tumors. Cancer-specific mortality of high-risk prostate cancer after carbon-ion radiotherapy plus long-term androgen deprivation therapy. Cancer Sci. 2017;108:2422–9. doi: 10.1111/cas.13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. .Sugahara S, Kamada T, Imai R, Tsuji H, Kameda N, Okada T, Tsujii H, Tatezaki S. Working Group for the Bone and Soft Tissue Sarcomas. Carbon ion radiotherapy for localized primary sarcoma of the extremities: results of a phase I/II trial. Radiother Oncol. 2012;105:226–31. doi: 10.1016/j.radonc.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 28. .Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, Kato H, Yamada S, Morita S, Yoshikawa K, Kandatsu S, Tateishi A. Working Group for the Bone and Soft Tissue Sarcomas. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–71. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 29. .Jensen AD, Nikoghosyan A, Windemuth-Kieselbach C, Debus J, Münter MW. Combined treatment of malignant salivary gland tumours with intensity-modulated radiation therapy (IMRT) and carbon ions: COSMIC. BMC Cancer. 2010;10:546. doi: 10.1186/1471-2407-10-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. .Koto M, Hasegawa A, Takagi R, Sasahara G, Ikawa H, Mizoe JE, Jingu K, Tsujii H, Kamada T, Okamoto Y. Organizing Committee for the Working Group for Head-and-Neck Cancer. Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother Oncol. 2014;113:60–5. doi: 10.1016/j.radonc.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 31. .Akbaba S, Held T, Lang K, Forster T, Federspil P, Herfarth K, Hafner M, Plinkert P, Rieken S, Debus J, Adeberg S. Bimodal radiotherapy with active raster-scanning carbon ion radiotherapy and intensity-modulated radiotherapy in high-risk nasopharyngeal carcinoma results in excellent local control. Cancers (Basel) 2019;11:379. doi: 10.3390/cancers11030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. .Akbaba S, Ahmed D, Lang K, Held T, Mattke M, Hoerner-Rieber J, Herfarth K, Rieken S, Plinkert P, Debus J, Adeberg S. Results of a combination treatment with intensity modulated radiotherapy and active raster-scanning carbon ion boost for adenoid cystic carcinoma of the minor salivary glands of the nasopharynx. Oral Oncol. 2019;91:39–46. doi: 10.1016/j.oraloncology.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 33. .Gifu Prefecture. Interim report on introduction of advanced cancer treatment facilities in Gifu Prefecture. [in Japanese] http://www.pref.gifu.lg.jp/kodomo/iryo/saigai-iryo/11229/ganryushisen-public.data/tyuukanhoukoku.pdf Accessed November 15, 2019.
- 34. .Pan HY, Haffty BG, Falit BP, Buchholz TA, Wilson LD, Hahn SM, Smith BD. Supply and demand for radiation oncology in the United States: updated projections for 2015 to 2025. Int J Radiat Oncol Biol Phys. 2016;96:493–500. doi: 10.1016/j.ijrobp.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 35. .Mayer R, Mock U, Jager R, Potter R, Vutuc C, Eiter H, Krugmann K, Hammer J, Hirn B, Hawliczek R, Knocke-Abulesz TH, Lukas P, Nechville E, Pakisch B, Papauschek M, Raunik W, Rhomberg W, Sabitzer H, Schratter-Sehn A, Sedlmayer F, Wedrich I, Auberger T. Epidemiological aspects of hadron therapy: a prospective nationwide study of the Austrian project MedAustron and the Austrian Society of Radiooncology (OEGRO) Radiother Oncol. 2004;73(suppl 2):S24–8. doi: 10.1016/s0167-8140(04)80008-2. [DOI] [PubMed] [Google Scholar]
- 36. .Tsujii H. Overview of carbon-ion radiotherapy (Micro-Mini & Nano-Dosimetry & Innovative Technologies in Radiation Therapy Conference; January 26–28, 2016; Tasmania, Australia) J Phys. 2017;777:012032. [Google Scholar]
- 37. .Peeters A, Grutters JP, Pijls-Johannesma M, Reimoser S, De Ruysscher D, Severens JL, Joore MA, Lambin P. How costly is particle therapy? cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol. 2010;95:45–53. doi: 10.1016/j.radonc.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 38. .Nakagawa Y, Yoshihara H, Kageji T, Matsuoka R, Nakagawa Y. Cost analysis of radiotherapy, carbon ion therapy, proton therapy and BNCT in Japan. Appl Radiat Isot. 2009;67(7–8 suppl):S80–3. doi: 10.1016/j.apradiso.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 39. .Kavanagh B. Radiation oncology APM: why us? why now? Int J Radiat Oncol Biol Phys. 2019;105:22–4. doi: 10.1016/j.ijrobp.2019.07.002. [editorial] [DOI] [PubMed] [Google Scholar]
- 40. .Gupta A, Khan AJ, Goyal S, Millevoi R, Elsebai N, Jabbour SK, Yue NJ, Haffty BG, Parikh RR. Insurance approval for proton beam therapy and its impact on delays in treatment. Int J Radiat Oncol Biol Phys. 2019;104:714–23. doi: 10.1016/j.ijrobp.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. .Jakel O, Land B, Combs SE, Schulz-Ertner D, Debus J. On the cost-effectiveness of carbon ion radiation therapy for skull base chordoma. Radiother Oncol. 2007;83:133–8. doi: 10.1016/j.radonc.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 42. .Mobaraki A, Ohno T, Yamada S, Sakurai H, Nakano T. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 2010;101:1834–9. doi: 10.1111/j.1349-7006.2010.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. .Sprave T, Verma V, Sterzing F, Bruckner T, Hees K, Land B, Jakel O, Herfarth K, Debus J, Uhl M. Cost-effectiveness of carbon ion radiation therapy for skull base chordoma utilizing long-term (10-year) outcome data. Anticancer Res. 2018;38:4853–8. doi: 10.21873/anticanres.12797. [DOI] [PubMed] [Google Scholar]
- 44. .Lazar AA, Schulte R, Faddegon B, Blakely EA, Roach M., III Clinical trials involving carbon-ion radiation therapy and the path forward. Cancer. 2018;124:4467–76. doi: 10.1002/cncr.31662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. .Fossati P, Matsufuji N, Kamada T, Karger CP. Radiobiological issues in prospective carbon ion therapy trials. Med Phys. 2018;45:e1096–110. doi: 10.1002/mp.12506. [DOI] [PubMed] [Google Scholar]
- 46. .Durante M, Debus J. Heavy charged particles: does improved precision and higher biological effectiveness translate to better outcome in patients? Semin Radiat Oncol. 2018;28:160–7. [Google Scholar]
- 47. .Pompos A, Durante M, Choy H. Heavy ions in cancer therapy. JAMA Oncol. 2016;2:1539–40. doi: 10.1001/jamaoncol.2016.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. .Mizoe JE, Tsujii H, Hasegawa A, Yanagi T, Takagi R, Kamada T, Tsuji H, Takakura K. Organizing Committee of the Central Nervous System Tumor Working Group. Phase I/II clinical trial of carbon ion radiotherapy for malignant gliomas: combined X-ray radiotherapy, chemotherapy, and carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:390–6. doi: 10.1016/j.ijrobp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 49. .Hadziahmetovic M, Shirai K, Chakravarti A. Recent advancements in multimodality treatment of gliomas. Future Oncol. 2011;7:1169–83. doi: 10.2217/fon.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. .Takahashi S, Kawase T, Yoshida K, Hasegawa A, Mizoe JE. Skull base chordomas: efficacy of surgery followed by carbon ion radiotherapy. Acta Neurochir (Wien) 2009;151:759–69. doi: 10.1007/s00701-009-0383-5. [DOI] [PubMed] [Google Scholar]
- 51. .Shirai K, Koto M, Demizu Y, Suefuji H, Ohno T, Tsuji H, Okimoto T, Shioyama Y, Saitoh JI, Nemoto K, Nakano T, Kamada T. Japan Carbon-Ion Radiation Oncology Study Group. Multi-institutional retrospective study of mucoepidermoid carcinoma treated with carbon-ion radiotherapy. Cancer Sci. 2017;108:1447–51. doi: 10.1111/cas.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. .Shirai K, Kubota Y, Ohno T. Saitoh J-i, Abe T, Mizukami T, Mori Y, Kawamura H, Akahane K, Nakano T. Carbon-ion radiotherapy for isolated lymph node metastasis after surgery or radiotherapy for lung cancer. Front Oncol. 2019;9:731. doi: 10.3389/fonc.2019.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. .Shirai K, Ohno T, Saitoh JI, Okamoto M, Katoh H, Murata K, Kawamura H, Musha A, Abe T, Mizukami T, Akahane K, Nakano T. Prospective study of isolated recurrent tumor re-irradiation with carbon-ion beams. Front Oncol. 2019;9:181. doi: 10.3389/fonc.2019.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. .Shirai K, Saitoh JI, Musha A, Abe T, Kobayashi D, Takahashi T, Tamaki T, Kawamura H, Takayasu Y, Shino M, Toyoda M, Takahashi K, Hirato J, Yokoo S, Chikamatsu K, Ohno T, Nakano T. Working Group on H, Neck T. Prospective observational study of carbon-ion radiotherapy for non-squamous cell carcinoma of the head and neck. Cancer Sci. 2017;108:2039–44. doi: 10.1111/cas.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. .Kawashiro S, Yamada S, Isozaki Y, Nemoto K, Tsuji H, Kamada T. Carbon-ion radiotherapy for locoregional recurrence after primary surgery for pancreatic cancer. Radiother Oncol. 2018;129:101–4. doi: 10.1016/j.radonc.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 56. .Kawashiro S, Yamada S, Okamoto M, Ohno T, Nakano T, Shinoto M, Shioyama Y, Nemoto K, Isozaki Y, Tsuji H, Kamada T. Multi-institutional study of carbon-ion radiotherapy for locally advanced pancreatic cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) study 1403 pancreas. Int J Radiat Oncol Biol Phys. 2018;101:1212–21. doi: 10.1016/j.ijrobp.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 57. .Shinoto M, Yamada S, Okamoto M, Shioyama Y, Ohno T, Nakano T, Nemoto K, Isozaki Y, Kawashiro S, Tsuji H, Kamada T. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) study 1404 rectum. Radiother Oncol. 2019;132:236–40. doi: 10.1016/j.radonc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 58. .Wang L, Wang X, Zhang Q, Ran J, Geng Y, Feng S, Li C, Zhao X. Is there a role for carbon therapy in the treatment of gynecological carcinomas? a systematic review. Future Oncol. 2019;15:3081–95. doi: 10.2217/fon-2019-0187. [DOI] [PubMed] [Google Scholar]
- 59. .Kato S, Ohno T, Tsujii H, Nakano T, Mizoe JE, Kamada T, Miyamoto T, Tsuji H, Kato H, Yamada S, Kandatsu S, Yoshikawa K, Ezawa H, Suzuki M. Working Group of the Gynecological Tumor. Dose escalation study of carbon ion radiotherapy for locally advanced carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2006;65:388–97. doi: 10.1016/j.ijrobp.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 60. .Irie D, Okonogi N, Wakatsuki M, Kato S, Ohno T, Karasawa K, Kiyohara H, Kobayashi D, Tsuji H, Nakano T, Kamada T, Shozu M. Working Group of the Gynecological Tumor. Carbon-ion radiotherapy for inoperable endometrial carcinoma. J Radiat Res. 2018;59:309–15. doi: 10.1093/jrr/rry003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. .Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS, Trifiletti DM. Carbon ion therapy: a modern review of an emerging technology. Front Oncol. 2020;10:82. doi: 10.3389/fonc.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]


