Abstract
Purpose
To clarify the dose distribution characteristics for early-stage glottic cancer by comparing the dose distribution between intensity-modulated radiation therapy (IMRT) and passive scattering proton therapy (PSPT) and to examine the usefulness of PSPT for early-stage glottic cancer.
Materials and Methods
Computed tomography datasets of 8 patients with T1-2 glottic cancer who had been treated by PSPT were used to create an IMRT plan in Eclipse with 7 fields and a PSPT plan in XiO-M with 2 fields. Organs at risk (OARs) included the carotid arteries, arytenoids, inferior constrictor muscles, strap muscles, thyroid cartilage, cricoid cartilage, and spinal cord. The prescription dose was 66 GyRBE in 33 fractions to the planning target volume (PTV). All plans were optimized such that 95% of the PTV received 90% of the prescription dose considering that the skin was slightly spared.
Results
The superiority of the PSPT was confirmed in all OARs. In the PSPT, the dose to the contralateral carotid artery and the spinal cord, which is slightly distant from the PTV, was dramatically reduced while maintaining the dose distribution uniformity of the PTV by comparison with IMRT.
Conclusion
PSPT for early-stage glottic cancer resulted in good target dose homogeneity and significantly spared the OARs as compared with the IMRT. PSPT is expected to be effective in reducing late effects and particularly useful for young people.
Keywords: carotid artery, glottic cancer, intensity-modulated radiation therapy, passive scattering proton therapy
Introduction
Early-stage glottic cancer is highly curable with conventional parallel opposed radiation therapy (RT) and is expected to have a long survival. Therefore, late side effects are not ignored especially in patients younger than 60 years [1]. Conventional RT preserves laryngeal function, but bilateral carotid arteries are usually injured from high radiation doses. It is hypothesized that the radiation disrupts the endothelial barrier of carotid vessels. Recently, Fokkema et al [2] examined histologic characteristics of carotid plaques from patients with prior RT and found more fibrous and less inflammatory plaques compared with those derived from non-RT patients. Radiation therapy is one of the risk factors accelerating carotid atherogenesis that may raise cerebrovascular events.
Recent advances in RT technology such as intensity-modulated radiation therapy (IMRT) or proton therapy (PT) are making it possible for us to concentrate dose to specific targets without sacrificing safety. Therefore, making full use of these technologies may reduce the exposure of the carotid arteries or other surrounding normal tissue while maintaining the same therapeutic effect as conventional parallel opposed RT. In addition, because secondary cancer risk in head and neck cancer is not small [3–5], it may be necessary to consider re-irradiation in cases with a history of irradiation. Limiting the dose to the carotid arteries may also allow re-irradiation when necessary. Consideration of advanced RT technologies with minimal impact to surrounding normal tissues is worthwhile in such cases. There are many reports on IMRT for early-stage glottic cancer [6–21], but no reports have yet detailed the dose distribution characteristics of PT for early-stage glottic cancer. In this study, we therefore aimed to clarify the dose distribution between IMRT and PT for early-stage glottic cancer and examined the usefulness of PT for early-stage glottic cancer.
Materials and Methods
Patients
Eight patients (7 males and 1 female) who had been treated by passive scattering PT (PSPT) at the Southern Tohoku Proton Therapy Center (STPTC) for T1-2 glottic cancer were included in the present study. Our study was approved by the institutional review board of STPTC. All patients were informed about standard treatments such as conventional RT or laryngectomy. They agreed to participate in the study, and all participants gave informed consent. Patients were aged 48 to 74 years (median: 65 years).
Computed Tomography Simulation and Target Delineation
All patients underwent computed tomography (CT) simulation at STPTC with Aquilion LB (Canon Medical Systems, Otawara, Japan). Patients were trained not to swallow during the CT imaging. The CT images were obtained along with a conventional scan with 1-mm-thick slices. Patients were immobilized with custom-made thermoplastic casts to provide high reproducibility.
The gross tumor volume was contoured on the basis of electronic endoscope examination and positron emission tomography scans or magnetic resonance imaging or contrast-enhanced CT to obtain accurate tumor delineation. The clinical target volume (CTV) was defined as the gross tumor volume plus a 5-mm margin in all directions except for air cavities. The planning target volume (PTV) was defined as the CTV plus a 3-mm margin in all directions, and an additional 2-mm margin was added in the craniocaudal direction. The organs at risk (OARs) included the carotid arteries, arytenoids, inferior constrictor muscles, strap muscles, thyroid cartilage, cricoid cartilage, and spinal cord. The OAR volumes of the spinal cord and carotid arteries extended 1 cm superiorly and inferiorly beyond the PTV.
Treatment Planning and Plan Evaluation
All calculations for the IMRT plans were performed for 6-MV photons with a calculation grid spacing of 2.5 mm. The accelerator used was Clinac iX (Varian Medical Systems, Palo Alto, California) with 120 multileaf collimator (MLC) leaf pairs, and the leaf widths at the isocenter were 5 mm. IMRT treatment plans were calculated by using the anisotropic analytic algorithm in the Eclipse treatment planning system (Varian Medical Systems). The prescription dose was 66 Gy in 33 fractions to the PTV. All plans were optimized such that 95% of the PTV received 90% of the prescription dose considering that the skin was slightly spared. The dose delivery technique was a dynamic MLC method with 7 fixed ports. The gantry angle was based on uniform arrangement but was slightly fine-tuned for each case.
The PT machine used was a Proton Type (Hitachi, Kashiwa, Japan) with 40 MLC leaf pairs, and the leaf widths at the isocenter were approximately 5 mm. This system comprises an ion source, a 3-MeV radiofrequency quadrupole linear accelerator, a 235-MeV synchrotron, a high-energy beam transport line, and gantry irradiation rooms. The proton beam delivery system includes beam-wobbling magnets, a lead scatterer, main dose monitor, ridge filter, range shifter, backup monitor, flatness monitor, block collimator, MLC, and range compensator. The ridge filters were installed downstream of the main dose monitor to produce spread-out Bragg peaks with lengths ranging from 2 to 14 cm in increments of 1 cm. The wobbler system comprises 2 dipole magnets, and the scattering elements create a broad, flattened beam at the final aperture. This method is known as the single-ring wobbling method, which is one of the PSPT [22, 23]. A commercially available treatment planning system (XiO-M, Elekta, Stockholm, Sweden), was used to calculate the dose distributions for PSPT. PSPT plans were calculated by using a pencil beam algorithm with a calculation grid spacing of 2 mm. At STPTC, we constructed a dose verification system based on Particle Therapy Simulation Framework [24] and we calculated and confirmed that the accuracy was within the clinically acceptable level. Prescription dose was defined similarly to that of IMRT. Irradiation was performed in 2 anterior oblique fields. The angle of fields depended on the patient's body contour and the location of carotid arteries. The margins placed on the CTV were mathematically calculated in PSPT. The expansion of margin placed on the CTV needed to take into account not only penumbra (7 mm) and setup uncertainty (3 mm), but also additional uncertainty (3 mm), and Hounsfield unit uncertainty (3.5%). Here, additional uncertainty accounts for the proton beam energy, the design of the range compensator, and determining the skin surface. Distal margin (DM), proximal margin (PM), lateral margin (LM), and smearing margin (SM) on the CTV for each beam were calculated by using the following formulas as reported by Moyers et al [25]. The radiation field was formed by using the MLC built in the snout, and the LM was set to 10 mm from CTV.
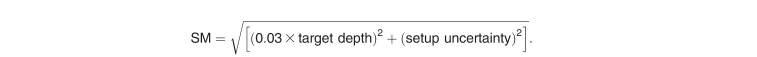 |
Median DM and PM were 3.8 mm (range, 3.3–4.5 mm) and 3.1 mm (range, 3.0–3.3 mm), respectively. Dose-volume data for all contoured structures were evaluated. The maximum dose and homogeneity index were used to evaluate the PTV dose heterogeneity. Homogeneity index was calculated by dividing the maximum dose of PTV by the minimum dose. Emerging data suggest that there is a dose-response threshold for radiation effects on the carotid arteries. Martin et al [26] observed that intimal-medial thickness was only statistically significant at doses between 35 and 50 Gy. Consequently, for this series, we selected the fractional volume of the carotid arteries receiving 35 GyRBE (V35) or 50 GyRBE (V50) as reference dose-volume parameters as Rosenthal et al [6] reported. Average carotid artery doses were also evaluated for reference. For other OARs, averaged maximum dose was evaluated. A 2-tailed t test was used for comparison. P values <.05 were considered statistically significant. All statistical analyses were performed by using JMP Pro version 12 (SAS Institute Inc, Cary, North Carolina).
Results
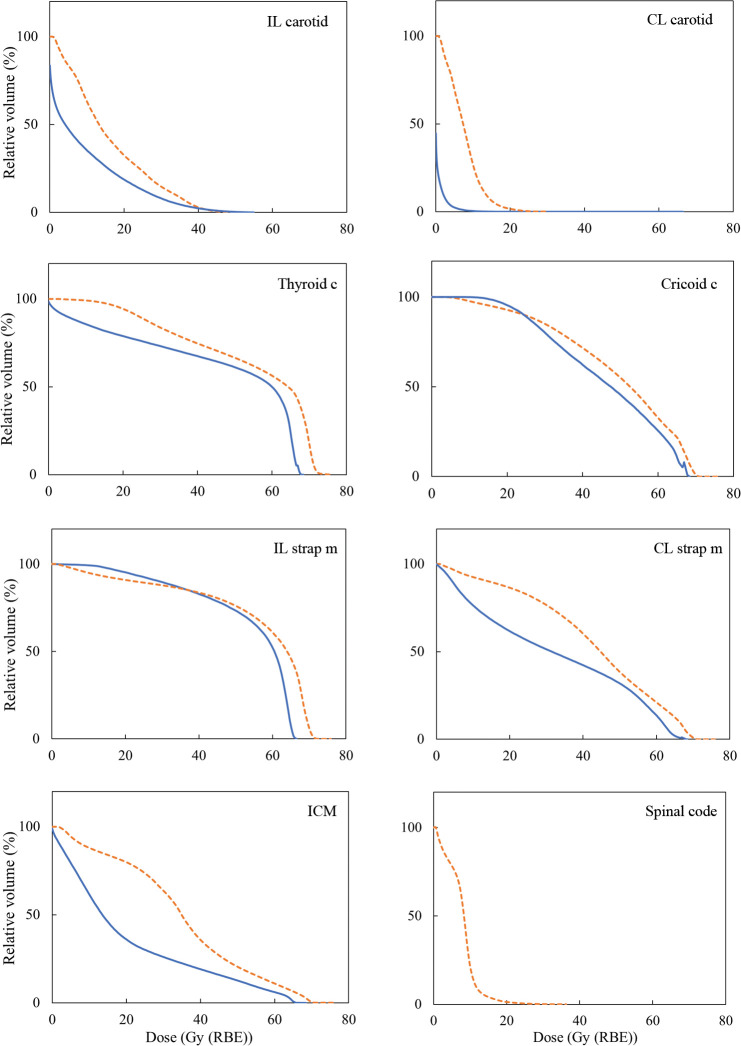
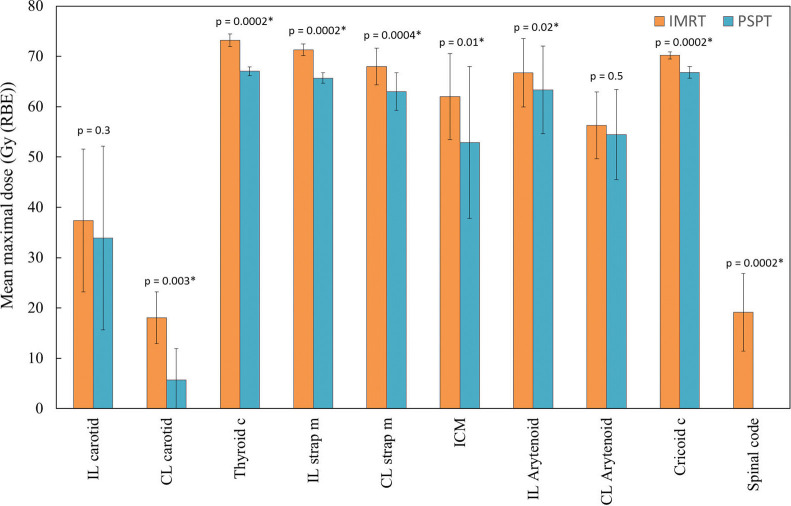
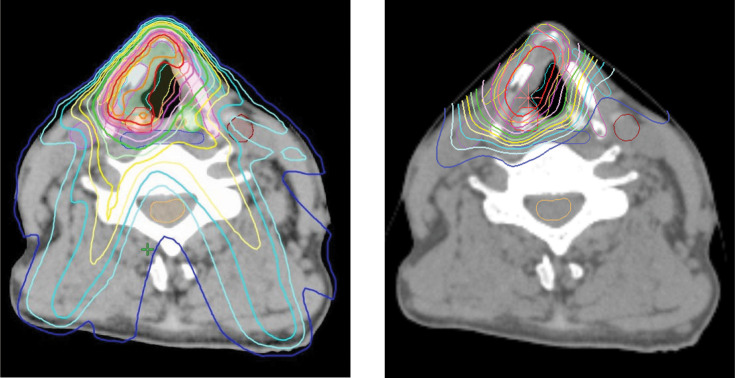
Figure 1 shows mean dose-volume histogram comparisons illustrating the doses received by carotid arteries, thyroid cartilage, cricoid cartilage, strap muscles, inferior constrictor muscles, and spinal cords when using IMRT (dotted lines) or PSPT (solid lines). Figure 2 shows the mean maximal dose for each OAR, and PSPT was superior to IMRT in all OARs. Figure 3 shows an example of typical dose distributions. Dose distribution was very limited in the PT, while the middle- to low-dose range tended to spread with IMRT. Main dose-volume parameters are tabulated in the Table. The V35 of both carotid arteries showed no significant difference between IMRT and PT, and both methods contributed to reduction of the carotid artery dose. The V50 of both carotid arteries was confirmed to be almost zero in each method. In IMRT, the average maximum dose to the contralateral carotid artery was 18.1 Gy, and although the effect of reduction was observed, the PTV tended to have a small hot spot. By contrast, in the PT, the dose to the contralateral carotid artery and spinal cord, which is slightly distant from the PTV, was dramatically reduced while the dose distribution homogeneity of the PTV was maintained.
Figure 1.
Population mean dose-volume histograms (n = 8) from 2 planning techniques shown for selected structures. Orange dotted lines and blue solid lines indicate intensity-modulated radiation therapy and passive scattering proton therapy, respectively. Abbreviations: c, cartilage; CL, contralateral; ICM, inferior constrictor muscle; IL, ipsilateral; m, muscle.
Figure 2.
Mean maximal doses to different structures from 2 planning techniques. Error bars represent standard deviation. *Statistically significant. Abbreviations: c, cartilage; CL, contralateral; ICM, inferior constrictor muscle; IL, ipsilateral; IMRT, intensity-modulated radiation therapy; m, muscle; PSPT, passive scattering proton therapy.
Figure 3.
Dose distributions at the level of the true vocal cords of IMRT (left) and PSPT (right) plans. The 105%, 100%, 95%, 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20%, and 10% isodose lines are denoted in sequential order. Abbreviations: IMRT, intensity-modulated radiation therapy; PSPT, passive scattering proton therapy.
Table.
Dosimetric parameters for IMRT and PSPT plans.
|
IMRT |
PSPT |
P
value |
|
| PTV | |||
| Max dose (GyRBE) | 73.7 ± 1.3 | 67.5 ± 0.9 | <.0001 |
| HI | 2.1 ± 0.6 | 1.3 ± 0.1 | .0017 |
| IL carotid | |||
| V35 (cm3) | 0.5 ± 0.5 | 0.3 ± 0.5 | .45 |
| Mean dose (GyRBE) | 17.0 ± 7.5 | 9.5 ± 6.7 | .053 |
| CL carotid | |||
| V35 (cm3) | 0.0 ± 0.0 | 0.0 ± 0.0 | N/A |
| Mean dose (GyRBE) | 8.1 ± 2.9 | 0.7 ± 0.7 | .00013 |
Abbreviations: IMRT, intensity-modulated radiation therapy; PSPT, passive scattering proton therapy; PTV, planning target volume; Max, maximum; GyRBE, gray relative biological effectiveness; HI, homogeneity index; IL, ipsilateral; V35, volume receiving dose ≥35 GyRBE; CL, contralateral; N/A, not applicable.
Discussion
Recently, several studies have highlighted the increased risk of stroke and direct carotid artery injury during high-dose irradiation of the carotid arteries [1, 2, 27]. Dorresteijn et al [1] reported that the relative risk of ischemic stroke after radiation treatments to the neck was 10.1 times greater in patients aged <60 years than that of an age- and sex-matched population-based cohort. High-dose irradiation of the carotid arteries may have some late side effects especially for patients with early-stage glottic cancer with expected disease-specific survival of >5 years. By contrast, substantial increased risk of stroke was experienced in non–high-dose irradiation cases such as treatment of Hodgkin lymphoma [28, 29]. Radiation vasculopathy is thought to be both time and dose dependent, so one would expect that the larger the dose, the greater the effect and the longer the time after exposure, the more severe the expected stenosis [30]. In view of these previous reports, efforts shall be made to keep the dose to the carotid artery to a minimum as much as possible. Many investigators have reported that IMRT techniques significantly reduce unnecessary radiation to the carotid arteries [6–21]. Although PT is another promising technology, few previously published studies have explored PT for early-stage glottic cancer. Therefore, this study attempted to clarify the dose distribution between IMRT and PT for early-stage glottic cancer and examined the usefulness of PT for early-stage glottic cancer.
As a result, we found that all of our cases contained an ipsilateral carotid artery mean maximum dose of 33.9 GyRBE (range, 4.9–54.3 GyRBE), and that to the contralateral carotid artery was 5.7 GyRBE (range, 0.5–17.4 GyRBE) in PT. The average maximum dose to contralateral carotid artery in IMRT was 18.1 Gy (range, 10.6–27.1 Gy) and there was a significant difference in the results with PSPT. It was found that the difference of the average maximum dose to contralateral carotid artery between IMRT and PT was 12.4 GyRBE (range, 1.7–26.6 GyRBE), and the impact varies greatly depending on the case. Although the clinical significance of these differences is unknown at this time, it would be better that the carotid artery dose be as low as possible, and the PSPT has advantages over IMRT.
IMRT was also found to be sufficiently durable for clinical application; however, a trade-off is required to reduce the dose; a small increase in dose to spinal cord was observed. In addition, Asher et al [31] also discussed the influence of air cavities within PTV on IMRT planning for glottic cancer. Although dose-volume histogram results suggest that IMRT performs at a clinically acceptable level, it must be applied carefully with thorough follow-up after irradiation in clinical practice. Our results also showed that the dose reaching the spinal cord with PSPT is zero. Rosenthal et al [6] stated that dosimetric advantages may be especially important for younger patients at risk for subsequent development of a metachronous second head and neck cancer. In this context, lower doses to spinal cord are highly advantageous. Proton therapy may therefore gain better tumor control with less collateral damage to normal tissues. However, owing to the limited resources of PT, further increase in the number of PT facilities will be required for this to be actively used in the future.
As shown in Figure 2, the mean maximal doses of OARs from 2 planning techniques tended to be higher in the present study than in that of Osman et al [9]. This may be because the CTV and PTV margins in this study are relatively large. The length of the irradiated carotid arteries depends on the length of the margin caused by breathing and swallowing. Osman et al [32] reported that the larynx moves by an average of 2 mm owing to respiration, through analysis using 4-dimensional CT; therefore, care must be taken in setting the safety margin in the craniocaudal direction. It is important to note that the margin sizes were set wider in this study as a conservative measure. A more important factor affecting spatial uncertainty is swallowing control. Instructing patients not to swallow during irradiation is insufficient, and it is desirable to take some aggressive measures. At our institution, organ motion problems were excellently solved by using a respiratory gating system. Figure 4 shows how the larynx movement was monitored during the actual PT for glottic cancer. By applying the respiratory monitoring system AZ-733V (Anzai Medical, Tokyo, Japan), it became possible to treat while monitoring the absence of swallowing in real time. Radiation therapists stop the proton beam immediately when they detect swallowing with this system, and patients are also able to press the bottom to notify them of the swallowing. Therefore, no swallowing was observed during the entire course of PT in our study.
Figure 4.
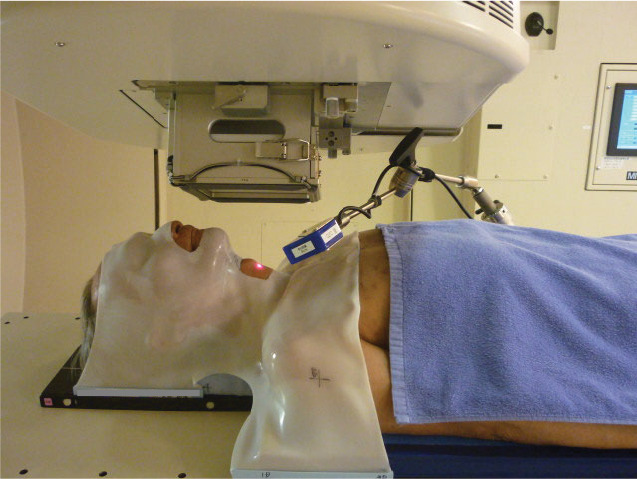
An overview of the real-time monitoring of larynx movement by applying the respiratory monitoring system during PSPT. Abbreviation: PSPT, passive scattering proton therapy.
In recent years, IMPT with pencil beam scanning has become widely used [33], but no report actively describes this for early-stage glottic cancer. Because glottic cancer is located in a shallow region, the incident energy of the proton beam must be reduced. However, the beam spot size increases as the energy decreases [34], and as a result the dose distribution may be worse than that of PSPT in some cases. The use of a universal bolus to reduce the beam spot size is being actively studied in the head and neck region [35], and this method is considered better in terms of dose distribution when applied to glottic cancer. However, because the universal bolus needs to be installed to cover the entire target, the method for monitoring the larynx in real time as described above may be difficult to apply, so another countermeasure must be considered. In IMRT, a bolus may be required as in electron therapy to increase the surface dose especially for anterior tumors [6, 10], but similar problems arise. By contrast, the contours of the cervix vary greatly between individuals, and in some cases, the skin cannot be adequately spared by PSPT; thus, pencil beam scanning may be effective in such cases. Moreover, the beam spot size has become smaller owing to the improved beamlines, and collimation such as using aperture for IMPT is also practical [36]. IMPT technology is still evolving and how the dose distribution changes when IMPT is used for early-stage glottic cancer is a topic for future studies. While PT is expected to be particularly effective for early-stage glottic cancer in young people, the optimal treatment parameters for PT, such as the number of fractions, the dose per fraction, and how to delineate the CTV, have not yet been established. Therefore, further investigation of this issue is necessary.
Conclusion
The dose distribution characteristics of PSPT for early-stage glottic cancer were investigated by comparison with IMRT. Using PSPT, the homogeneity of dose distribution in PTV was maintained, and the contralateral carotid artery was adequately spared without increasing the spinal cord dose, thus confirming its superiority. Although further investigation of the optimal dose fractionation will be necessary, the use of PT for early-stage glottic cancer is effective in reducing late effects, and it is expected that this will be particularly useful for young people.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
Funding: The authors have no funding to disclose.
Ethical Approval: All patient data have been collected under internal review board (IRB)–approved protocol.
References
- 1. .Dorresteijn LD, Kappelle AC, Boogerd W, Klokman WJ, Balm AJ, Keus RB, van Leeuwen FE, Bartelink H. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–8. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 2. .Fokkema M, den Hartog AG, van Lammeren GW, Bots ML, Pasterkamp G, Vink A, Moll FL, de Borst GJ. Radiation-induced carotid stenotic lesions have a more stable phenotype than de novo atherosclerotic plaques. Eur J Vasc Endovasc Surg. 2012;43:643–8. doi: 10.1016/j.ejvs.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 3. .Fujita M, Rudoltz MS, Canady DJ, Patel P, Machtay M, Pittard MQ, Mohiuddin M, Regine WF. Second malignant neoplasia in patients with T1 glottic cancer treated with radiation. Laryngoscope. 1998;108:1853–5. doi: 10.1097/00005537-199812000-00016. [DOI] [PubMed] [Google Scholar]
- 4. .Narayana A, Vaughan AT, Fisher SG, Reddy SP. Second primary tumors in laryngeal cancer: results of long-term follow-up. Int J Radiat Oncol Biol Phys. 1998;42:557–62. doi: 10.1016/s0360-3016(98)00250-8. [DOI] [PubMed] [Google Scholar]
- 5. .Holland JM, Arsanjani A, Liem BJ, Hoffelt SC, Cohen JI, Stevens KR., Jr Second malignancies in early stage laryngeal carcinoma patients treated with radiotherapy. J Laryngol Otol. 2002;116:190–3. doi: 10.1258/0022215021910500. [DOI] [PubMed] [Google Scholar]
- 6. .Rosenthal DI, Fuller CD, Barker JL, Jr, Mason B, Garcia JA, Lewin JS, Holsinger FC, Stasney CR, Frank SJ, Schwartz DL, Morrison WH, Garden AS, Ang KK. Simple carotid-sparing intensity-modulated radiotherapy technique and preliminary experience for T1–2 glottic cancer. Int J Radiat Oncol Biol Phys. 2010;77:455–61. doi: 10.1016/j.ijrobp.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. .Chera BS, Amdur RJ, Morris CG, Mendenhall WM. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys. 2010;77:1380–5. doi: 10.1016/j.ijrobp.2009.07.1687. [DOI] [PubMed] [Google Scholar]
- 8. .Levendag PC, Teguh DN, Keskin-Cambay F, Al-Mamgani A, van Rooij P, Astreinidou E, Kwa SL, Heijmen B, Monserez DA, Osman SO. Single vocal cord irradiation: a competitive treatment strategy in early glottic cancer. Radiother Oncol. 2011;101:415–9. doi: 10.1016/j.radonc.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 9. .Osman SO, Astreinidou E, de Boer HC, Keskin-Cambay F, Breedveld S, Voet P, Al-Mamgani A, Heijmen BJ, Levendag PC. IMRT for image-guided single vocal cord irradiation. Int J Radiat Oncol Biol Phys. 2012;82:989–97. doi: 10.1016/j.ijrobp.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 10. .Camingue P, Christian R, Ng D, Williams P, Amin M, Roniger DL. Comparison of external beam treatment techniques for T1–2, N0, M0 glottic cancers. Med Dosim. 2012;37:221–4. doi: 10.1016/j.meddos.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 11. .Nguyen NP, Chi A, Betz M, Almeida F, Vos P, Davis R, Slane B, Ceizyk M, Abraham D, Smith-Raymond L, Stevie M, Jang S, Gelumbauskas S, Vinh-Hung V. Feasibility of intensity-modulated and image-guided radiotherapy for functional organ preservation in locally advanced laryngeal cancer. PLoS One. 2012;7:e42729. doi: 10.1371/journal.pone.0042729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. .Matthiesen C. De La Fuente Herman T, Singh H, Mascia A, Confer M, Simpson H, Higby C, Arain A, Keole S, Herman T, Bogardus C, Zhao YD, Ahmad S. Dosimetric and radiobiologic comparison of 3D conformal, IMRT, VMAT and proton therapy for the treatment of early-stage glottic cancer. J Med Imaging Radiat Oncol. 2015;59:221–8. doi: 10.1111/1754-9485.12227. [DOI] [PubMed] [Google Scholar]
- 13. .Zumsteg ZS, Riaz N, Jaffery S, Hu M, Gelblum D, Zhou Y, Mychalczak B, Zelefsky MJ, Wolden S, Rao S, Lee NY. Carotid sparing intensity-modulated radiation therapy achieves comparable locoregional control to conventional radiotherapy in T1–2N0 laryngeal carcinoma. Oral Oncol. 2015;51:716–23. doi: 10.1016/j.oraloncology.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. .Al-Mamgani A, Kwa SL, Tans L, Moring M, Fransen D, Mehilal R, Verduijn GM, Baatenburg de Jong RJ, Heijmen BJ, Levendag PC. Single vocal cord irradiation: image guided intensity modulated hypofractionated radiation therapy for T1a glottic cancer: early clinical results. Int J Radiat Oncol Biol Phys. 2015;93:337–43. doi: 10.1016/j.ijrobp.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 15. .Yeo SG. Volumetric modulated arc radiotherapy of the whole larynx, followed by a single affected vocal cord, for T1a glottic cancer: dosimetric analysis of a case. Mol Clin Oncol. 2016;4:429–32. doi: 10.3892/mco.2016.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. .Hong CS, Oh D, Ju SG, Ahn YC, Noh JM, Chung K, Kim JS, Suh TS. Carotid-sparing TomoHelical 3-dimensional conformal radiotherapy for early glottic cancer. Cancer Res Treat. 2016;48:63–70. doi: 10.4143/crt.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. .Choi HS, Jeong BK, Jeong H, Song JH, Kim JP, Park JJ, Woo SH, Kang KM. Carotid sparing intensity modulated radiotherapy on early glottic cancer: preliminary study. Radiat Oncol J. 2016;34:26–33. doi: 10.3857/roj.2016.34.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. .Rock K, Huang SH, Tiong A, Lu L, Xu W, Ringash J, Bratman SV, Tong L, Chan B, Cho J, Giuliani M, Hope A, Bayley A, Kim J, de Almeida JR, O'Sullivan B, Waldron J. Partial laryngeal IMRT for T2N0 glottic cancer: impact of image guidance and radiation therapy intensification. Int J Radiat Oncol Biol Phys. 2018;102:941–9. doi: 10.1016/j.ijrobp.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 19. .Zhang Y, Chiu T, Dubas J, Tian Z, Lee P, Gu X, Yan Y, Sher D, Timmerman R, Zhao B. Benchmarking techniques for stereotactic body radiotherapy for early-stage glottic laryngeal cancer: LINAC-based non-coplanar VMAT vs. Cyberknife planning. Radiat Oncol. 2019;14:193. doi: 10.1186/s13014-019-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. .Chung SY, Lee CG. Feasibility of single vocal cord irradiation as a treatment strategy for T1a glottic cancer. Head Neck. 2019;19:1–6. doi: 10.1002/hed.26052. [DOI] [PubMed] [Google Scholar]
- 21. .Cho IJ, Chung WK, Lee JK, Lee MC, Paek J, Kim YH, Jeong JU, Yoon MS, Song JY, Nam TK, Ahn SJ, Lee DH, Yoon TM, Lim SC. Intensity-modulated radiotherapy for stage I glottic cancer: a short-term outcomes compared with three-dimensional conformal radiotherapy. Radiat Oncol J. 2019;37:271–8. doi: 10.3857/roj.2019.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. .Himukai T, Takada Y, Hotta K, Hara Y, Komori M, Kanai T, Kohno R. Analytical design method of optimum ridge filters for wobbled and collimated proton beams. Jpn J Med Phys. 2008;28:57–69. [PubMed] [Google Scholar]
- 23. .Kase Y, Yamashita H, Numano M, Sakama M, Mizota M, Maeda Y, Tameshige Y, Murayama S. A model-based analysis of a simplified beam-specific dose output in proton therapy with a single-ring wobbling system. Phys Med Biol. 2015;60:359–74. doi: 10.1088/0031-9155/60/1/359. [DOI] [PubMed] [Google Scholar]
- 24. .Akagi T, Aso T, Faddegon B, Kimura A, Matsufuji N, Nishio T, Omachi C, Paganetti H, Perl J, Sasaki T, Sawkey D, Schümann J, Shin J, Toshito T, Yamashita T, Yoshida H. The PTSim and TOPAS projects, bringing Geant4 to the particle therapy clinic. Prog Nucl Sci Technol. 2011;2:912–7. [Google Scholar]
- 25. .Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–38. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 26. .Martin JD, Buckley AR, Graeb D, Walman B, Salvian A, Hay JH. Carotid artery stenosis in asymptomatic patients who have received unilateral head-and-neck irradiation. Int J Radiat Oncol Biol Phys. 2005;63:1197–205. doi: 10.1016/j.ijrobp.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 27. .Swisher-McClure S, Mitra N, Lin A, Ahn P, Wan F, O'Malley B, Weinstein GS, Bekelman JE. Risk of fatal cerebrovascular accidents after external beam radiation therapy for early-stage glottic laryngeal cancer. Head Neck. 2014;36:611–6. doi: 10.1002/hed.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. .Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–37. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 29. .De Bruin ML, Dorresteijn LDA, van't Veer MB, Krol ADG, van der Pal HJ, Kappelle AC, Boogerd W, Aleman BMP, van Leeuwen FE. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–37. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 30. .Scott AS, Parr LA, Johnstone PA. Risk of cerebrovascular events after neck and supraclavicular radiotherapy: a systematic review. Radiother Oncol. 2009;90:163–5. doi: 10.1016/j.radonc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 31. .Asher D, Amestoy W, Studenski MT, Samuels SE, Abramowitz MC, Freedman LM, Elsayyad N, Samuels MA. Dosimetric comparison of intensity-modulated radiation therapy for early-stage glottic cancers with and without the air cavity in the planning target volume. Med Dosim. 2019;44:405–8. doi: 10.1016/j.meddos.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 32. .Osman SO, de Boer HC, Heijmen BJ, Levendag PC. Four-dimensional CT analysis of vocal cords mobility for highly focused single vocal cord irradiation. Radiother Oncol. 2008;89:19–27. doi: 10.1016/j.radonc.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 33. .Yuan TZ, Zhan ZJ, Qian CN. New frontiers in proton therapy: applications in cancers. Cancer Commun (Lond) 2019;39:61. doi: 10.1186/s40880-019-0407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. .Rana S, Samuel EJJ. Feasibility study of utilizing XRV-124 scintillation detector for quality assurance of spot profile in pencil beam scanning proton therapy. Phys Med. 2019;66:15–20. doi: 10.1016/j.ejmp.2019.09.078. [DOI] [PubMed] [Google Scholar]
- 35. .Both S, Shen J, Kirk M, Lin L, Tang S, Alonso-Basanta M, Lustig R, Lin H, Deville C, Hill-Kayser C, Tochner Z, McDonough J. Development and clinical implementation of a universal bolus to maintain spot size during delivery of base of skull pencil beam scanning proton therapy. Int J Radiat Oncol Biol Phys. 2014;90:79–84. doi: 10.1016/j.ijrobp.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 36. .Bäumer C, Janson M, Timmermann B, Wulff J. Collimated proton pencil-beam scanning for superficial targets: impact of the order of range shifter and aperture. Phys Med Biol. 2018;63:085020. doi: 10.1088/1361-6560/aab79c. [DOI] [PubMed] [Google Scholar]