SUMMARY
Objective:
Viscosupplementation has been used for decades to treat mild to moderate osteoarthritis, yet it is unknown if the lubricating function of different pathological synovial fluids (SF) vary, or if they respond differentially to viscosupplementation. The objectives of this study were to (i) evaluate the friction coefficients and induced shear strains in articular cartilage when lubricated with pathological SF, (ii) identify the effect of hyaluronic acid (HA) supplementation on friction coefficients and shear strains, and (iii) identify SF biomarkers that correlate with lubricating function.
Method:
Human pathological SF was grouped by white blood cell count (inflammatory: >2000 cells/mm3, n = 6; non-inflammatory: <2000 cells/mm3, n = 6). Compositional analyses for lubricin and cytokines were performed. Friction coefficients and local tissue shear strain measurements were coupled using new, microscale rheological analyses by lubricating neonatal bovine cartilage explants with SF alone and in a 1:1 ratio with HA (Hymovis®).
Results:
Friction coefficients were not significantly different between the inflammatory and non-inflammatory pathologies (p = 0.09), and were poorly correlated with peak tissue strains at the cartilage articular surface (R2 = 0.34). A subset of inflammatory SF samples induced higher tissue strains, and HA supplementation was most effective at lowering friction and tissue strains in this inflammatory subset. Across all pathologies there were clear relationships between polymorphonuclear neutrophil (PMN), IL-8, and lubricin concentrations with cartilage tissue strains.
Conclusion:
These results suggest that pathological SF is characterized by distinct tribological endotypes where SF lubricating behaviors are differentially modified by viscosupplementation and are identifiable by biomarkers.
Keywords: Arthritis, Osteoarthritis, Friction, Hyaluronic acid, Lubricin, Inflammation
Introduction
Hyaluronic acid (HA) is a glycosaminoglycan that is essential for proper synovial fluid (SF) lubrication, and viscosupplementation with HA has been used for decades to treat mild to moderate osteoarthritis (OA)1. OA progression leads to a decrease in SF viscosity2 and inferior joint lubrication. Poorer lubrication in turn results in increased cartilage tissue strains, and results in subsequent chondrocyte death, mitochondrial dysfunction, and apoptosis3–5. Maintaining lower shear strains via lubrication therapies, like viscosupplementation, may prevent or decrease the negative biological consequences of SF pathology. However, clinical success of viscosupplementation is mixed with insufficient evidence to determine how the efficacy of HA injections vary in OA subpopulations6. More recently, the term endotype has been used to describe disease subtypes that result from specific pathobiological mechanisms7.
Numerous studies have investigated the effect of HA injections on knee OA8,9, yet meta-analyses have yielded conflicting conclusions10–12. Based on such analyses, the American Academy of Orthopaedic Surgeons does not recommend viscosupplementation due to evidence suggesting an insufficient number of patients are likely to benefit from the treatment13, and the OA Research Society International recommendation is uncertain based on evidence14. In contrast, the American Medical Society for Sports Medicine endorses viscosupplementation for Kellgren and Lawrence (KL) grades II and III15. Meta-analyses offer the advantage of combining studies to increase sample size and power of statistical measures, but the interpretation of such results may be confounded by the variation in disease states that are pooled together. There has been growing appreciation that OA subpopulations exist as identified by a variety of biomarkers16. As such, the approach of considering all OA patients as similarly responsive to all treatments might mask subset populations of knee OA that have differential responses to lubrication therapies.
Biochemical evaluations have elucidated populations of patients with distinct SF pathologies17,18 where biomarkers have been predictive of patient outcomes19. White blood cell (WBC) count in SF is used to distinguish between inflammatory and non-inflammatory SF pathologies20. Within the non-inflammatory range (<500 cells/mm3), WBC count can identify patients with increased synovial tissue volume, pain, and patients that are likely to respond to anti-inflammatory treatment21. The concentration of lubricin, a critical boundary lubricant in SF, may vary with joint injuries22,23, and numerous animal studies also report both increases and decreases in lubricin concentration from various OA models24,25. These compositional assessments of SF have identified potential therapeutic targets, yet only one study has investigated the effect of lubricin and lubricin + HA supplementation on friction coefficients in OA SF samples26. It remains unknown if biomarkers other than lubricin and HA are indicative of SF lubricating function.
The current protocol for determining if a patient should receive viscosupplementation does not account for variation in the lubricating ability of SF27. Recommendation for viscosupplementation treatment is based on the KL grading system, but a recent study found that the concentration of lubricin did not correlate with KL grading27. An in vitro analysis found that articular cartilage tissue strains were reduced when equine SF from acutely injured joints was supplemented with HA28. However, no study to date has evaluated functional lubrication outcomes of SF supplemented with HA from different human SF pathologies.
Lubrication is typically quantified using the coefficient of friction, a metric that describes the load transfer to the articular cartilage surface. Cartilage tissue has non-linear, heterogeneous properties, and therefore the coefficient of friction doesn’t necessarily translate to chondrocyte damage or pathology. Recently, local tissue strains have been found to be directly correlated with chondrocyte damage in articular cartilage rather than the coefficient of friction3,4. However, it is unknown if human SF pathology affects local tissue strains under shear loading or if viscosupplementation alters these tissue strains. Further, it is unknown if distinct pathological SF respond differently to lubrication therapies.
With this in mind, the objectives of this study were to (1) evaluate the friction coefficients and induced shear strains in articular cartilage when lubricated with inflammatory and non-inflammatory human SF, (2) identify the effect of viscosupplementation on friction coefficients and shear strains, and (3) identify SF biomarkers that correlate with SF lubricating function.
Methods
Human SF collection and analysis
Human SF samples were obtained as part of routine treatment with patient consent under the approval of the University of Padova review board (N.0039872/2015). SF was obtained by arthrocentesis from the knee joints of six patients with OA and six patients with inflammatory arthritis. After aspiration, a subset of the fluid was placed in tubes containing ethylenediaminetetraacetic acid (EDTA) and plain tubes for optical light microscopy routine analysis. The total WBC count was determined using a standard hematological counting chamber, and the polymorphonuclear neutrophil (PMN) composition was determined from supravital staining. The remaining SF was centrifuged at 3000 rpm for 10 min and stored at −80°C for further analysis.
SF classification
For the general classification of SF inflammatory degree, the WBC count of 2000 cells/mm3 represents the cut-off to distinguish inflammatory (>2000) from non-inflammatory (<2000) SF. Additionally, a cut-off of 20% PMN was used in this study29. Consistently, SF from patients with OA (n = 6, age 59 ± 10) had WBC counts less than 2000 cells/mm3 and a PMN composition below 20%. The remaining samples were collected from patients with inflammatory arthritis (n = 6, age 59 ± 10; WBC count >2000 cells/mm3; PMN > 20%) and were classified as inflammatory. Further characterization of inflammatory SF samples is described in Supplemental Table 1.
SF compositional analysis
Cytokine profiles of the SF samples were performed in duplicate as previously described30. The levels of C-C motif chemokine ligand 2 (CCL2), transforming growth factor beta (TGFβ), interleuken 1 beta (IL-1β), interleuken 6 (IL-6), interleuken 8 (IL-8), and interleuken 10 (IL-10) were determined by enzyme-linked immunosorbent assays (ELISA) according to manufacturer specifications (eBioscience, San Diego, California, USA). The sensitivities for the cytokines were 7.81 pg/mL (CCL2), 7.81 pg/mL (TGFβ), 2.34 pg/mL (IL-1β), 1.60 pg/mL (IL-6), 1.20 pg/mL (IL-8), 2.34 pg/mL (IL-10).
Lubricin concentration was quantified in triplicate using a peanut agglutinin sandwich ELISA (Sigma—Aldrich, St. Louis, MO) with an anti-lubricin monoclonal antibody 9G3 (MABT401; Millipore Sigma, Burlington, MA) as previously described24,25. Purified lubricin from bovine SF was used as the standard. HA concentration was quantified in triplicate using a hyaluronan DuoSet ELISA (Bio-Techne Corporation, Minneapolis, MN) as previously described24,25.
HA (Hymovis®)
The HA used in this study (Hymovis®, Fidia Farmaceutici S.p.A.) is a modified HA (700 kDa HA hexadecylamide, 72,000 mPa•s at 1 s−1 shear rate)31 with 1–3 hexadecyl side chains every 100 sugar residues32. This substitution results in increased viscosity due to hydrophobic interactions involving the aliphatic side chains without the addition of permanent crosslinks.
Tissue preparation
The goal of this study was to characterize the lubricating function of human SF, and therefore a reliable source of cartilage was used to remove any effect of differences caused by tissue variation. Neonatal bovid cartilage was chosen as a model because it is a reliable source of tissue with consistent friction properties, and has revealed different lubricant efficacies in a consistent and reproducible manner31,33,34. Cartilage was harvested from the femoral condyles of 1—3 day old bovids (Gold Medal Packing, Rome, NY, USA) and stored at −20°C until testing.
Microscale strain mapping
Articular cartilage explants were lubricated by phosphate buffered saline (PBS) alone, HA alone, a SF sample alone, or by a 1:1 SF:HA mixture to analyze friction coefficients and microscale shear strains adapted from previous studies3,35. Loading parameters were chosen to match those from Bonnevie et al. to enable more direct comparisons of outcomes3. Six mm diameter and 2 mm thick cartilage plugs were bisected and stained with 7 μg/mL of 5-DTAF (Molecular Probes1, Grand Island, NY, USA) for general protein fluorescence for 1 h followed by a PBS rinse. A cartilage hemicylinder was adhered to a fixed back plate on a tissue deformation imaging stage (TDIS) and submerged in the lubricant. The TDIS is a microscale test frame that allows for the application of oscillatory displacements and simultaneous imaging of tissue deformations through a glass slide (Fig. 1). For testing, the TDIS was mounted on an inverted Zeiss laser scanning microscope 510 live confocal microscope and imaged using a 488 nm laser. The tissue was axially compressed 15% and allowed 30 min to stress relax. Once at equilibrium, five lines were photo-bleached perpendicular to the tissue surface through the full tissue depth to allow for displacement tracking35. Shear strains were applied by a glass plate articulated parallel to the cartilage surface at a rate of 1 mm/s with images captured in real time at 60 frames per second. This corresponded to a waveform frequency of 0.625 Hz and amplitude of 400 mm. Care was taken to ensure tissue remained hydrated and submerged in the lubricant through testing duration. After testing the SF alone, the SF was mixed in a 1:1 ratio with HA and the same experiment was repeated with a new cartilage explant.
Fig. 1.
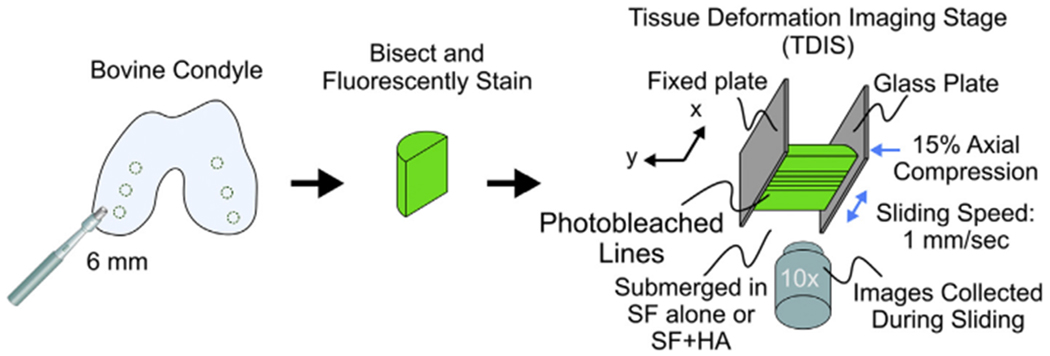
Cartilage tissue sliding methods. Neonatal bovine cartilage was dissected from the femoral condyles, bisected, and fluorescently stained. The tissue was mounted onto the TDIS where the deep zone was glued to a fixed backplate and underwent 15% axial compression, allowed to fully stress relax, and was subjected to an oscillatory displacement at a sliding speed of 1 mm/s. Images of tissue displacements were tracked from photobleached lines in the sample. The tissue remained hydrated and submerged in the lubricant during testing.
Through differentiation of the photo-bleached line displacements in Matlab, local shear strains were calculated as previously described35. From displacement tracking, regimes of static friction (where the cartilage was in static contact with the glass plate) and kinetic friction (where the cartilage was sliding against the glass plate) were observed (Fig. 1AB). The ratio of time spent in the static friction regime over the shear cycle period was defined as the adhesion time ratio (tμ static/tcycle), and was calculated by averaging over 10 cycles.
Friction coefficient analysis
Kinetic coefficients of friction were calculated as the ratio of shear force over axial force. Shear forces were calculated from the TDIS backplate deformations using a spring constant of 0.00138 N/μm. Axial forces were calculated from applied axial strains and tissue cross sectional areas assuming a modulus of 0.5 MPa36. While this optical analysis allows for microscale strain quantification through the tissue depth, some SF samples visually obstructed tissue fluorescence and the backplate displacements could not be accurately tracked. These samples were excluded from the friction coefficient analysis.
Statistical analyses
Lubricin concentration, HA concentration, kinetic friction coefficients, and adhesion time ratios were compared between inflammatory and non-inflammatory SF pathologies using a t-test. A linear mixed-effects model with repeated measures was used to examine the effect of pathology (inflammatory vs non-inflammatory) and HA treatment on maximum shear strain, with patient as a random effect. The model was followed with a post-hoc comparison for SF pathologies using Kenward-Roger’s method for estimation of degrees of freedom, and was corrected for multiple comparisons. Linear regression analyses were performed for patient age and all quantified compositional parameters with functional lubrication (assessed as the tissue surface shear strain) as the dependent variable. Non-linear regression was also performed on the same set of data to fit a dose—response curve [Y=Bottom + (Top-Bottom)/(1+(X/IC50))], as lubricating ability has been shown to have a dose—response relationship with lubricin composition33. Both linear and non-linear regression analyses were performed using Prism software. A pair-wise comparison was performed to assess collinearity between any sample parameters. Surface shear strains were compared between PBS, inflammatory SF, inflammatory SF with HA, non-inflammatory SF, and non-inflammatory SF with HA using a mixed effects model. Lubricant and the random effect of patient were fixed factors, as pathological SF samples were tested both alone and with HA supplementation. The model was followed with a post-hoc comparison to compare strains between the lubricant groups with a Tukey adjustment for multiple comparisons. P-values less than 0.05 were considered statistically significant.
Results
Adhesion time ratios explain tissue strain variability better than friction coefficients
In this study we examined if friction measurements correlated with local shear strains, as shear strains have been strongly correlated with chondrocyte responses. Under shear loading, tissue deformations revealed regimes of static friction (when the tissue was in static contact with the opposing glass plate) and kinetic friction (when the tissue began to slide with no further increase in displacement, Fig 2AB). The relative time the tissue was in static friction per shear cycle, or the adhesion time ratio (tμ static/tcycle), was strongly correlated with maximum tissue shear strain at the surface (R2 = 0.619, p < 0.0001, Fig. 2(C)). In contrast, the friction coefficient was weakly correlated with the tissue surface shear strain (R2 = 0.342, p < 0.05, Fig. 2(D)).
Fig. 2.
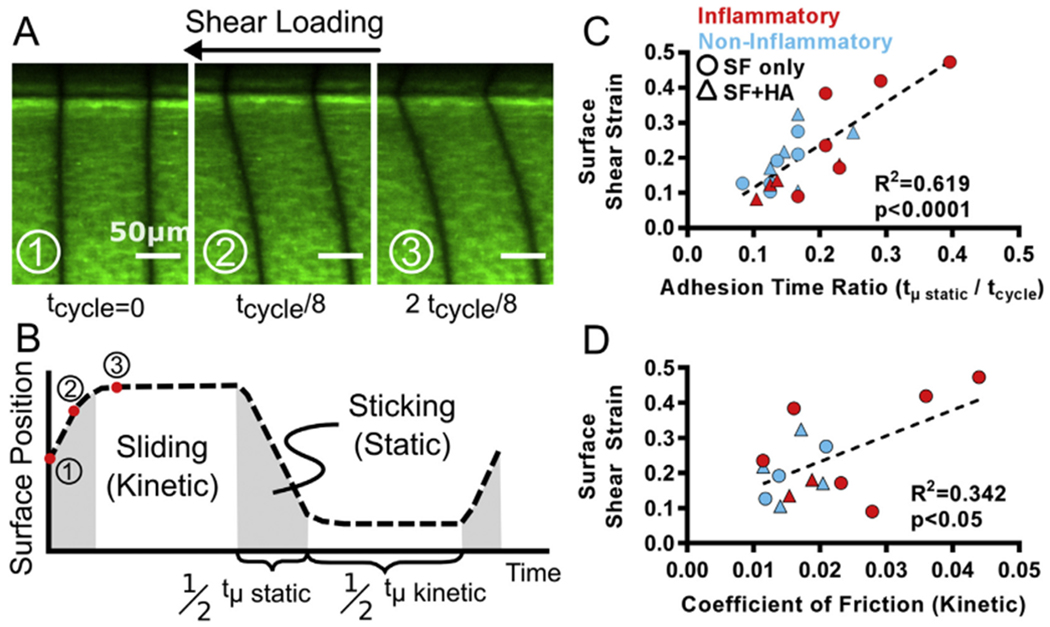
Coefficient of friction is poorly correlated with tissue strains (A) Articular cartilage fluorescently stained under shear loading at three separate time points in the first half of the shear cycle (B) Calculation of time in static friction (tμ static) and time in kinetic friction (tμ kinetic) over one shear cycle (C) Adhesion time ratio is strongly correlated with peak shear strains at the tissue surface, while (D) the coefficient of friction is weakly correlated.
Adhesion time ratios were significantly different between the inflammatory (0.25 ± 0.08, n = 6) and non-inflammatory (0.13 ± 0.03, n = 6) pathological SF (p < 0.05). The coefficients of friction for inflammatory samples were higher on average (0.026 ± 0.012, n = 6) compared to non-inflammatory samples (0.016 ± 0.004, n = 3), but the difference was not significant (p = 0.09), likely due to the large variability within the inflammatory SF group.
Shear strain measurements reveal SF tribological endotypes
Shear strains analyzed from tissue displacements [Fig. 3(A)] revealed differential lubricating abilities and responses to HA supplementation. For all samples, tissue strains were highest at the tissue surface and decreased with depth. Cartilage lubricated with HA alone revealed peak strains between 0.11 and 0.19 at the tissue surface, decaying to below 0.01 strain at depths greater than 225 μm [Fig. 3(B)]. Two distinct behaviors within the inflammatory pathology were observed. In one group, surface strains ranged from 0.09 to 0.24 (light red), while a second group had higher strains ranging from 0.38 to 0.47 (dark red, Fig. 3(C)). The addition of HA reduced surface shear strains below 0.20 for all inflammatory SF samples [Fig. 3(D)]. In contrast, the non-inflammatory group showed peak strains ranging from 0.11 to 0.28 at the tissue surface [Fig. 3(E)]. Supplementing these non-inflammatory samples with HA did not alter the surface strains, revealing no effect on lubricating performance [Fig. 3(F)].
Fig. 3.
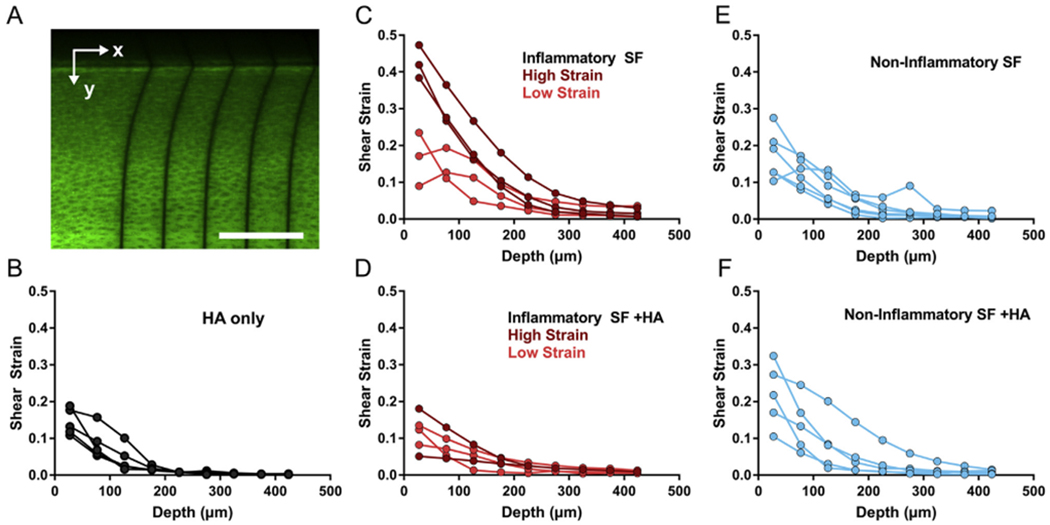
Local shear strain measurements reveal tribological endotypes of pathological SF (A) Representative image of tissue lubricated with inflammatory SF where photobleached lines are perpendicular to the tissue surface. Scale bar = 200 μm. Shear strains through tissue depth for tissue lubricated with (B) HA alone (C) inflammatory SF (D) inflammatory SF with HA (E) non-inflammatory SF, or (F) non-inflammatory SF with HA.
Maximum shear strains at the surface of the cartilage explants were significantly reduced in the inflammatory pathology with HA supplementation (p < 0.05, Fig. 4), identifying a distinct tribological endotype. HA supplementation had no effect on the maximum surface shear strains for the non-inflammatory pathology (p = 0.93). On average, the strains for the inflammatory pathology decreased by −0.16 with the addition of HA (Supplemental Fig. 1). For the non-inflammatory pathology, there was almost no change in tissue strains on average (+0.03). Overall, HA appears to be effective at lowering tissue strains in the inflammatory pathology, especially samples with higher strains (dark red), but not for the non-inflammatory OA pathology.
Fig. 4.
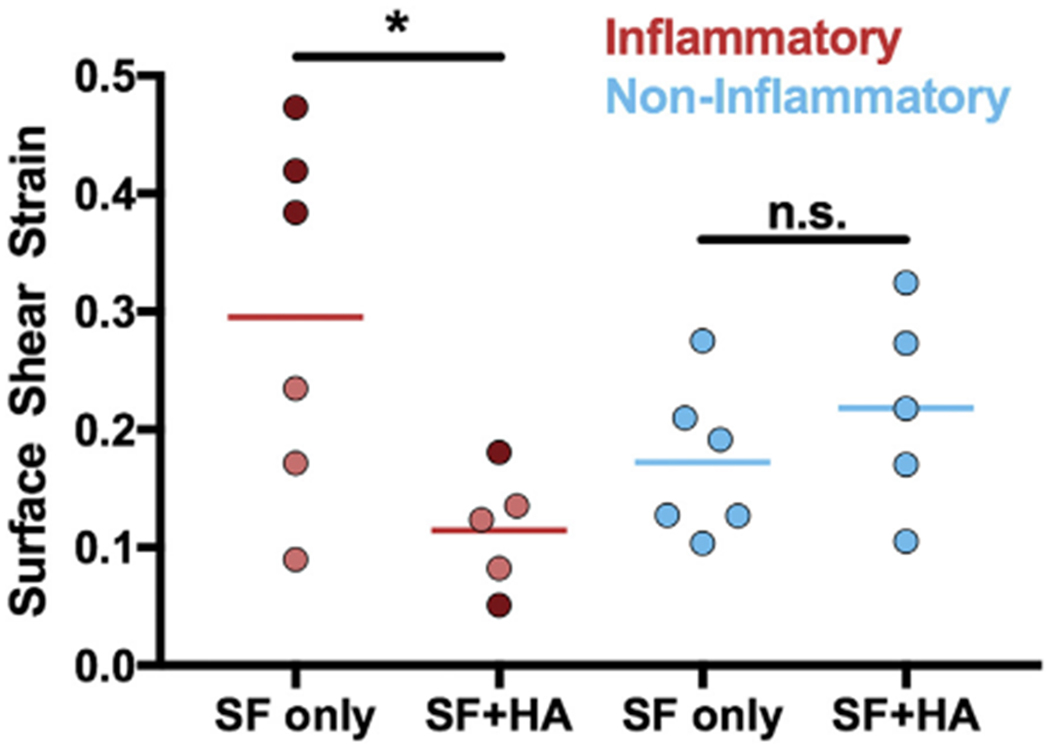
HA supplementation had varying efficacy based on SF pathology. Surface shear strain values for each individual SF sample. Each point is an individual sample. For inflammatory samples, dark red = high strain subset; light red = low strain subset.
HA concentration does not explain shear strain variation
HA concentration was not statistically different between inflammatory and non-inflammatory SF pathologies [Fig. 5(A)]. Additionally, HA concentration was poorly correlated with surface shear strain measurements (R2 = 0.002, Fig. 5(B), Table I) and the change in surface shear strains with HA supplementation for all samples (R2 = 0.117, Fig. 5(C)). As such, HA concentration did not explain the observed variation in surface shear strain measurements.
Fig. 5.
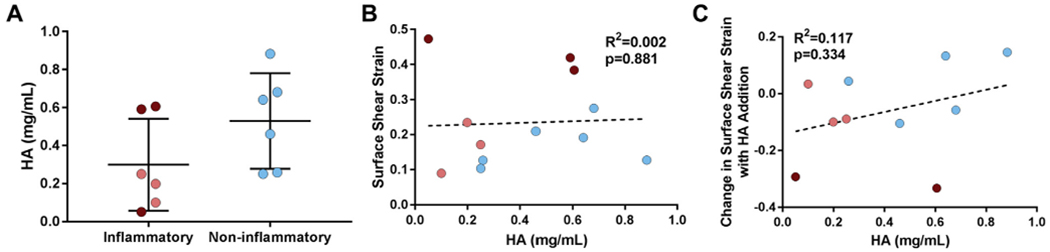
HA concentration does not explain variation in tissue shear strains (A) HA concentration was not different between inflammatory (red) and non-inflammatory (blue) SF pathologies (P = 0.138). HA concentration was poorly correlated with (B) surface shear strains and (C) the change in surface shear strains with HA supplementation. Each point is an individual sample. For inflammatory samples, dark red = high strain subset; light red = low strain subset.
Table I.
Fit parameters for patient age and SF compositional features as independent variables and tissue surface shear strain as the dependent variable
| Sample Parameters | Linear Fit |
Dose—Response Fit |
||
|---|---|---|---|---|
| R2 | p-value | R2 | p-value | |
| Age | 0.12 | 0.2653 | 0.12 | 0.2928 |
| WBC | 0.23 | 0.1137 | 0.23 | 0.1327 |
| PMN | 0.43 | 0.0201 | 0.43 | 0.0275 |
| CCL2 | 0.18 | 0.1755 | Did not converge | |
| TGFβ | 0.18 | 0.1736 | 0.18 | 0.1976 |
| IL-1β | 0.12 | 0.2654 | 0.17 | 0.2033 |
| IL-6 | 0.07 | 0.4116 | 0.27 | 0.1055 |
| IL-8 | 0.73 | 0.0004 | 0.74 | 0.0007 |
| IL-10 | 0.21 | 0.1377 | 0.20 | 0.1679 |
| HA | 0.00 | 0.8805 | 0.11 | 0.7555 |
| Lubricin | 0.11 | 0.2928 | 0.37 | 0.0490 |
Linear and dose—response relationships were fit for each sample parameter with reported R2 and p-values. PMN, IL-8, and lubricin concentration all had a significant relationship with surface shear strain. The p-values for significant relationships are bolded.
Inflammatory markers and lubricin concentrations identify SF tribological endotypes
An array of cytokines and lubricin concentration were quantified for each SF sample and were assessed via linear and non-linear regression to explain variability in tissue strains (Table I). These results revealed that three compositional features (PMN, IL-8, and lubricin) explained the functional lubricating differences across both groups of pathological SF. PMN composition had a moderate correlation with functional lubricating ability, with shear strains increasing with PMN count (R2 = 0.43, p < 0.05, Fig. 6(A)). IL-8 concentrations had a strong correlation with surface strains (R2 = 0.73, p < 0.001), where IL-8 levels in the high strain inflammatory subset (dark red) were up to two orders of magnitude greater than all other samples [Fig. 6(B)]. Lubricin concentration explained tissue surface strain variabilities following a dose—response relationship (R2 = 0.37, p < 0.05, Fig. 6(C)), where the lowest lubricin concentrations were observed for the high strain subset of samples in the inflammatory group (dark red). Patient age and all other cytokines did not show significant correlations with tissue strains (Table II). While not significant, patient age did show a mild correlation with decreased lubricin content (R2 = 0.287, p = 0.0724, Supplemental Fig. 2).
Fig. 6.
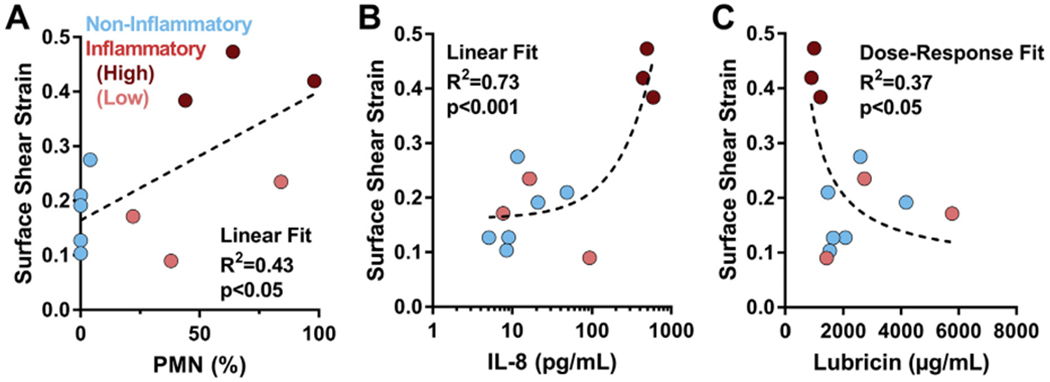
PMN, IL-8, and lubricin concentrations explain maximum shear strains at tissue surface. Surface shear strain variability for both inflammatory (red) and non-inflammatory (blue) samples were explained by (A) PMN concentration (B) IL-8 concentration, and (C) lubricin concentration. PMN and IL-8 data fit to linear curves, and lubricin data fit to a dose—response curve. For inflammatory samples, dark red = high strain subset; light red = low strain subset.
Table II.
R2 values for pair-wise comparisons of patient age, inflammatory classification parameters, inflammatory markers, lubricin concentration, and tissue surface shear strain
| Volume | WBC | PMN | CCL2 | TGFβ | IL-1β | IL-6 | IL-8 | IL-10 | HA | Lubricin | Shear Strain | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic | Age | 0.01 | 0.05 | 0.00 | 0.00 | 0.08 | 0.07 | 0.01 | 0.13 | 0.08 | 0.19 | 0.29 | 0.12 |
| SF Aspiration | Volume | 0.00 | 0.03 | 0.06 | 0.03 | 0.01 | 0.00 | 0.09 | 0.06 | 0.00 | 0.10 | 0.04 | |
| Inflammatory Classification | WBC | 0.78 | 0.08 | 0.02 | 0.62 | 0.73 | 0.21 | 0.11 | 0.00 | 0.03 | 0.23 | ||
| PMN | 0.00 | 0.00 | 0.69 | 0.74 | 0.38 | 0.35 | 0.08 | 0.07 | 0.43 | ||||
| Inflammatory Markers | CCL2 | 0.41 | 0.03 | 0.03 | 0.06 | 0.29 | 0.11 | 0.06 | 0.17* | ||||
| TGFβ | 0.00 | 0.06 | 0.08 | 0.20 | 0.04 | 0.09 | 0.18 | ||||||
| IL-1β | 0.69 | 0.11 | 0.35 | 0.20 | 0.00 | 0.17 | |||||||
| IL-6 | 0.04 | 0.19 | 0.08 | 0.03 | 0.26 | ||||||||
| IL-8 | 0.23 | 0.00 | 0.26 | 0.74 | |||||||||
| IL-10 | 0.36 | 0.09 | 0.20 | ||||||||||
| Lubrication | HA | 0.00 | 0.00 | ||||||||||
| Lubricin | 0.37 | ||||||||||||
Grey indicates poor correlations and green indicates strong correlations. Of note is the correlation between PMN, IL-8, and lubricin with the functional assessment of tissue shear strain. All shear strain correlation values were taken from the dose—response fit non-linear regression. The dose—response fit did not converge for one sample (denoted by *), so the linear fit R2 value was used. Relationships that are significant are in bolded text and outlined.
Discussion
This study evaluated the lubricating function of pathological SF from inflammatory and non-inflammatory populations. Friction coefficients were not significantly different between the populations, and were poorly correlated with tissue level strains. Both friction coefficients and tissue strains for an individual sample were obtained from a single experiment using a new microrheological analysis technique, reducing time and the volume of SF needed. A subset of inflammatory SF samples induced higher adhesion time ratios and tissue surface strains. Further, HA supplementation was most effective at lowering friction, adhesion time ratios, and surface strains in this subset of inflammatory SF. Across all samples there were clear relationships between PMN, IL-8, and lubricin concentrations and shear strains. Notably, the subset of inflammatory SF with the poorest lubrication had the highest PMN and IL-8, and the lowest lubricin. These results suggest that pathological SF is characterized by distinct tribological endotypes. These endotypes are differentially modified by viscosupplementation and are identifiable by biomarkers.
Poor lubrication is damaging to cartilage both by direct tissue wear37 as well as through damage to chondrocytes3,5. The effects on tissue damage and cell behavior are both tied to tissue strain, although most studies use friction coefficients as a predictor of tissue or cell damage. Here, we found that the friction coefficient is only weakly correlated with tissue shear strains. In contrast, adhesion time ratios proved to be more strongly correlated with maximum tissue shear strains at the articular surface. This metric quantifies the time the tissue is in contact with the opposing surface before overcoming static friction and beginning to slide in the kinetic friction mode. Studies of cartilage deformation under adhesive contact demonstrated that the articular surface undergoes significant strain stiffening38. This observation implies that the tissue surface deforms most during the initial application of force, which is true during static friction. Therefore lubricants that allow for a quicker transition from static to kinetic friction may be beneficial to cartilage by decreasing tissue strains.
These data highlight the importance of monitoring both friction coefficients and tissue shear strains to evaluate cartilage lubricants. Previous work used the TDIS technique to monitor tissue shear strains in adhesive contact35,39 as well as sliding contact3. The current study coupled these techniques with force measurements to calculate the friction coefficient, and these friction coefficients match those seen in macro-scale studies of lubrication of cartilage on glass by SF3,33,40,41. Collectively, these data support the idea of using microscale tribological techniques to study tissue lubrication.
The strains measured here were below tissue failure thresholds, as no tissue cracking or tearing was observed during testing. However, there are potential biological consequences of high tissue strain at sub-failure magnitudes. Thresholds for tissue and cellular level damage have been characterized by in vitro studies3,4, where strains greater than 30% resulted in widespread cell death. Lubrication by healthy equine SF has been shown to result in significantly less chondrocyte death, apoptosis, and mitochondrial dysfunction compared to PBS3. In the present study, lubrication with the inflammatory SF resulted in peak strain magnitudes greater than 30% and were similar to PBS (p = 0.29, Supplemental Fig. 3), indicating the cartilage tissue would incur biological detriments similar to that with PBS lubrication. However the addition of HA to the inflammatory SF reduced strains below PBS levels (p < 0.05, Supplemental Fig. 3), thereby potentially lessening negative cellular responses.
In the present study, classic inflammatory markers (PMN and IL-8) and lubricin concentration explained the functional lubricating abilities of human pathological SF whereas HA concentration did not. Our measured HA concentrations were below values found in healthy SF, but consistent with previous reports of HA in disease22,42–44. Therefore while HA concentrations were decreased in both inflammatory and non-inflammatory SF pathologies, this did not explain the variability observed in tissue shear strains. In contrast, lubricin was found to be significantly correlated with tissue shear strains. Lubricin is a critical boundary lubricant in SF, demonstrated by lubricin-deficient SF showing increased friction coefficients compared to healthy SF in humans37. Notably in this study, lower lubricin levels correlated with higher tissue strains regardless of SF pathology. Lubricin concentrations may be lower especially in the inflammatory SF samples as the presence of inflammatory cytokines is known to lower lubricin levels, both through degradation by neutrophil-derived enzymes45 and suppression of lubricin synthesis46.
Pathological SF that specifically had elevated levels of IL-8 and PMN correlated with inferior lubrication of their SF, identifying an inflammatory subset that characterizes a tribological endotype. IL-8 attracts and activates neutrophils with long-lasting effects47, suggesting IL-8 supports continued neutrophil presence and release of lubricin degradation products. As such, IL-8 could be a therapeutic target to preserve lubricating function of pathological SF, as well as serve as a marker that could inform appropriate lubrication therapy.
The inflammatory degree of the pathological SF had a clear effect on the cartilage tissue response, and HA was found to reduce tissue strains that were elevated in the inflammatory SF pathology. However viscosupplementation is not indicated for an inflammatory flare of OA as enzymes and oxidants present in the inflamed joint degrade the HA chains, rendering the therapy less effective48,49. Effective inflammatory arthritis treatments target inflammatory processes at the synovium level (e.g., tumor necrosis factor alpha inhibitors50), but these molecules don’t penetrate into cartilage51. Here, we identified viscosupplementation to have an effect at the cartilage tissue level, but did not investigate any synovium-level responses or the pharmacokinetics of the viscosupplement in different SF inflammatory environments. This highlights the complexity of the joint environment and the challenge to target both the synovium and cartilage in disease therapies.
Our results are the first to show distinct tribological endotypes of human pathological SF with viscosupplementation having varying efficacy on lubricating function. This is not the first characterization of pathological SF subpopulations, as previous studies have identified compositional phenotypes16 and temporal changes in SF composition23. Here, we have connected compositional features to a functional endotype, where IL-8, PMN, and lubricin concentrations explain the variability in lubricating function of pathological SF. Such data suggest that biomarker analyses may provide enough information to elucidate functional endotypes with further characterization, allowing for more specified clinical diagnoses that inform patient treatment.
When interpreting the results of this study, there are several limitations to take into account. We compared two distinct classes of pathological SF based on their inflammatory degree, but no healthy control was evaluated. There were low sample numbers within each pathological SF group, and even smaller samples within the inflammatory SF pathology to compare between the high and low strain subsets. Several other factors (patient age, WBC, IL-6, IL-10) all showed weak and not–significant correlations with lubricating ability (R2 < 0.3), but a larger sample size may yield statistically significant relationships. Despite the low sample numbers, we observed biomarkers that correlate with lubricating function. Future studies plan on obtaining healthy SF and expanding the number of pathological SF assessed to more fully characterize the tribological endotypes relating to OA.
Our reported lubricin concentrations are supraphysiologic compared to other reports from human SF22,23. This discrepancy likely results from the purity of the bovine lubricin standard. While the lubricin concentration magnitudes reported here may be above the range of human SF concentrations, they still allow for a relative comparison between samples in this study. Our experimental design assessed cartilage on glass lubrication outcomes. Therefore if articular cartilage was slid against a counter face of another material besides glass, the sticking (i.e., static friction mode) and resultant shear strain magnitudes may differ. However, our cartilage-glass kinetic friction coefficients are similar to reported cartilage—cartilage friction coefficients26,52. Additional limitations include that this in vitro study was performed using healthy neonatal bovine femoral cartilage, and therefore does not capture how damaged human cartilage may behave in vivo when lubricated with pathological SF. However, the cartilage source was kept consistent to identify differences between the SF samples independent of the tissue condition, and the comparison between neonatal bovine and human tissue has been extensively characterized and share similar features quantitatively35,53. Further, the use of the neonatal bovine cartilage on glass system has been shown to measure frictional behavior of lubricants that are predictive of human clinical efficacy54.
Our results reveal the existence of pathological SF tribological endotypes with implications for lubrication therapies. SF samples that generated high tissue strains were characterized by low lubricin levels and elevated inflammatory markers. Notably, viscosupplementation was effective at reducing strains in this subset. Damaging mechanobiological consequences have been linked to high tissue strains, suggesting that patients within the HA responsive tribological endotype may benefit more from viscosupplementation therapy.
Supplementary Material
Acknowledgments
The authors would like to graciously thank Heidi Reesink VMD, PhD, DACVS-LA, Jin Su PhD, and Yuyan Wang for their assistance with lubricin quantification, and the Cornell Statistical Consulting Unit for their assistance with statistical analyses.
Role of funding sources
This work was supported in part by the NIH (R01AR071394-01), the NSF CMMI (1536463) the Department of Education (GAANN Fellowship P200A150273 J), an institutional research grant from the University of Padova (DOR1790217/17), and Fidia Farmaceutici S.p.A. Study sponsors included co-authors CS and DG who were involved in the conception and study design, interpretation of data, drafting and revision of the manuscript, and approved the final version of the manuscript for publication.
Footnotes
Declaration of competing interest
LB is a paid consultant for and DG and CS are employees of Fidia Farmaceutici S.p.A.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2020.02.029.
References
- 1.Marshall KW. Viscosupplementation for osteoarthritis: current status, unresolved issues, and future directions. J Rheumatol 1998;25(11):2056e8. [PubMed] [Google Scholar]
- 2.Jebens E, Monk-Jones M. On the viscosity and pH of synovial fluid and the pH of blood. J Bone Joint Surg Br 1959;41-B(2):388–400. [DOI] [PubMed] [Google Scholar]
- 3.Bonnevie ED, Delco ML, Bartell LR, Jasty N, Cohen I, Fortier LA, et al. Microscale frictional strains determine chondrocyte fate in loaded cartilage. J Biomech 2018;74:72–8, 10.1016/j.jbiomech.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartell LR, Fortier LA, Bonassar LJ, Cohen I. Measuring microscale strain fields in articular cartilage during rapid impact reveals thresholds for chondrocyte death and a protective role for the superficial layer. J Biomech 2015;48(12):3440–6, 10.1016/J.JBIOMECH.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci Unit States Am 2013;110(15):5852–7, 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhadra AK, Altman R, Dasa V, Myrick K, Rosen J, Vad V, et al. Appropriate use criteria for hyaluronic acid in the treatment of knee osteoarthritis in the United States. Cartilage 2017;8(3): 234–54, 10.1177/1947603516662503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deveza LA, Nelson AE, Loeser RF. Phenotypes of osteoarthritis: current state and future implications. Clin Exp Rheumatol 2019;37(5):64–72. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee PB, Kim YC, Lim YJ, Lee CJ, Sim WS, Ha CW, et al. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label, randomized, multicenter clinical trial. J Int Med Res 2006;34(1):77–87, 10.1177/147323000603400110. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson J, Sj€ogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology 2002;41(11):1240–8. [DOI] [PubMed] [Google Scholar]
- 10.Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am 2015;97(24): 2047–60, 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA.Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev April 2006, 10.1002/14651858.CD005321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller L, Strand V, McIntyre L, Beach W, Block J. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res 2015;8:217–28, 10.2147/JPR.S83076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013;21(9):571–6, 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 14.Mcalindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI Guidelines for the Non-surgical Management of Knee Osteoarthritis 2014, 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Trojian TH, Concoff AL, Joy SM, Hatzenbuehler JR, Saulsberry WJ, Coleman CI, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med 2016;50(2):84–92, 10.1097/JSM.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 16.Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage 2017;25(12):1926–41, 10.1016/j.joca.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Egsgaard LL, Eskehave TN, Bay-Jensen AC, Hoeck HC, Arendt-Nielsen L. Identifying specific profiles in patients with different degrees of painful knee osteoarthritis based on serological biochemical and mechanistic pain biomarkers. Pain 2015;156(1):96–107, 10.1016/j.pain.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs CA, Vranceanu A-M, Thompson KL, Lattermann C. Rapid progression of knee pain and osteoarthritis biomarkers greatest for patients with combined obesity and depression: data from the osteoarthritis initiative. Cartilage. June 2018, 194760351877757, 10.1177/1947603518777577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuéllar VG, Cuéllar JM, Kirsch T, Strauss EJ. Correlation of synovial fluid biomarkers with cartilage pathology and associated outcomes in knee arthroscopy. Arthrosc J Arthrosc Relat Surg 2016;32(3):475–85, 10.1016/J.ARTHRO.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Hinton R, Moody RL, Davis AW, Thomas SF, David Eisenhower D. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician 2002;65(5). [PubMed] [Google Scholar]
- 21.McCabe PS, Parkes MJ, Maricar N, Hutchinson CE, Freemont A, O’Neill TW, et al. Synovial fluid white blood cell count in knee osteoarthritis: association with structural findings and treatment response. Arthritis Rheum 2017;69(1):103–7, 10.1002/art.39829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballard BL, Antonacci JM, Temple-Wong MM, Hui AY, Schumacher BL, Bugbee WD, et al. Effect of tibial plateau fracture on lubrication function and composition of synovial fluid. J Bone Joint Surg Am 2012;94(10):e64, 10.2106/JBJS.K.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum 2008;58(6):1707–15, 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reesink HL, Watts AE, Mohammed HO, Jay GD, Nixon AJ. Lubricin/proteoglycan 4 increases in both experimental and naturally occurring equine osteoarthritis. Osteoarthritis Cartilage 2017;25(1):128–37, 10.1016/J.JOCA.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor α on chondroprotection in an animal model. Arthritis Rheum 2009;60(10):2997–3006, 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis Rheum 2012;64(12):3963–71, 10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa H, Matsumoto K, Terabayashi N, Kawashima K, Takeuchi K, Akiyama H. Association of lubricin concentration in synovial fluid and clinical status of osteoarthritic knee. Mod Rheumatol 2017;27(3):489–92, 10.1080/14397595.2016.1209829. [DOI] [PubMed] [Google Scholar]
- 28.Wong BL, Hyun S, Kim C, Antonacci JM, Mcilwraith CW, Sah RL, et al. Cartilage shear kinematics during tibio-femoral articulation: effect of acute joint injury & tribosupplementation on synovial fluid lubrication. Osteoarthritis Cartilage 2010;18(3):464–71, 10.1016/j.joca.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punzi L, Oliviero F, Plebani M. New biochemical insights into the pathogenesis of osteoarthritis and the role of laboratory investigations in clinical assessment. Crit Rev Clin Lab Sci 2005;42(4):279–309, 10.1080/10408360591001886. [DOI] [PubMed] [Google Scholar]
- 30.Scanu A, Oliviero F, Ramonda R, Frallonardo P, Dayer J-M, Punzi L. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor β1 in the resolution phase. Ann Rheum Dis 2012;71(4):621–4, 10.1136/annrheumdis-2011-200711. [DOI] [PubMed] [Google Scholar]
- 31.Bonnevie ED, Galesso D, Secchieri C, Cohen I, Bonassar LJ. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PloS One 2015;10(11), e0143415, 10.1371/journal.pone.0143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finelli I, Chiessi E, Galesso D, Renier D, Paradossi G. Gel-like structure of a hexadecyl derivative of hyaluronic acid for the treatment of osteoarthritis. Macromol Biosci 2009;9(7):646–53, 10.1002/mabi.200900009. [DOI] [PubMed] [Google Scholar]
- 33.Gleghorn JP, Jones ARC, Flannery CR, Bonassar LJ. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res 2009, 10.1002/jor.20798. [DOI] [PubMed] [Google Scholar]
- 34.Samaroo KJ, Tan M, Putnam D, Bonassar LJ. Binding and lubrication of biomimetic boundary lubricants on articular cartilage. J Orthop Res 2016;(March):548–57, 10.1002/jor.23370. [DOI] [PubMed] [Google Scholar]
- 35.Buckley MR, Bergou AJ, Fouchard J, Bonassar LJ, Cohen I. High-resolution spatial mapping of shear properties in cartilage. J Biomech 2010;43(4):796–800, 10.1016/j.jbiomech.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S, Hung C, Ateshian G. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage 2004;12(1):65–73, 10.1016/J.JOCA.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum 2007;56(11):3662–9, 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley MR, Gleghorn JP, Bonassar LJ, Cohen I. Mapping the depth dependence of shear properties in articular cartilage. J Biomech 2008;41(11):2430–7, 10.1016/j.jbiomech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Middendorf JM, Griffin DJ, Shortkroff S, Dugopolski C, Kennedy S, Siemiatkoski J, et al. Mechanical properties and structure-function relationships of human chondrocyte-seeded cartilage constructs after in vitro culture. J Orthop Res 2017;35(10):2298–306, 10.1002/jor.23535. [DOI] [PubMed] [Google Scholar]
- 40.Caligaris M, Ateshian GA. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthritis Cartilage 2008;16(10):1220–7, 10.1016/J.JOCA.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forster H, Fisher J. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proc Inst Mech Eng Part H J Eng Med 1999;213(4):329–45, 10.1243/0954411991535167. [DOI] [PubMed] [Google Scholar]
- 42.Dahl LB, Dahl IMS, Engstrom-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis 1985;44(12):817–22, 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kongtawelert P, Ghosh P. A method for the quantitation of hyaluronan (hyaluronic acid) in biological fluids using a labeled avidin-biotin technique. Anal Biochem 1990;185(2): 313–8, 10.1016/0003-2697(90)90300-X. [DOI] [PubMed] [Google Scholar]
- 44.Balazs E The physical properties of synovial fluid and the special role of hyaluronic acid. Disord Knee 1974;2:61–74. [Google Scholar]
- 45.Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum 2007;56(1):108–16, 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 46.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 1999;254(3):535–41, 10.1006/BBRC.1998.0104. [DOI] [PubMed] [Google Scholar]
- 47.Bickel M The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol 1993;64(5 Suppl l): 456–60. [PubMed] [Google Scholar]
- 48.Legré-Boyer, Viscosupplementation V. Techniques, indications, results. Orthop Traumatol Surg Res 2015;101(1):S101–8, 10.1016/J.OTSR.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 49.Fraser JRE, Kimpton WG, Pierscionek BK, Cahill RNP. The kinetics of hyaluronan in normal and acutely inflamed synovial joints: observations with experimental arthritis in sheep. Semin Arthritis Rheum 1993;22(6):9–17, 10.1016/S0049-0172(10)80015-0. [DOI] [PubMed] [Google Scholar]
- 50.Feldmann M Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol 2002;2(5):364–71, 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 51.DiDomenico CD, Lintz M, Bonassar LJ. Molecular transport in articular cartilage — what have we learned from the past 50 years? Nat Rev Rheumatol 2018;14(7):393–403, 10.1038/s41584-018-0033-5. [DOI] [PubMed] [Google Scholar]
- 52.Abubacker S, Dorosz SG, Ponjevic D, Jay GD, Matyas JR, Schmidt TA. Full-length recombinant human proteoglycan 4 interacts with hyaluronan to provide cartilage boundary lubrication. Ann Biomed Eng 2016;44(4):1128–37, 10.1007/s10439-015-1390-8. [DOI] [PubMed] [Google Scholar]
- 53.Henak CR, Ross KA, Bonnevie ED, Fortier LA, Cohen I, Kennedy JG, et al. Human talar and femoral cartilage have distinct mechanical properties near the articular surface. J Biomech 2016;49(14):3320–7, 10.1016/J.JBIOMECH.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Bonnevie ED, Galesso D, Secchieri C, Bonassar LJ. Frictional Characterization of Injectable Hyaluronic Acids Is More Predictive of Clinical Outcomes than Traditional Rheological or Viscoelastic Characterization In: Awad HA, Ed. PloS One 2019;vol. 14(5), e0216702, 10.1371/journal.pone.0216702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


