Abstract
A persistent challenge in enhancing gene therapy is the transient availability of the target gene product. This is particularly true in tissue engineering applications. The transient exposure of cells to the product could be insufficient to promote tissue regeneration. Here we report the development of a new material engineered to have a high affinity for a therapeutic gene product. We focus on insulin-like growth factor–I (IGF-I) for its highly anabolic effects on many tissues such as spinal cord, heart, brain and cartilage. One of the ways that tissues store IGF-I is through a group of insulin like growth factor binding proteins (IGFBPs), such as IGFBP-5. We grafted the IGF-I binding peptide sequence from IGFBP-5 onto alginate in order to retain the endogenous IGF-I produced by transfected chondrocytes. This novel material bound IGF-I and released the growth factor for at least 30 days in culture. We found that this binding enhanced the biosynthesis of transfected cells up to 19-fold. These data demonstrate the coordinated engineering of cell behavior and material chemistry to greatly enhance extracellular matrix synthesis and tissue assembly, and can serve as a template for the enhanced performance of other therapeutic proteins.
Keywords: Osteoarthritis, Growth Factor, IGF-I, Binding Peptide
Graphical Abstract

1. INTRODUCTION
A number of recent studies have focused on delivering therapeutic proteins using gene therapy to enhance the repair of skeletal muscle, brain, spinal cord, and cartilage[1–3]. Many of these studies have focused on a variety of membrane repair proteins, transcription factors, and growth factors to increase cellular synthesis and control paracrine cascades[1,4,5]. However, one of the main limiting factors in the efficacy of gene therapy is the transient availability of the gene product, regardless of the vector used. For example the use of gene therapy in cartilage repair has targeted increased growth factor production; however, the desired gene product is typically upregulated for up to two weeks[6,7]. Further, the highest concentrations of growth factors are in the first days after transfection, and have the potential to produce supra-therapeutic, or toxic levels of the growth factor[8].
One of the proteins commonly targeted for cartilage repair in gene therapy is insulin-like growth factor–I (IGF-I)[7–10]. Various viral and nonviral vectors have been used to transduce/transfect chondrocytes to upregulate IGF-I synthesis prior to therapeutic cell delivery[7,11–13]. Although IGF-I expression can remain elevated for a month or more[14], this expression decreases with time, and is often low by 2 weeks[7,15]. This is an extremely short time compared to the 6–12 months typically needed for effective cartilage repair[16], and may not induce optimal repair in vivo. As such, there is a great need for approaches to extend the availability of IGF-I for cartilage therapy.
In tissues such as cartilage, IGF-I is retained in the tissue by a family of binding proteins called insulin-like growth factor binding proteins (IGFBPs). These binding proteins are highly specific to IGF-I, with binding affinities of 1–10 nM[17]. IGFBPs also bind to cartilage enhanced cellular matrix (ECM), acting as both a sink and source for the growth factor as needed[18]. It has been shown that IGFBP-5 has a specific localized small domain that is largely responsible for its high affinity binding to IGF-I[17].
Cell-based gene therapy for articular cartilage repair requires a means of delivering cells to the site in need of repair[19]. There has been increased attention to the development of biomaterials that prolong release and expression of gene products. Some of those biomaterials are chitosan, poly-L-lactic acid (PLLA), poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx), polyethyleminine (PEI), and polyethyleneglycol (PEG). Chondrocytes have been cultured in chitosan-fabricate and a plasmid DNA scaffold to study cell proliferation, adherence, and transforming growth factor-ß1 (TGF-ß1) expression[20] and chitosan-pEGFP nanoparticles have been used to transfer exogenous genes into primary chondrocytes for the treatment of joint diseases[21]. Trimethylated chitosan (TMO) was synthesized from oligometric chitosan to deliver luciferase plasmid DNA to epithelial cells[22]. PLLA has been used with mesenchymal stem cells (MSCs) transfected with adenoviral SOX-9 to differentiate monolayer MSCs into chondrocyte-like cells[23]. PHBHHx scaffolds have been seeded with chondrocytes which employed tetracycline (Tet-on) to induce Sox9 expression[24]. Modified polyethylenimine (PEI) exhibited lower toxicity and higher gene expression of plasmid DNA in COS-7 cells and HepG2cells[25]. Polyethylene glycol-grafted polyethylenimine (PEG-PEI) has been used as a vector to deliver gene products to adipose stem cells to differentiate them to cartilage or osteoblast cells[26]. Hydrogels, including alginate, have also been shown to serve this purpose[27]. There are numerous examples of short peptide sequences being grafted to materials to enhance cell adhesion[17,28,29]. Similarly, materials have been modified to contain heparin-like carbohydrate components which have been shown to enhance binding of growth factors such as FGF-2[30]. All of the previous materials have been used to promote gene delivery and to prolong the release and expression of the gene product, but none of these materials have interacted in any specific way with the proteins that are targeted for production by the cells. The goal of this study was to develop a material that could bind to the targeted gene product and assess whether that binding will enhance matrix production. To our knowledge, peptide-based modification of materials to localize growth factors has not been reported.
Here we demonstrate the development of a new material with high affinity for IGF-I by grafting a binding peptide sequence from IGFBP-5 onto alginate. This new material greatly extends the availability of the growth factor during chondrocyte culture and enhances cartilage matrix biosynthesis up to 19-fold.
2. MATERIALS AND METHODS
2.1. Chemistry
UP LVG alginate (from NovaMatrix®) was modified with carbodiimide chemistry[28]. 100 mg alginate (1% w/v alginate solution) was dissolved in 0.1 M of 1-(N-morpholino) ethanesulfonic acid (MES) buffer (Sigma-Aldrich) at pH 6.5. The reaction scheme is shown in Figure 1 where amide linkages were obtained by adding 2.5 mM of 1-ethyl-(dimethylaminopropyl) carbodiimide (EDC) (Sigma-Aldrich) and 1.2 mM of sulfo-N-hydroxysuccinimide (NHS) to raise the efficiency of amide bond formation (Pierce Chemical)[28]. 1 mg of GGG-K(ivDde)PLALL peptide sequence (INNOVAGEN) was added after 5 minutes; the solution was left to react for 20 hrs. The reaction was quenched with 0.026 mM of hydroxyamine hydrochloride (Sigma-Aldrich) at 20 hrs. Alginate solution was purified by dialysis (3500 MWCO) (Fisher Scientific) with different salt concentrations varying 115 mM to 0 mM. Samples were stored at −80 °F for 24 hours and then they were lyophilized for 24 hours. The additional 3 glycines at the N-terminus of this synthetic peptide sequence were added in order to provide a linker layer that would more fully expose the active peptides in the sequence once the peptide was bound to a surface. The lysine on this sequence was protected from unwanted chemical reactions by an ivDde protecting group; this was removed using 5% hydrazine, anhydrous (Sigma) in dimethylformamide (DMF). Following the deprotection step, the modified alginate solution GGG-KPLALL (KPLHALL) was purified by dialysis (3500 MWCO) (Fisher Scientific) with DI H2O for 24 hours. Samples were stored at −80 °F for 24 hours and then they were lyophilized for 24 hours.
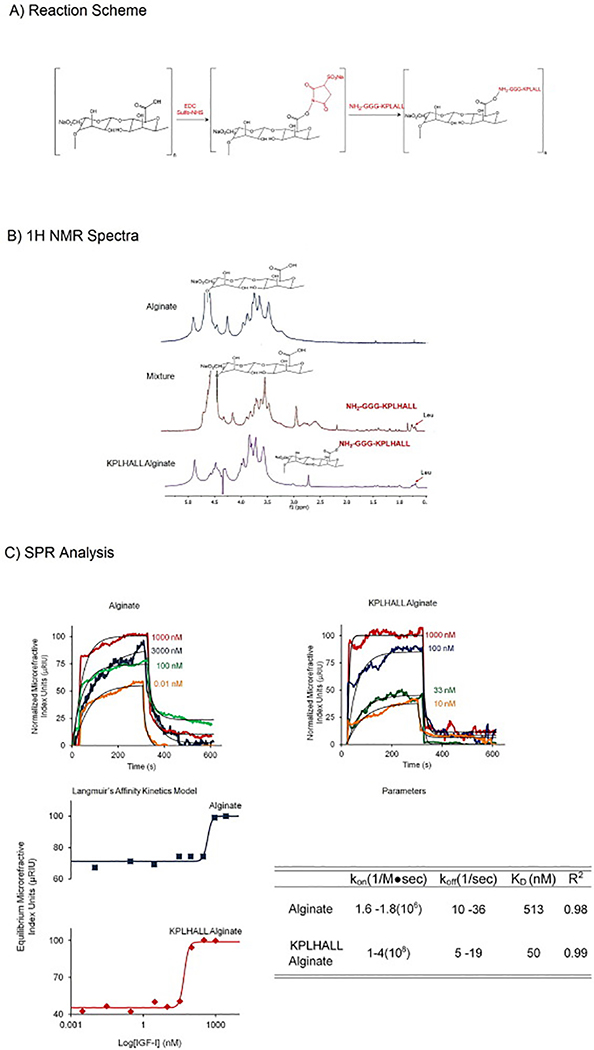
Figure 1.
1A Reaction Scheme: EDC and Sulfo-NHS activate the alginate forming a carboxylate carbon intermediate. The carboxylate carbon intermediate is attacked by the primary amine nitrogen forming the amide bond to the alginate backbone.
1B NMR Spectra: First spectra shows the alginate sample with broad peaks for a polysaccharide, from 4.8 to 3.2, and distinct peak for the anomeric proton[59]. Second spectrum, mixture of the alginate and peptide, shows both different peaks for alginate and high leucine amino acids peaks. The third spectrum shows the modified alginate with KPLHALL where the chemical shift in the leucine peaks and the height of the peaks differs from the mixture.
1C SPR Spectra and Langmuir’s Affinity Kinetics Model: Representative examples of curve fits for the affinity kinetic analysis of control (alginate) and modified alginate (KPLHALL alginate). Concentrations of IGF-I for alginate varied from 3000 nM to 0 nM. Concentrations of IGF-I for KPLHALL alginate varied from 1000 nM to 0 nM.
Langmuir’s Affinity Kinetics Model for alginate is the top one (blue) and KPLHALL alginate is on the bottom (red). KD shifted 10 times, where KD is 50 nM sin KPLHALL alginate (KD of 50 nM) when compared to alginate (KD of 513 nM). KPLHALL alginate had a kon ranging from 1 to 4(108/M●sec) and a koff from 5/s to 19/s (R2: 0.40 to 0.90).The parameters for alginate kon and koff ranged from 1.6 to 1.8(108/Ms) and koff 10/s to 29/s (R2: 0.74 to 0.9).
2.2. Nuclear Magnetic Resonance Studies
The freeze-dried KPLHALL alginate and unmodified alginate were dissolved in D2O at 0.0012% w/v. There were three groups: alginate, a mixture of alginate with free KPLHALL peptide, and KPLHALL-grafted alginate. 1H nuclear magnetic resonance (1H NMR) spectra were recorded on 600MHz Varian Inova NMR. 1H NMR was normalized to residual solvent D2O. The final molar concentration of peptide KPLHALL grafted in alginate was obtained by integrating the area under the curve with MNova NMR Software, 100 μM of binding sites of KPLHALL (0.3% of degree of graft on alginate backbone). Also, diffusion ordered spectroscopy (DOSY) was performed to demonstrate stable attachment of the peptide sequence to the alginate.
2.3. Surface Plasmon Resonance Studies
Surface Plasmon Resonance (SPR) measurements were performed with an SPR Refractometer Instrument (Reichert, Inc., Depew, NY). A range of KPLHALL concentrations from 0 to 100 μM was achieved by mixing the 100 μM KPLHALL with unmodified alginate in varying ratios. Modified and unmodified alginate were bound to 50 nm thick gold chips (Fischer Scientific, Pittsburg, PA) using carbodiimide chemistry. Samples were run at 25° C in buffer for 10 minutes per sample, using a constant flow rate of 25 μl /min over the surface of the SPR chip. Concentrations of IGF-I ranged from 5 to 3000 nM. PBST (PBS plus 0.05% v/v Tween 20) was used as both the sample and flow buffer. After each binding experiment, the surface was regenerated with 40 mM HCl. The sensorgram profiles were analyzed using SigmaPlot where response to equilibrium was calculated with an three parameter exponential rise to maximum model and then a Langumir binding model was used to determine the binding constant (KD) [31].
2.4. Analysis
The microrefractive index unit at equilibrium was measured for each concentration of IGF-I as it flowed through the chip (Equation 1).
Where, Req is the refractive index unit at equilibrium, t is time, t0 is the time after baseline, and Ʈ is the exponential time constant.
KD was calculated by fitting the data to Langmuir’s Affinity Kinetics Model [31] (Equation 2)
Req is the response at equilibrium for each curve; [IGF-I] is the concentration of IGF-I; Rmax is the maximum equilibrium response and KD is the binding affinity constant. The dissociation rate constant (koff) was calculated by fitting the dissociation phase[31] (Equation 3)
Where, RT is the total response where it is assumed that the total complex concentration of IGF-I and KPLHALL [IGF-I*KPLHALL]TOTAL is proportional to the total response (RT). Req is the response at equilibrium for each curve and Roff is the equilibrium response at the end of the dissociation phase. The experiment was measured at different times, t (time where dissociation finished) and t1 (time where dissociation started).
Furthermore, the association rate constant (kon) of each IGF-I concentration was calculated as (Equation 4)
2.5. Cell Culture and Matrix Synthesis
Chondrocytes were isolated as previously described[7,32]. Articular cartilage was harvested from stifle (knee) condyles of 1–3 day old bovids (Bos taurus). Articular cartilage was washed with PBS and 1% antibiotics and antimycotic. Chondrocytes were then isolated by adding 0.3% type 2 collagenase (catalogue number LS004177, Worthington Biochemical, Lakewood, NJ) overnight. Isolated chondrocytes were washed and suspended in DMEM containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS) and placed in T-75 flasks plates at 60% confluency at 37˚C in 5% CO2. After 48 hours, cells were transfected using two different complexes, made 30 minutes before transfection, at a ratio of 3:1[7,10,33]: FuGENE®6 (Roche Applied Science, Indianapolis, IN) + pAAV/IGF-I or FuGENE®6+pAAV/ MCS (Empty). After 16 hours, the transfection was stopped by replacing the culture medium with 5 mL of fresh media without FBS, and 100 U/mL penicillin, 100 μg/mL streptomycin. Afterwards, cells were trypsinized and mixed with 2% (w/v) modified or unmodified alginate. These culture, encapsulation, and transfection methods were selected based on previous work [9] demonstrating the successful formation of tissue in vivo using these protocols.
A range of KPLHALL concentrations from 0 to 100 μM was achieved by mixing the 100 μM KPLHALL with unmodified alginate in varying ratios. Cells were encapsulated in beads formed by extrusion through a 22-gauge needle into a 102 mM CaCl2 solution. Beads were incubated with DMEM without FBS for 30 days; beads were collected every six days.
2.6. Biochemical Analysis
Beads collected every six days for 30 days were used to measure DNA, glycosaminoglycan (GAG) and hydroxyproline (HYPRO) content. DNA content was measured via Hoechst dye assay[34]. GAG content was measured using the DMMB dye-binding assay[35], and HYPRO content was measured using DMAB dye assay[36]. Syntheses of GAG [35] and HYPRO [36] were used as the primary measure of chondrocyte metabolic activity. The kinetics of GAG and HYPRO accumulation were fitted to an established model of matrix synthesis to calculate steady-state GAG and HYPRO content [37] (Equation 5).
Where, [ECM] is the matrix synthesized by the transfected or control of chondrocytes in the different concentrations of KPLHALL. [ECM]SS is steady state matrix production produced by the chondrocytes at different concentrations of KPLHALL. Each steady state value was normalized to alginate (0 μM) steady state value for GAG and HYPRO. These normalized steady-state values of GAG and HYPRO synthesis were used to determine the effect of KPLHALL content on chondrocyte matrix synthesis using a generalized variable slope concentration–response model (Equation 6) [37].
Where, [ECM]SS is the steady state matrix production, [ECM0μM]SS is the steady state matrix production, [ECM]max is the maximum stimulation, [ECM]min is the minimum stimulation, [KPLHALL] is the concentration of KPLHALL-modified alginate, and EC50 is the dose required to produce 50% response.
Beads from day 30 were collected for immunohistochemistry analysis. Samples were treated with citrate antigen retrieval buffer for 10 minutes at 90°C. Slides were then washed with TBS/TWEEN20 and PBS. Samples were placed in humidity chambers for 30 minutes with 3% hydrogen peroxide. Blocking solution (goat serum) was added and incubated for 1 hour at room temperature. Afterwards the primary antibody Rb pAb for IGF-I (ab40657) was applied and left overnight at 4°C. Negative controls were obtained by omitting the primary antibody to a section on each slide (See supplementary figure S1). Next Rabbit IgG for secondary antibody was applied for 30 minutes at room temperature, followed by ABC reagent (Vectastain PK-4000, Vector Labs). Slides were developed with DAB peroxidase (Vector Labs) for approximately 3 minutes.
2.7. Statistical Analysis
GAG and HYPRO data are expressed as mean ±SD. The effect of culture time and transfection were analyzed by 2-way ANOVA using Tukey’s t-test for post hoc analysis performed in SigmaPlot 11 (SYSTAT, Chicago, IL), with significance determined with p<0.05. The temporal patterns in GAG and HYPRO data were then fit to equation 5 using SigmaPlot 11, which calculated best fit values of [ECM]SS and τ, with the uncertainties in these fits expressed as standard error.
Each steady state values and standard errors were normalized to alginate (0 μM) steady state value for GAG and HYPRO. The normalized values were fitted to Equation 6 using software SigmaPlot 11, which enabled the calculation of best fit values for EC50, [ECM]max, and [ECM]min values and their uncertainty, expressed as standard error. Statistical differences between EC50, [ECM]max, and [ECM]min were determined by an unpaired t test using GradPad Prism (GraphPad, Inc., La Jolla, CA).
3. RESULTS
3.1. Generation and Characterization of Hydrogels with Affinity for IGF-I
The peptide sequence KPLHALL from the hydrophobic binding pocket of IGFBP-5 was chosen as a candidate for enhancing IGF-I binding based on analysis of the crystal structure of IGFBP-5 /IGF-I complex. These data showed that 6 of the 7 amino acids in this peptide sequence were within 4Å of IGF-I when the complex is formed, enabling a high level of affinity [38].
The high affinity sequence was synthesized and grafted to alginate via previously established carbodiimide/sulfo-NHS chemistry[39](Fig 1A). The modification of alginate was confirmed by 1H NMR analysis, using the leucine peaks at ~0.7 ppm to both demonstrate successful grafting and measure the amount of conjugated peptide (Fig 1B). Additionally the stability of the peptide graft was demonstrated by diffusion ordered NMR (DOSY), which showed the persistence of leucine peaks in the grafted material (See supplementary figure S2).
Analysis of IGF-I binding to modified and control alginate via surface plasmon resonance (SPR) demonstrated significant enhancement of binding by the modified material (Fig1C). The dissociation constant (KD) decreased from 513 nM to 50 nM due to modification with KPLHALL (Fig1C). This level of affinity for IGF-I was lower than that for full length of IGFBP-5 (KD=3.7 nM), but similar to a truncated form of IGFBP-5 containing only the binding pocket (KD=37 nM)[17].
3.2. Enhancement of IGF-I Binding and Matrix Production
The enhanced binding demonstrated by KPLHALL-modified alginate motivated the hypothesis that this material would retain IGF-I produced by encapsulated chondrocytes. To test this hypothesis, articular chondrocytes were transfected with an adeno-associated virus-based plasmid vector (pAAV/IGF-I), carrying human IGF-I cDNA (transfected chondrocytes) or with empty vector (control chondrocytes) and encapsulated as previously described[33,39] in modified and unmodified alginate and cultured for 30 days.
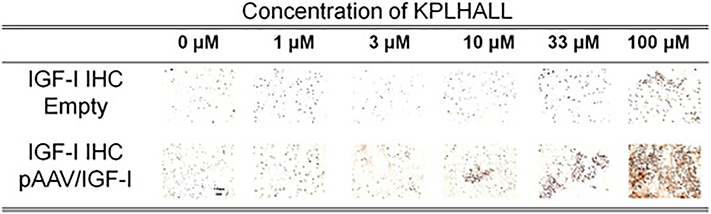
We have previously shown that IGF-I transfection is highly efficient, but also transient, with enhanced IGF-I expression subsiding within 6 days[7,33]. Consistent with this data, immunohistochemistry staining of unmodified alginate (i.e. 0 μM binding peptide) cultures showed minimal presence of IGF-I at 30 days (Fig.2). In contrast, control chondrocytes cultured in alginate modified with KPLHALL showed enhanced retention of IGF-I at 30 days, with IGF-I retention proportional to the concentration of the grafted binding peptide. This enhancement was greater in chondrocytes transfected with pAAV/IGF-I, where the highest concentrations of binding peptide (i.e. 33μM and 100 μM) resulted in robust staining for IGF-I at 30 days (Fig.2).
Figure 2. IGF-I Binding.
Immunohistochemistry of constructs for IGF-I at Day 30. Scale bar = 100 μm. Alginate does not show differences in immunolocalization staining at day 30. Immunolocalization staining for IGF-I changes as the concentration of binding sites (KPLHALL) increases in the constructs. The constructs with 0 μM of binding sites barely show retention of f IGF-I; and constructs with 1 and 3 μM of binding sites show some retention of IGF-I. The highest immunolocalization of IGF-I is at 100 μM.
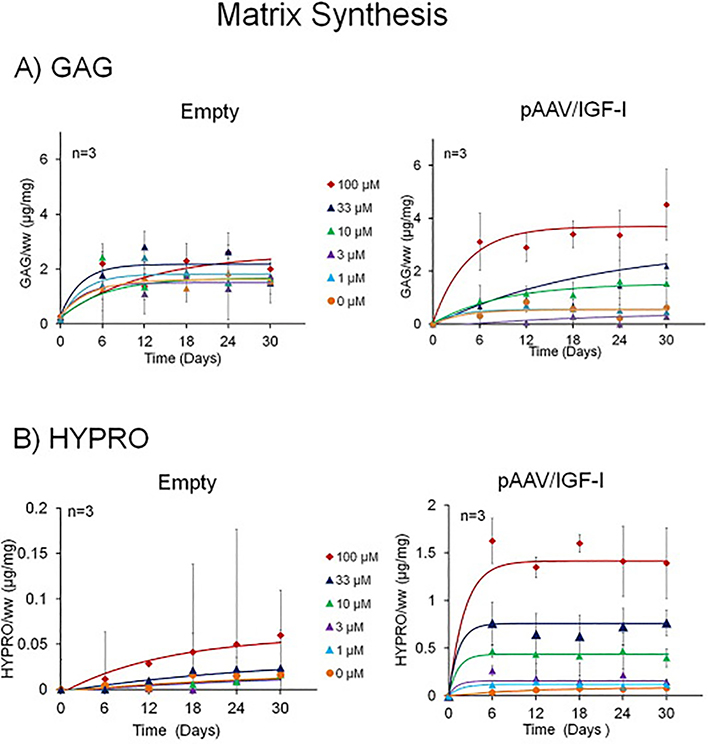
Both empty vector control and pAAV/IGF-I transfected chondrocytes showed accumulation of the principal cartilage matrix components with time. Transfection with p/AAV/IGF-I enhanced accumulation of glycosaminoglycan (GAG) (p<0.001 by 2-way ANOVA) (Fig3A) and collagen, as indicated by hyproxyproline (HYPRO) (p<0.001) when compared to empty vector controls (Fig.3B). These typically reached steady state after two weeks in culture. The average of the time constants (τ) varied from 5.4 ± 3.6 hr to 10.0 ± 8.0 hr for GAG; tau values varied from 3.0 ± 3.7 hr to 27.9 ± 7.2 hr for HYPRO. The presence of peptide binding enhanced matrix synthesis in empty vector and pAAV/IGF-I transfected cultures (*p<0.001 by 2-way ANOVA) for both GAG and HYPRO. In IGF-I transfection had a dramatic effect on HYPRO production both in the presence and absence of the binding peptide (*p<0.01 by 2-way ANOVA).
Figure 3.
3A GAG matrix accumulation kinetic profiles: The production of GAG increases in both transfected and control groups. Control chondrocytes have smaller changes in kinetic profiles between the differences in KPLHALL concentrations. pAAV/IGF-I transfected chondrocytes have a greater effect (*p<0.001 by 2-way ANOVA) between the differences in KPLHALL concentrations.
3B HYPRO matrix accumulation kinetic profiles: The production of HYPRO increases in both pAAV/IGF-I transfected chondrocytes and control chondrocytes. The effect in chondrocytes that are transfected with pAAV/IGF-I is greater than the effect on those transfected with pAAV/MCS (Empty) (p<0.001 by 2-way ANOVA). This effect is greater in concentrations of 10, 33 and 100 μM binding sites (p<0.001 by 2-way ANOVA). When alginate is compared with the highest concentration of binding sites (100 μM) there is an increase from 0.08 μg/mg at 0 uM to 1.4 μg/mg at 100 μM (p<0.001 by 2-way ANOVA)in pAAV/IGF-I transfected chondrocytes.
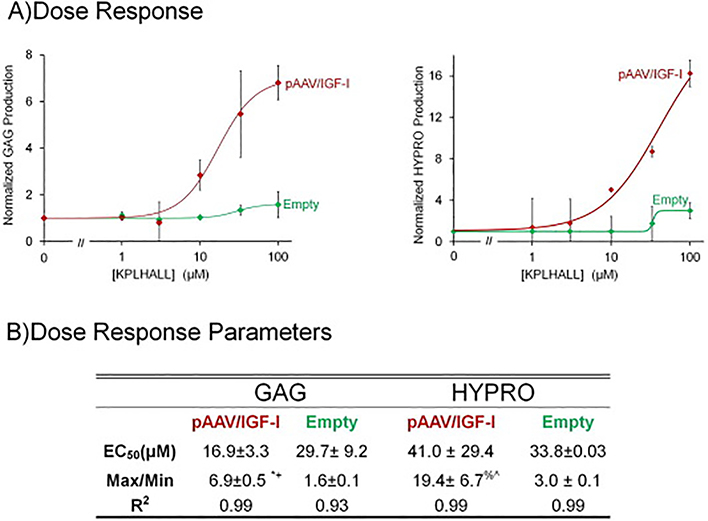
To understand the effect of IGF-I retention via binding peptide sites, the steady-state matrix contents were calculated using an established kinetic model [37]. For each culture condition, steady state matrix contents were normalized to the unmodified alginate (0 μM), which revealed that matrix synthesis was clearly dose-dependent (R2>0.93 for all fits) on binding peptide density (Fig.4A). For the control cells, enhancement of GAG and HYPRO synthesis were 1.6 and 3-fold, respectively. This effect was more dramatic in transfected cells, with GAG and HYPRO synthesis upregulated 6.9 (*p<0.0001 by unpaired t test) and 19.4-fold (%p<0.02 by unpaired t test), respectively. For all cultures, EC50 for enhancement of synthesis ranged from 17 to 41 μM KPLHALL.
Figure 4.
4A GAG and HYPRO Dose Response: GAG at the steady state concentration of each concentration is plotted against the different concentrations of binding sites of KPLHALL covalently attached to alginate. Transfected chondrocytes with pAAV/IGF-I produce 600% GAG, than the control chondrocytes transfected with pAAV/ MCS (Empty) (*p<0.0001 by unpaired t test). pAAV/IGF-I transfected chondrocytes required 16.9 μM of binding sites in the alginate to produce an increase in GAG of 6.9 (Table 2). Control chondrocytes required almost twice the amount of KPLHALL modified alginate only to produce 60% (Table 2). *p<0.001 when compared to min. +p<0.001 when compared to max empty.
4B Dose Response: The production of HYPRO increases both in pAAV/IGF-I transfected chondrocytes and control chondrocytes. The effect in chondrocytes that are transfected with pAAV/IGF-I is greater than the effect in cells transfected with pAAV/ MCS (Empty). pAAV/IGF-I increased HYPRO accumulation by 20 fold at the maximum effective concentration of IGF-I binding sites(p<0.02 by unpaired t test). The parameters are summarized on table 2 where all R2 are higher than 0.93 (Table 2). %p<0.02 when compared to min. ^p<0.02 when compared to max empty.
4. DISCUSSION
These data illustrate that the biomimetic modification of alginate with a binding peptide from IGFBP-5 enables high affinity binding of IGF-I. The data further demonstrate that this modification extend the presence of the growth factor in cell-seeded constructs to at least one month, and that this extended presence of IGF-I in turn substantially enhances cartilage matrix synthesis.
Producing materials that specifically bind IGF-I present specific technical challenges. Proteins that are known to bind IGF-I have complex 3D configurations that stabilize the formation of the binding protein-IGF-I complex[38]. IGFBP-5, which has 252 residues, and mini-IGFBP-5, which has 52 residues, have binding affinities of 3.7 nM and 37 nM respectively. Grafting such proteins to a material to enhance IGF-I binding would be technically challenging and expensive, due to the cost of producing the protein recombinantly. Additionally, stability of the grafted material would be of great concern. Grafting shorter peptides would solve this problem; however it is not clear whether shorter peptides would produce high affinity binding. Here we showed that grafting a seven peptide sequence from the binding pocket of IGFBP-5 to alginate produced a material with high affinity (KD = 50 nM). This peptide sequence is short enough that folding is likely absent or minimally important. However, it is important to note that grafting to alginate via the Ɛ amino group in the lysine residue of the peptide rather than the glycine leader sequence eliminated the beneficial effect on matrix synthesis (data not shown). This data suggests that some amount of leader sequence is necessary for proper binding of IGF-I to the modified alginate. Additionally, the affinity may be aided by the fact that alginate is a polyanion and IGF-I is positively charged (pI = 8.5) [40], and charge based interaction may play a role in aiding binding. Nevertheless the significant enhancement of IGF-I binding by the addition of a short peptide may provide a template for targeted modification of biomaterials for growth factor binding.
Other studies have explored alternative approaches for expanding the time of IGF-I action through controlled delivery. For example, the interaction of IGF-I with the cartilage ECM has been altered by generating a recombinant fusion protein of IGF-I with a heparin binding domain[41]. This modified IGF-I bound to heparin sulfate and chondroitin sulfate with high affinities (21 nM and 172 nM) and extended the retention time of the growth factor in cartilage[41]. Other approaches for delivery include gene-activated matrix (GAM) [42] materials, in which a material is loaded with a plasmid that is released slowly to the cells. Similarly IGF-I peptide was released from degradable microparticles to achieve delivery over ~2 weeks[43]. The approach described in the current paper compliments these previously described approaches and provides additional flexibility for tuning both cellular and matrix responses over many weeks. Additionally, we can use our modified alginate with other methods of gene therapy.
Similarly this modified material may be helpful in enhancing other methods of gene therapy. Many studies on cartilage gene therapy have focused on extending the time of production and availability of IGF-I. Adeno-associated virus (AAV), a non-pathologic human parvovirus, is capable of transfecting non-dividing cells for extended periods of time and can transduce normal and osteoarthritic articular cartilage in vitro[44]. Adenoviral vectors (Ade.IGF-I) have been used to transduce cells where production of IGF-I ranges from 21 days up to 150 days[45,46]. Also, chondrocytes transduced with recombinant adeno-associated virus (rAAV)[47] have produced IGF-I for over 20 days. While our approach described here focused on plasmid transfection, such an approach can also be used with any of the above vectors. Tuning both IGF-I production and binding could enable even more extended availability of the growth factor.
Both IGF-I as a target growth factor[48,49] and alginate as a delivery vehicle[50,51] have been used in vivo to enhance the repair of cartilage. The modified alginate presented here shows great promise to further improve chondrocyte matrix production and cartilage repair in vivo[50]. We noted a robust staining for type II collagen, particularly at higher concentrations of KPLHALL constructs (See supplementary figure S3). Furthermore, the DNA analysis showed similar amounts of DNA throughout all the groups throughout the duration of the experiments. This data, in combination with the matrix synthesis data suggests that a relatively uniform number of cells was maintained during culture (See supplementary figure S4). This new biomaterial can be incorporated into other studies that have used alginate as a scaffold or have used other vectors[48,52]. Overall, these data suggest that combining gene therapy with targeted modification of scaffold material provides a platform where cellular production and extracellular binding of IGF-I act synergistically to enhance cellular biosynthesis. IGF-I regulates many other tissues including spinal cord[53], heart[54] and brain[55,56]. As such, these studies suggest the possibility of using such modified biomaterial not only with chondrocytes, but with other cell types. Furthermore, this approach can be extracted for use with other growth factors, such as TGF-β and BMP-2, that also have known protein binding sites in extracellular matrix[57,58].
5. CONCLUSION
Modifying alginate with the peptide KPLHALL from the binding pocket of IGFBP-5 enhanced binding affinity more than 10-fold, extended IGF-I availability over 30 days and increased GAG and HYPRO synthesis 7 and 20 fold respectively. The approach of controlling growth factor binding, by the grafting of small peptides for biomaterials represents an important new approach to drug delivery and tissue engineering.
Supplementary Material
Supplemental 1
Negative Control for IGF-I IHC in 100μM KPLHALL Alginate with pAAV/IGF-I transfected chondrocytes. Scale bar = 100 μm
Supplemental 2
Diffusion NMR was performed to see the stability of conjugation in the alginate; the NMR signal of the leucines is present even as the gradient increases. In contrast to the mixture sample, the leucine signals disappear as the gradient increases.
Supplemental 3
Immunohistochemistry of constructs for type II collagen at Day 30. Scale bar = 100 μm Robust staining for type II collagen, particularly at higher concentrations of KPLHALL constructs.
Supplemental 4
Amount of DNA via Hoechst DNA assay throughout the duration of the experiment.
SIGNIFICANCE.
The present manuscript focuses on the enhancement of chondrocyte gene therapy through the modification of scaffold materials to enhance the retention of targeted gene products. This study combined tissue engineering and gene therapy, where customized biomaterials augmented the action of IGF-I by enhancing the retention of protein produced by transfection of the IGF-I gene. This approach enabled tuning of binding of IGF-I to alginate, which increased GAG and HYPRO production by transfected chondrocytes. To our knowledge, peptide-based modification of materials to augment growth factor-targeted gene therapy has not been reported previously.
ACKNOWLEDGMENTS
This research is supported by Alfred P. Sloan Foundation, Coleman Foundation, Award NIH/NIAMS F31AR061982, NIH Grant R01 AR047702, and the Department of Veterans Affairs. We would like to thank Dr. Keresztes for his help with the NMR, Dr. Parker for all her help with the SPR and Ms. Michele Karr for all her help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen P-J, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao R-P, Ma J, Recombinant MG53 Protein Modulates Therapeutic Cell Membrane Repair in Treatment of Muscular Dystrophy, Sci. Transl. Med 4 (2012) 139ra85–139. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nagahara AH, a Merrill D, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, V Chao M, Koo EH, Geschwind D, Masliah E, a Chiba A, Tuszynski MH, Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease., Nat. Med 15 (2009) 331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ, Large-Scale Chondroitin Sulfate Proteoglycan Digestion with Chondroitinase Gene Therapy Leads to Reduced Pathology and Modulates Macrophage Phenotype following Spinal Cord Contusion Injury., J. Neurosci 34 (2014) 4822–36. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kafienah W, Al-Fayez F, Hollander AP, Barker MD, Inhibition of cartilage degradation: A combined tissue engineering and gene therapy approach, Arthritis Rheum. 48 (2003) 709–718. doi: 10.1002/art.10842. [DOI] [PubMed] [Google Scholar]
- [5].Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC, Osteoarthritis gene therapy., Gene Ther. 11 (2004) 379–89. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- [6].Nixon a J., Saxer R. a, Brower-Toland BD, Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-I autocrine/paracrine response in chondrocytes., J. Orthop. Res 19 (2001) 26–32. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- [7].Shi S, Mercer S, Trippel SB, Effect of transfection strategy on growth factor overexpression by articular chondrocytes., J. Orthop. Res 28 (2010) 103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu P, Wang X, Fu YX, Enhanced local delivery with reduced systemic toxicity: delivery, delivery, and delivery., Gene Ther. 13 (2006) 1131–1132. doi: 10.1038/sj.gt.3302760. [DOI] [PubMed] [Google Scholar]
- [9].Madry H, Zurakowski D, Trippel SB, Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation., Gene Ther. 8 (2001) 1443–9. [DOI] [PubMed] [Google Scholar]
- [10].Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB, Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I)., Gene Ther. 12 (2005) 1171–9. [DOI] [PubMed] [Google Scholar]
- [11].Saxer RA, Bent SJ, Brower-Toland BD, Mi Z, Robbins PD, Evans CH, a J. Nixon, Gene mediated insulin-like growth factor-I delivery to the synovium., J. Orthop. Res 19 (2001) 759–67. [DOI] [PubMed] [Google Scholar]
- [12].Madry H, Cucchiarini M, Advances and challenges in gene-based approaches for osteoarthritis., J. Gene Med 15 (2013) 343–55. doi: 10.1002/jgm.2741. [DOI] [PubMed] [Google Scholar]
- [13].Hellgren I, Drvota V, Pieper R, Enoksson S, Blomberg P, Islam KB, Sylvén C, Highly efficient cell-mediated gene transfer using non-viral vectors and FuGene6: in vitro and in vivo studies., Cell. Mol. Life Sci 57 (2000) 1326–33. http://www.ncbi.nlm.nih.gov/pubmed/11028922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ, Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model., J. Bone Joint Surg. Br 89 (2007) 672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- [15].Brower-Toland BD, a Saxer R, Goodrich LR, Mi Z, Robbins PD, Evans CH, Nixon a J., Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function., Hum. Gene Ther 12 (2001) 117–29. [DOI] [PubMed] [Google Scholar]
- [16].Brigham, Standard of Care : Autologous Chondrocyte Implantation ( ACI ), 34 (2007) 1–8. [Google Scholar]
- [17].Kalus W, Zweckstetter M, Renner C, Sanchez Y, Georgescu J, Grol M, Demuth D, Schumacher R, Dony C, Lang K, a Holak T, Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions., EMBO J. 17 (1998) 6558–72. doi: 10.1093/emboj/17.22.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jones JI, Gockerman A, Busby WH, Camacho-Hubner C, Clemmons DR, Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I., J. Cell Biol 121 (1993) 679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ducheyne P, Mauck RL, Smith DH, Biomaterials in the repair of sports injuries., Nat. Mater 11 (2012) 652–4. doi: 10.1038/nmat3392. [DOI] [PubMed] [Google Scholar]
- [20].Guo T, Zhao J, Chang J, Ding Z, Hong H, Chen J, Zhang J, Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-??1 for chondrocytes proliferation, Biomaterials. 27 (2006) 1095–1103. doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [21].Zhao X, Yu SB, Wu FL, Bin Mao Z, Yu CL, Transfection of primary chondrocytes using chitosan-pEGFP nanoparticles, J. Control. Release 112 (2006) 223–228. doi: 10.1016/j.jconrel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [22].Thanou M, Florea BI, Geldof M, Junginger HE, Borchard G, Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines, Biomaterials. 23 (2002) 153–159. [DOI] [PubMed] [Google Scholar]
- [23].Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt J. a., Freemont AJ, Hoyland JA, The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-l-lactic acid (PLLA) scaffolds, Biomaterials. 27 (2006) 4069–4078. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [24].Yao Y, He Y, Guan Q, Wu Q, Biomaterials A tetracycline expression system in combination with Sox9 for cartilage tissue engineering, Biomaterials. 35 (2014) 1898–1906. doi: 10.1016/j.biomaterials.2013.11.043. [DOI] [PubMed] [Google Scholar]
- [25].Liu C, Zhang P, Zhai X, Tian F, Li W, Yang J, Liu Y, Wang H, Wang W, Liu W, Biomaterials Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fl uorescence, Biomaterials. 33 (2012) 3604–3613. doi: 10.1016/j.biomaterials.2012.01.052. [DOI] [PubMed] [Google Scholar]
- [26].Chen XA, Zhang LJ, He ZJ, Wang WW, Xu B, Zhong Q, Shuai XT, Yang LQ, Bin Deng Y, Plasmid-encapsulated polyethylene glycol-grafted polyethylenimine nanoparticles for gene delivery into rat mesenchymal stem cells., Int. J. Nanomedicine 6 (2011) 843–853. doi: 10.2147/IJN.S17155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Madry H, Cucchiarini M, Stein U, Remberger K, Menger MD, Kohn D, Trippel SB, Sustained transgene expression in cartilage defects in vivo after transplantation of articular chondrocytes modified by lipid-mediated gene transfer in a gel suspension delivery system., J. Gene Med 5 (2003) 502–9. [DOI] [PubMed] [Google Scholar]
- [28].a Rowley J, Madlambayan G, Mooney DJ, Alginate hydrogels as synthetic extracellular matrix materials., Biomaterials. 20 (1999) 45–53. [DOI] [PubMed] [Google Scholar]
- [29].Hern DL, Hubbell J. a., Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing, J. Biomed. Mater. Res 39 (1998) 266–276. doi:. [DOI] [PubMed] [Google Scholar]
- [30].a Marcum J, Rosenberg RD, Anticoagulantly active heparin-like molecules from vascular tissue., Biochemistry. 23 (1984) 1730–1737. doi: 10.1016/0014-4827(86)90525-2. [DOI] [PubMed] [Google Scholar]
- [31].Packer N, Karlsson N, Surface Plasmon Resonance Methods and Protocols, Methods in Molecular Biology, 2010. doi: 10.1007/978-1-60761-670-2. [DOI] [Google Scholar]
- [32].Ballyns JJ, Wright TM, Bonassar LJ, Effect of media mixing on ECM assembly and mechanical properties of anatomically-shaped tissue engineered meniscus., Biomaterials. 31 (2010) 6756–63. doi: 10.1016/j.biomaterials.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aguilar IN, Trippel S, Bonassar LJ, Comparison of Efficacy of Endogenous and Exogenous IGF-I in Stimulating Matrix Production in Neonatal and Mature Chondrocytes, 14853 (2015) 1–22. doi: 10.1177/1947603515578691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim GA YJ, Sah R, Doongoe-Yuan H, Fluorometric Assay of DNA in Cartilage Explants Using Hoechst 33258 Cartilage Explant Sample Preparation, Anal. Bochemistry 174 (1988) 168–176. [DOI] [PubMed] [Google Scholar]
- [35].Enobakhare BO, Bader DL, Lee DA, Quantification of Sulfated Glycosaminoglycans in Chondrocyte / Alginate Cultures , by Use of 1,9 Dimethylmethylene Blue, Anal. Bochemistry 243 (1996) 191–194. [DOI] [PubMed] [Google Scholar]
- [36].Neuman RE, Logan MA, The determination of hydroxyproline, J. Biol. Chem (1949) 229–306. doi: 10.1016/0009-8981(65)90038-0. [DOI] [PubMed] [Google Scholar]
- [37].Sauermann W, Feuerstein TJ, Some Mathematical Models for Concentration-Response Relationships, Biometrical J. 40 (1998) 865–881. [Google Scholar]
- [38].Zesławski W, Beisel HG, Kamionka M, Kalus W, a Engh R, Huber R, Lang K, a Holak T, The interaction of insulin-like growth factor-I with the N-terminal domain of IGFBP-5., EMBO J. 20 (2001) 3638–44. doi: 10.1093/emboj/20.14.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Genes NG, a Rowley J, Mooney DJ, Bonassar LJ, Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces., Arch. Biochem. Biophys 422 (2004) 161–7. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- [40].Mullen LM, Best SM, a Brooks R, Ghose S, Gwynne JH, Wardale J, Rushton N, Cameron RE, Binding and release characteristics of insulin-like growth factor-1 from a collagen-glycosaminoglycan scaffold., Tissue Eng. Part C. Methods 16 (2010) 1439–48. doi: 10.1089/ten.TEC.2009.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miller R, Grodzinsky AJ, Cummings K, Plaas A, Cole A, Lee RT, Patwari P, Sc MDD, Intra-articular Injection of HB-IGF-1 Sustains Delivery of IGF-1 to Cartilage through Binding to Chondroitin Sulfate, 62 (2011) 3686–3694. doi: 10.1002/art.27709.Intra-articular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bonadio J, Tissue engineering via local gene delivery, J. Mol. Med 78 (2000) 303–311. doi: 10.1007/s001090000118. [DOI] [PubMed] [Google Scholar]
- [43].Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R, Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering., J. Orthop. Res 19 (2001) 1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- [44].Madry H, Cucchiarini M, Terwilliger EF, Trippel SB, Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage., Hum. Gene Ther 14 (2003) 393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- [45].Steinert AF, Palmer GD, Pilapil C, Nöth U, Evans CH, Ghivizzani SC, Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer., Tissue Eng. Part A. 15 (2009) 1127–1139. doi: 10.1089/ten.tea.2007.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Madry H, Cucchiarini M, Terwilliger EF, Trippel SB, Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage., Hum. Gene Ther 14 (2003) 393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- [47].Weimer A, Madry H, Venkatesan JK, Schmitt G, Frisch J, Wezel A, Jung J, Kohn D, Terwilliger EF, Trippel SB, Cucchiarini M, Benefits of recombinant adeno-associated virus (rAAV)-mediated insulinlike growth factor I (IGF-I) overexpression for the long-term reconstruction of human osteoarthritic cartilage by modulation of the IGF-I axis., Mol. Med 18 (2012) 346–58. doi: 10.2119/molmed.2011.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nixon AJ, Fortier LA, Williams J, Mohammed H, Enhanced Repair of Extensive Articular Defects by Insulin-like Growth Factor-I-laden Fibrin Composites, (1999). [DOI] [PubMed]
- [49].Bonassar LJ, Grodzinsky a J., Frank EH, Davila SG, Bhaktav NR, Trippel SB, The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I., J. Orthop. Res 19 (2001) 11–7. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- [50].Kaul G, Cucchiarini M, Arntzen D, Zurakowski D, Menger MD, Kohn D, Trippel SB, Madry H, Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo., J. Gene Med 8 (2006) 100–11. doi: 10.1002/jgm.819. [DOI] [PubMed] [Google Scholar]
- [51].Lee KY, Peters MC, Anderson KW, Mooney DJ, Controlled growth factor release from synthetic extracellular matrices., Nature. 408 (2000) 998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- [52].Madry H, Cucchiarini M, Kaul G, Kohn D, Terwilliger EF, Trippel SB, SB T, Menisci Are Efficiently Transduced by Recombinant Adeno-Associated Virus Vectors In Vitro and In Vivo, Am. J. Sports Med 32 (2004) 1860–1865. [DOI] [PubMed] [Google Scholar]
- [53].Hinks GL, Franklin RJ, Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination., Mol. Cell. Neurosci 14 (1999) 153–168. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- [54].Opgaard OS, Wang PH, IGF-I is a matter of heart, Growth Horm. IGF Res 15 (2005) 89–94. doi: 10.1016/j.ghir.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [55].Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I, Serum insulin-like growth factor I regulates brain amyloid-beta levels., Nat. Med 8 (2002) 1390–1397. doi: 10.1038/nm793. [DOI] [PubMed] [Google Scholar]
- [56].Doré S, Kar S, Quirion R, Rediscovering an old friend, IGF-I: potential use in the treatment of neurodegenerative diseases., Trends Neurosci. 20 (1997) 326–331. doi:S0166223696010363 [pii]. [DOI] [PubMed] [Google Scholar]
- [57].Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-ullrich JE, Thrombospondin Binds and Activates the Small and Large Forms of Latent Transforming Growth Factor-b in a Chemically Defined System, J. Biol. Chem 269 (1994) 26775–26782. [PubMed] [Google Scholar]
- [58].Sweatt a., Sane DC, Hutson SM, Wallin R, Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats, J. Thromb. Haemost 1 (2003) 178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- [59].Mooney DJ, Presentation of BMP - 2 Mimicking Peptides in 3D Hydrogels Directs Cell Fate Commitment in Osteoblasts and Mesenchymal Stem Cells, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental 1
Negative Control for IGF-I IHC in 100μM KPLHALL Alginate with pAAV/IGF-I transfected chondrocytes. Scale bar = 100 μm
Supplemental 2
Diffusion NMR was performed to see the stability of conjugation in the alginate; the NMR signal of the leucines is present even as the gradient increases. In contrast to the mixture sample, the leucine signals disappear as the gradient increases.
Supplemental 3
Immunohistochemistry of constructs for type II collagen at Day 30. Scale bar = 100 μm Robust staining for type II collagen, particularly at higher concentrations of KPLHALL constructs.
Supplemental 4
Amount of DNA via Hoechst DNA assay throughout the duration of the experiment.