Abstract
Systemic therapy options nowadays for advanced hepatocellular carcinoma (HCC) are either immunotherapy with immune checkpoint inhibitors or targeted therapy. As the incidence of liver cancer is much higher in developing countries, these new medications are not readily accessible for most of the patients. Cytotoxic chemotherapy agents are more available and affordable in developing countries. We are trying to explore the effectiveness of the newer cytotoxic agents in the systematic treatment for advanced HCC. This is a systematic review of all randomized controlled trials since 1997 that utilized systemic cytotoxic chemotherapy agents in the systemic treatment for advanced HCC using Scopus, PubMed, and Cochrane library up to February 2020. Six randomized trials were found. Different drugs and dosages were used, so it was statistically inappropriate to conduct a meta-analysis. No Phase III trial showed statistically significant overall survival (OS) benefit for cytotoxic chemotherapy, except subgroup analysis of Chinese patients in one study who had leucovorin, fluorouracil, and oxaliplatin (FOLFOX) regimen. There was no significant progression-free survival (PFS) or response rate in the Phase II trials. There are not enough data to infer the actual benefits of systemic cytotoxic chemotherapy in advanced HCC. However, oxaliplatin-based regimens may give feasible results. Health systems with limited access to targeted therapy and immunotherapy agents may use oxaliplatin-based regimens in clinical trials for advanced HCC. These results should be confirmed in multiple future randomized clinical trials.
Keywords: HCC, randomized trials, sorafenib, oxaliplatin
Introduction
Liver cancer is the sixth most common cancer and the fourth most common cause of death from cancer in the world.1,2 The median survival of advanced hepatocellular carcinoma (HCC) is seven and six months for Barcelona Clinic. Liver Cancer (BCLC) stage C and D, respectively, for untreated patients.3 For about ten years, sorafenib was the only standard systemic treatment for patients with HCC who are not candidates for resection or local treatment. That was based mainly on SHARP and Asia-Pacific trials.4,5 Recently, there are multiple targeted therapies, and immunotherapy agents were approved for the systemic treatment for HCC. Lenvatinib was approved by the food and drug administration (FDA) in August 2018 as a first-line treatment for patients with unresectable HCC based on REFLECT trial.6 Regorafenib, nivolumab, pembrolizumab, and cabozantinib were approved as second-line therapy for HCC, while ramucirumab was approved as second-line therapy for patients with alpha-fetoprotein ≥400.7 A recent study using atezolizumab with bevacizumab showed an overall survival and progression-free survival benefits over sorafenib for the first time.8
The cost and availability of targeted therapy in developing countries are significant problems. Eighty-three percent of estimated new liver cancer cases were found in less developed countries in 2012.9 When targeted therapy is not available, the other option will be supportive care only for most of the patients, as historically cytotoxic chemotherapy did not have remarkable results in the treatment of advanced HCC.10 In a systematic review of randomized controlled trials for chemotherapy in advanced HCC till 1997, almost all the trails used doxorubicin or doxorubicin analogs. The results were mixed, but no remarkable benefits were found, and toxicity was significant.11 Since that time, many cytotoxic agents were developed, but no precise data about their effect on systemic treatment for HCC. In this review, we will explore all randomized trials since 1997 that used cytotoxic chemotherapy drugs, single or combined, in the systemic treatment of advanced HCC in adults in comparison to other systemic treatments. We will review study design, patients’ characteristics, overall survival benefits, tumor response, progression-free survival, and toxicity from cytotoxic agents.
Methods
A search was conducted in the electronic database using Scopus, PubMed, and Cochrane library. A review of the reference lists of retrieved articles was done as well. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed.12 The search was done through February 29th, 2020.
For Scopus, the keywords used are Hepatocellular carcinoma, randomized, systemic chemotherapy, gemcitabine, oxaliplatin, doxorubicin, capecitabine, and cisplatin. Not locoregional or chemoembolization.
Inclusion Criteria
All randomized controlled trials that are in English and published as a full article since 1997.
Systemic cytotoxic chemotherapy is used exclusively as one of the arms of the study for the treatment for advanced HCC (single of multiple cytotoxic chemotherapy agents as one of the arms of the study).
Participants are adult patients with advanced hepatocellular carcinoma with any etiology.
The study should have an independent concurrent control arm.
Exclusion Criteria
Chemotherapy was used as adjuvant or neoadjuvant therapy.
Chemotherapy is used as a local injection (Transarterial chemoembolization TACE).
Studies that were published as abstract only.
Non-cytotoxic chemotherapy agents are used with cytotoxic chemotherapy arm.
Quality Assessment
The authors included only the articles that fulfilled the above criteria. Both authors reviewed and assessed the data independently.
Analysis of Data
Studies design and characteristics (Table 1),
Patients’ characteristics (Table 2),
Outcome: Median overall survival, progression-free survival, objective response, and side effects (Table 3).
As different drugs and dosing regimens were used, it was not appropriate to conduct a Meta-Analyses.
Table 1.
Studies Design and Characteristics
| Study | Abdel-Rahman et al13 | Qin et al15 | Gish et al14 | Yeo et al16 | Abou Alfa et al17 | Mok et al18 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Study | Phase II Randomized Open Label |
Phase III Randomized Open Label |
Phase III Randomized Open Label |
Phase III Randomized Open Label |
Phase II Randomized Double-Blind |
Phase II Randomized Open Label | |||||||
| Study arms | Sorafenib | Capecitabine | FOLFOX4 | Doxorubicin | Nolatrexed | Doxorubicin | Doxorubicin | PIAF | Doxorubicin + sorafenib | Doxorubicin +placebo | Doxorubicin | Nolatrexed | |
| Dosage/frequency | 400mg BID daily | 1000mg/m2bid d 1–14 | Oxa: 85mg/m2 d1 LV200mg/m2 d1and2, FU 400mg/m2 then 600mg/m2 over 22hr every 2 wks |
Doxorubicin 50mg/m2 every 3 weeks | 800mg/m2 CI over 5 days/3ws | 60mg/m2 every 3ws | 60mg/m2 every 3 weeks | P: 20 mg/m 2, d1-4 I:5 MU/m2 d 1–4 A: 40mg/m2 D1, F:400 mg/m2 D1-4 every Wk |
Doxorubicin 60 mg/m2 every 21 days + sorafenib 400mg BID daily | 60 mg/m2 every 21 days | 60mg/m2 d1 every 3 weeks | 725 mg/m2 over 5 days every 3 weeks | |
| Number in each arm | 26 | 26 | 184 | 187 | 222 | 223 | 94 | 94 | 47 | 49 | 18 | 36 | |
| Main inclusion criteria |
|
|
LVEF ≥50% |
|
LVEF ≥45% |
KPS≥ 70 Bilirubin No prior CTX within 4 weeks only |
|||||||
| Main exclusion criteria |
|
|
Transplant candidate (included later)
|
|
|
|
|||||||
| Methods of assessment |
Baseline | Three-phase CT or MRI | CT or MRI | NS | CT ± US | NS | CT or US | ||||||
| Follow-up | Repeat imaging every 8w | 6w±1 during treatment 2ms ±1w in follow-up |
NS | After 3 and 6 cycles of treatment | NS | Repeat imaging with every cycle | |||||||
| Response criteria | RECIST 1.0 for response | RECIST 1.0 | WHO criteria for partial and complete response | WHO criteria | RECIST 1.0 | WHO criteria for partial and complete response | |||||||
Abbreviations: CLIP, cancer of the liver Italian program; CI, continuous infusion; FU, 5 fluorouracil; LV, leucovorin; mets, metastases; NS, non-specified; PIAF, cisplatin/interferon α−2b/doxorubicin/fluorouracil; KPS, Karnofesky performance status; RECIST, response evaluation criteria in solid tumors; Oxa, oxaliplatin; TA, transarterial.
Table 2.
Patients’ Characteristics (Age, Sex, Status, Child-Pugh Score, Stage, Etiology)
| Study | Abdel-Rahman et al.13 | Qin et al15 | Gish et al14 | Yeo et al16 | Abou Alfa et al.17 | Mok et al18 | |||
|---|---|---|---|---|---|---|---|---|---|
| Nolatrexed | Doxorubicin | Doxorubicin | PIAF | Doxorubicin+ Sorafenib | Doxorubicin | ||||
| Age | 53.5 (median) | 18–75 | NS | 15–75 | 38–82 (median 65y) | >18y | |||
| Sex | Mainly males | 88.7%males | NS | 91% males | 76% males | 90.7% males | |||
| Baseline status | ECOG PST 0–2 | KPS ≥70 | KPS ≥ 60 | ECOG PST 0–2 | ECOG PST 0–2 | KPS ≥ 70 | |||
| Child-Pugh | A-B | A(87.9%)-B(12.1%) | A 74.8% B 24.3% |
A 73.1% B 26.9% |
A 87.2% B 12.8% |
A 83% B 17% |
A | NS | |
| Stage | BCLC C | BCLC B(20%) or C(80%) | Using CLIP score 35.1% score 2 29.3% score 3 27.9% score 1 |
35.9% score 2 28.7% score 2 27.4% score 2 |
Okuda staging I 9% II 87% II 4% |
I 9% II 87% III 4% |
NS | NS | |
| Possible etiology | HCV 96% HBV 2% NASH 2% |
HBV 91.4% HCV 6.7% |
HCV 35.6% HBV26.1% History of Alcoholism29.3% |
HCV 43% HBV18.4% History of Alcoholism 26% |
HBV 85% HCV 9% |
HBV 87% HCV 4% |
HBV 6.4% HCV 21.3% |
HBV 14.3% HCV 14.3% |
HBV 78% |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CLIP, Cancer of the Liver Italian Program; KPS, Karnofesky performance status; NASH, non-alcoholic steatohepatitis; PIAF, cisplatin/interferon α−2b/doxorubicin/fluorouracil; PST, performance status.
Table 3.
Outcome Median Overall Survival, Progression-Free Survival, Objective Response and Side Effects
| Study | Abdel-Rahman et al13 | Qin et al15 | Gish et al14 | Yeo et al16 | Abou Alfa et al17 | Mok et al18 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study arm | Sorafenib | Capecitabine | FOLFOX | Doxorubicin | Nolatrexed | Doxorubicin | Doxorubicin | PIAF | D+S | D+P | Nolatrexed | Doxorubicin |
| Progression-free survival | 6m | 4m | 2.93m | 1.77m | 2.76m | 2.3m | NS | NS | 6m | 2.7m | 1.58m | 1.55m |
| p < 0.005, HR 2.708 | p < 0.001, HR 0.62 | p= 0.709 | p= 0.006, HR: 0.45 | |||||||||
| Median overall survival | 7.05m | 5.07m | 6.4m | 4.79m | 5.13m | 7.43m | 6.83m | 8.67m | 13.7m | 6.5m | 4.6m | 3.4m |
| p < 0.016, HR 2.36 | p= 0.07, HR 0.8 | p=0.0068, HR 0.753 | p= 0.83, HR 0.97 | p= 0.006, HR 0.49 | p = 0.9843 | |||||||
| Main adverse event | Hand and foot syndrome, gastrointestinal | Hyperbilirubinemia, gastrointestinal | Neutropenia Nausea ↑ AST anorexia |
Alopecia ↑ AST Nausea |
Stomatitis Vomiting Diarrhea Thrombocytopenia |
Alopecia Neutropenia Anemia |
Neutropenia Anemia Thrombocytopenia |
Neutropenia Thrombocytopenia Hypokalemia |
Leucopenia Hand & foot skin reaction Diarrhea Systolic dysfunction |
Fatigue Neutropenia Diarrhea |
Neutropenia Stomatitis Anemia vomiting |
Neutropenia Anemia Alopecia |
| Partial response | 3pts | 1pt | 15 | 5 | NS | NS | 9 | 19 | 2 | 0 | 0 | 0 |
| Complete response | 1pt | 0 | 0 | 0 | NS | NS | 0 | 0 | 0 | 1 | 0 | 0 |
| Response rate | 14.5% | 3% | 8.15% | 2.67% | 1.4% | 4% | 10.5% | 20.9% | 4% | 2% | 0 | 0 |
Abbreviations: m, month; HR, hazard ratio; D+S, doxorubicin+sorafenib; D+P, doxorubicin+placebo; NS, not specified.
Results
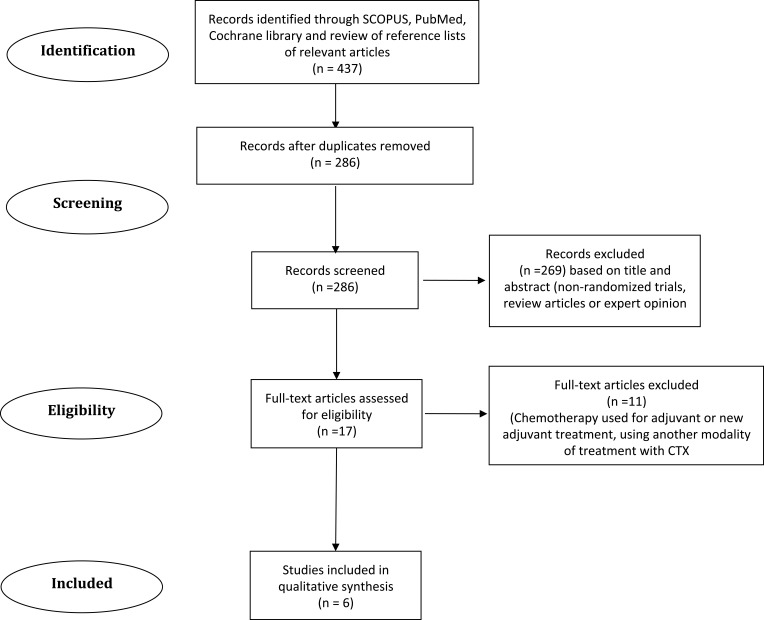
Four hundred thirty-seven articles were found with the initial search, 151 were excluded as they were duplicate. Two hundred eighty-six articles were examined; 269 articles were excluded as they were either non-randomized trials, systematic reviews, or expert opinion. Seventeen articles were examined in detail, 11 were excluded as they did not use cytotoxic chemotherapy exclusively at least in one of the study arms or used chemotherapy as adjuvant or neoadjuvant treatment.
Study Characteristics
Six studies have met the criteria (Figure 1). Three of these studies were Phase II, and the others were Phase III. All, except one,13 were designed before the approval of sorafenib. A single study was double-blinded,14 while the others were open-label trials. All of them used doxorubicin as a control arm, except one used sorafenib.13 Three of these studies used the combination regimen.15–17 To evaluate the response, three of them used WHO criteria,14,16,18 the other three used Response Evaluation Criteria in Solid Tumors (RECIST) 1.0.13,15,17 In the studies that gave patients’ characteristics, most of their patients were male. Baseline performance either between 0–2 according to ECOG performance status or above 60% according to Karnofesky Performance status (KPS) scale. All were Child-Paugh A or B. The studies used different staging systems but mainly Barcelona Clinic Liver Cancer staging system. For possible underlying etiology, patients with HBV infection were the majority in three of the studies.15,16,18 The majority of patients had HCV infection in one study.13
Figure 1.
Flow chart of study section.
Outcomes
It was challenging to do a statistically meaningful meta-analysis as these studies used different cytotoxic agents, and different doses (doxorubicin was used in most of these studies in different doses as the control arm, and it is known that doxorubicin has poor results in HCC). Median overall survival (OS) was the primary outcome of phase III studies.14–16 OS in these studies, for control arm (doxorubicin), was ranging between 4.79 and 7.43 months. Longest median overall survival was 8.67 months in Yeo et al study at cisplatin/interferon α-2b/doxorubicin/5-fluorouracil (PIAF) arm, but that was not statistically significant with p-value 0.86.16 In a large phase III trial, nolatrexed showed lower median overall survival than doxorubicin, as OS was 5.13 and 7.43 months, respectively, with p-value 0.0068.13 FOLFOX showed better OS than doxorubicin with OS 6.4 months and 4.79 months, respectively, but that study did not meet its primary endpoint with p = 0.07.15 The highest tumor response was at PIAF arm at Yeo et al study, as the response rate was 20.9%, but all responses were partial with no complete response.16 FOLFOX arm at Qin et al study showed a good partial response but no complete response; the response rate was 8.15%.15 In phase II trials, response rate and progression-free survival were not significant for capecitabine and nolatrexed.13,18 Side effects of doxorubicin in all studies were mainly neutropenia, anemia, alopecia, and fatigue. FOLFOX’s main non-hematological side effects were nausea, AST elevation, and anorexia. For hematological side effects, 30.6% had grade 3 to 4 neutropenia.15 PIAF regimen toxicities were significant and were mainly neutropenia, thrombocytopenia, and hypokalemia.16 Nolatrexed showed stomatitis, vomiting, diarrhea, and thrombocytopenia.14 Two studies were not completed; Mok et al study was discontinued as there was no objective response in nolatrexed arm.18 The other study was not completed after interim analysis, as it showed significant differences between sorafenib + doxorubicin arm and doxorubicin + placebo arm.17
Discussion
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death worldwide. More than 80% of HCC cases occur in low-resource and middle-resource countries where medical and social care resources are often constrained. One of the common risk factors for HCC is chronic hepatitis B and chronic hepatitis C (CHC) infection.19–26
Advanced hepatocellular carcinoma is a challenging disease with different epidemiology and risk factors based on demographics worldwide.27,28 There was no universal approved drug for systemic treatment of advanced HCC until the approval of sorafenib. As sorafenib showed short overall survival benefit and modest response, clinical trials have been ongoing in that field. Kinase inhibitors other than sorafenib, like Lenvatinib, cabozantinib, and regorafenib, showed some good results, but none were superior to sorafenib.29 As HCC is known to be a highly vascular tumor, drugs that act as vascular endothelium growth factor inhibitors with different mechanisms have been investigated as bevacizumab and Ramucirumab. Multiple drug categories have been investigated like a-Fetoprotein Targeted Drugs, and Glypican-3 Targeted Drugs.29
The liver is a central immunomodulator that keeps the balance between protection and immunotolerance.30,31 Deregulation of the liver immunological network is a hallmark of chronic liver disease and HCC.32–39 Immunotherapy has been recently used for multiple types of cancer and has raised hope for the successful treatment of advanced HCC. Immune Checkpoint inhibitors have been used for treatment as a single agent or in combination with targeted therapy or VEGF inhibitors with promising results, as atezolizumab, Pembrolizumab, and Nivolumab.40,41 Many clinical trials are ongoing to investigate the effect of Chimeric antigen receptor T (CAR-T) cell therapy on HCC.40
In developing countries, the cost of these new medications can be a challenge due to limited resources. Many studies showed that sorafenib is not a cost-effective option for advanced HCC in some developing countries.42–44 Cytotoxic chemotherapy drugs are more available and cheaper, but historically, systemic cytotoxic chemotherapy did not show noteworthy results, but local cytotoxic chemotherapy agents have been used frequently. Transarterial Chemoembolization (TACE) with cytotoxic chemotherapy drugs have been used as a single modality of treatment or in combination with other locoregional modalities, like three-dimensional conformal radiotherapy, percutaneous ethanol injection, percutaneous microwave coagulation therapy, and percutaneous acetic acid injection.45,46 The most common chemotherapy combinations that have been used for TACE are Fluorouracil, cisplatin, mitomycin, and epirubicin.47 Using systemic therapy (specifical sorafenib) with TACE is controversial. The side effects of the combination of sorafenib and the cytotoxic agents for TACE were significant, but the combination of hydroxycamptothecin plus pirarubicin or epirubicin did not increase the side effects with improving efficacy.48
The use of systemic cytotoxic chemotherapy alone in the treatment of HCC is rare since the approval of sorafenib. Doxorubicin was the most common drug to be used in these studies as a control arm, but there is no benefit of analyzing doxorubicin results together as it is evident from previous literature that it has little benefits with high toxicity. There are only three phase III trials that have used single cytotoxic chemotherapy drugs or in combination with other cytotoxic chemotherapy drugs as an arm of a trial in systemic treatment for advanced HCC over the last twenty years. PIAF study had a better OS over doxorubicin, but that was not statistically significant, and the toxicity was high.16 Nolatrexed did not show a survival benefit over doxorubicin.13 Although the third phase III study, which has used FOLFOX against doxorubicin, did not show statistically significant overall survival,15 in a subgroup analysis for Chinese patients in that study, there was a statistically significant overall survival. Median overall survival was 5.7 months and 4.3 months for FOLFOX4 and doxorubicin, respectively, HR 0.74; 95% CI: 0.55–0.98; p-value 0.03.49 Based on that, China Food and drug administration approved oxaliplatin for the treatment of advanced HCC patients who are not eligible for a transplant or local treatment.50 However, we should consider that most of the patients in that study had HBV infection, and that is not the most common etiology in different areas of the world. Phase II trials were included to examine response rate and progression-free survival. Capecitabine, nolatrexed, and doxorubicin did not show significant PFS or response rate in these studies. Other than these randomized trials, studies showed a survival benefit for some of the cytotoxic drugs as gemcitabine and oxaliplatin. In a retrospective study for two hundred and four patients who had Gemcitabine/Oxaliplatin combination (GEMOX) for HCC treatment, the median overall survival was 11 months (95% CI: 9–14), and the response rate was 22%.51 Other small trials using gemcitabine and oxaliplatin showed good tumor response.52,53 For toxicity, oxaliplatin-based regimens for the treatment of advanced HCC are relatively safe and well tolerated.54
Limitations
There are few randomized trials utilized systemic cytotoxic chemotherapy exclusively. Three of the trials that were retrieved had a small sample size that affected the statistical results of these studies. Also, no study mentioned the subtypes of HCC in their patients, and that can make a difference in the outcome.
Conclusions
There is no enough data to infer the actual benefits of systemic cytotoxic chemotherapy in advanced HCC. But, oxaliplatin-based regimens may give feasible results. Health systems with limited access to targeted therapy and immunotherapy agents may use oxaliplatin-based regimens in clinical trials for advanced HCC. These results should be confirmed in multiple future randomized clinical trials.
Funding Statement
There is no funding to report.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Hetta HF, Mekky MA, Zahran AM, et al. Regulatory B cells and their cytokine profile in HCV-related hepatocellular carcinoma: association with regulatory T cells and disease progression. Vaccines. 2020;8(3):380. doi: 10.3390/vaccines8030380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology (Baltimore, Md). 2015;61(1):184–190. doi: 10.1002/hep.27443 [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 7.Vogel A, Saborowski A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev. 2020;82:101946. doi: 10.1016/j.ctrv.2019.101946 [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 10.Nagahama H, Okada S, Okusaka T, et al. Predictive factors for tumor response to systemic chemotherapy in patients with hepatocellular carcinoma. Jpn J Clin Oncol. 1997;27(5):321–324. doi: 10.1093/jjco/27.5.321 [DOI] [PubMed] [Google Scholar]
- 11.Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8(2):117–136. doi: 10.1023/A:1008285123736 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Rahman O, Abdel-Wahab M, Shaker M, Abdel-Wahab S, Elbassiony M, Ellithy M. Sorafenib versus capecitabine in the management of advanced hepatocellular carcinoma. Med Oncol. 2013;30(3):655. doi: 10.1007/s12032-013-0655-z [DOI] [PubMed] [Google Scholar]
- 14.Gish RG, Porta C, Lazar L, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol. 2007;25(21):3069–3075. doi: 10.1200/JCO.2006.08.4046 [DOI] [PubMed] [Google Scholar]
- 15.Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501–3508. doi: 10.1200/JCO.2012.44.5643 [DOI] [PubMed] [Google Scholar]
- 16.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532–1538. doi: 10.1093/jnci/dji315 [DOI] [PubMed] [Google Scholar]
- 17.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304(19):2154–2160. doi: 10.1001/jama.2010.1672 [DOI] [PubMed] [Google Scholar]
- 18.Mok TS, Leung TW, Lee SD, et al. A multi-centre randomized phase II study of nolatrexed versus doxorubicin in treatment of Chinese patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 1999;44(4):307–311. doi: 10.1007/s002800050982 [DOI] [PubMed] [Google Scholar]
- 19.Mekky MA, Sayed HI, Abdelmalek MO, et al. Prevalence and predictors of occult hepatitis C virus infection among Egyptian patients who achieved sustained virologic response to sofosbuvir/daclatasvir therapy: a multi-center study. Infect Drug Resist. 2019;12:273. doi: 10.2147/IDR.S181638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekky MA, Abdel-Malek MO, Osman HA, et al. Efficacy of ombitasvir/paritaprevir/ritonavir/ribavirin in management of HCV genotype 4 and end-stage kidney disease. Clin Res Hepatol Gastroenterol. 2019;43(1):82–87. doi: 10.1016/j.clinre.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Elsherbiny NM, Rammadan M, Hassan EA, et al. Autoimmune hepatitis: shifts in gut microbiota and metabolic pathways among Egyptian patients. Microorganisms. 2020;8(7):1011. doi: 10.3390/microorganisms8071011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetta HF, Elkady A, Morsy KH, Mohamed IS, Ibrahim MA. Serum level of IL17a among cirrhotic hepatitis C virus infected patients with incidence of diabetes mellitus. Egypt J Immunol. 2017;24(1):79–88. [PubMed] [Google Scholar]
- 23.Hetta HF, Khairy H, Ismail S. Circulating IL17A and IFN-gamma serum levels in cirrhotic hepatitis C virus infected patients with autoimmune thyroiditis. Int J Curr Microbiol Appl Sci. 2017;6(3):1972–1983. doi: 10.20546/ijcmas.2017.603.225 [DOI] [Google Scholar]
- 24.Mehta M, Hetta HF, Abdel-Hameed EA, et al. Association between IL28b rs12979860 single nucleotide polymorphism and the frequency of colonic T reg in chronically HCV-infected patients. Arch Virol. 2016;161(11):3161–3169. doi: 10.1007/s00705-016-3015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hetta HF, Mekky MA, Khalil NK, et al. Extra-hepatic infection of hepatitis C virus in the colon tissue and its relationship with hepatitis C virus pathogenesis. J Med Microbiol. 2016;65(8):703. doi: 10.1099/jmm.0.000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Hameed EA, Rouster SD, Ji H, et al. Evaluating the role of cellular immune responses in the emergence of HCV NS3 resistance mutations during protease inhibitor therapy. Viral Immunol. 2016;29(4):252–258. doi: 10.1089/vim.2015.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB. 2005;7(1):35–41. doi: 10.1080/13651820410024058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abd El-Baky RM, Hetta HF, Koneru G, et al. Impact of interleukin IL-6 rs-1474347 and IL-10 rs-1800896 genetic polymorphisms on the susceptibility of HCV-infected Egyptian patients to hepatocellular carcinoma. Immunol Res. 2020;68(3):118–125. doi: 10.1007/s12026-020-09126-8 [DOI] [PubMed] [Google Scholar]
- 29.Ma YS, Liu JB, Wu TM, Fu D. New therapeutic options for advanced hepatocellular carcinoma. Cancer Control. 2020;27(3):1073274820945975. doi: 10.1177/1073274820945975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetta HF, Mehta MJ, Shata MTM. Gut immune response in the presence of hepatitis C virus infection. World J Immunol. 2014;4(2):52–62. doi: 10.5411/wji.v4.i2.52 [DOI] [Google Scholar]
- 31.Shata MTM, Abdel-Hameed EA, Hetta HF, Sherman KE. Immune activation in HIV/HCV-infected patients is associated with low-level expression of liver expressed antimicrobial peptide-2 (LEAP-2). J Clin Pathol. 2013;66(11):967–975. doi: 10.1136/jclinpath-2013-201581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahran AM, Nafady-Hego H, Mansor SG, et al. Increased frequency and FOXP3 expression of human CD8+ CD25High+ T lymphocytes and its relation to CD4 regulatory T cells in patients with hepatocellular carcinoma. Hum Immunol. 2019;80(7):510–516. doi: 10.1016/j.humimm.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 33.Hetta HF, Zahran AM, Mansor SG, Abdel‐Malek MO, Mekky MA, Abbas WA. Frequency and implications of myeloid-derived suppressor cells and lymphocyte subsets in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. J Med Virol. 2019;91(7):1319–1328. doi: 10.1002/jmv.25428 [DOI] [PubMed] [Google Scholar]
- 34.Zahran AM, Zahran ZAM, El-Badawy O, et al. Prognostic impact of toll-like receptors 2 and 4 expression on monocytes in Egyptian patients with hepatocellular carcinoma. Immunol Res. 2019;67(2–3):157–165. [DOI] [PubMed] [Google Scholar]
- 35.Abd Ellah NH, Tawfeek HM, John J, Hetta HF. Nanomedicine as a future therapeutic approach for hepatitis C virus. Nanomedicine. 2019;14(11):1471–1491. doi: 10.2217/nnm-2018-0348 [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi VK, Singh A, Dubey SK, Hetta HF, John J, Singh M. Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma. Microb Pathog. 2019;128:184–194. doi: 10.1016/j.micpath.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 37.Zahran AM, Ashmawy AM, Rayan A, Elkady A, Elsherbiny NM, Hetta HF. Frequency and implications of natural killer and natural killer T cells in hepatocellular carcinoma. Egypt J Immunol. 2018;25(2):45–52. [PubMed] [Google Scholar]
- 38.Hetta H, Elkady A, Tohamy T, Regulatory BM. B cells: key players in hepatocellular carcinoma progression. Gastroenterol Hepatol Open Access. 2016;5(2):00136. doi: 10.15406/ghoa.2016.05.00136 [DOI] [Google Scholar]
- 39.Hetta HF, Mekky MA, Khalil NK, et al. Association of colonic regulatory T cells with hepatitis C virus pathogenesis and liver pathology. J Gastroenterol Hepatol. 2015;30(10):1543–1551. doi: 10.1111/jgh.12936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Ding J, Li HY, Wang ZH, Wu J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim Biophys Acta Rev Cancer. 2020;1874:188441. doi: 10.1016/j.bbcan.2020.188441 [DOI] [PubMed] [Google Scholar]
- 41.Zahran AM, Hetta HF, Rayan A, et al. Differential expression of Tim-3, PD-1, and CCR5 on peripheral T and B lymphocytes in hepatitis C virus-related hepatocellular carcinoma and their impact on treatment outcomes. Cancer Immunol Immunother. 2020;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta N, Verma RK, Prinja S, Dhiman RK. Cost-effectiveness of sorafenib for treatment of advanced hepatocellular carcinoma in India. J Clin Exp Hepatol. 2019;9(4):468–475. doi: 10.1016/j.jceh.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasse A, Carmo R. P-239 Sorafenib for advanced hepatocellular carcinoma (HCC) in the public health setting in Brazil: a cost-effectiveness analysis. Ann Oncol. 2019;30(Supplement_4):mdz155.238. doi: 10.1093/annonc/mdz155.238 [DOI] [Google Scholar]
- 44.Zhang P, Yang Y, Wen F, et al. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27(7):853–859. doi: 10.1097/MEG.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 45.Feng F, Jiang Q, Jia H, et al. Which is the best combination of TACE and Sorafenib for advanced hepatocellular carcinoma treatment? A systematic review and network meta-analysis. Pharmacol Res. 2018;135:89–101. doi: 10.1016/j.phrs.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 46.Hassan EA, Ahmed EH, Nafee AM, El-Gafary N, Hetta HF, El-Mokhtar MA. Regulatory T cells, IL10 and IL6 in HCV related hepatocellular carcinoma after transarterial chemoembolization (TACE). Egypt J Immunol. 2019;26(1):69–78. [PubMed] [Google Scholar]
- 47.Ma X, Li RS, Wang J, et al. The therapeutic efficacy and safety of compound kushen injection combined with transarterial chemoembolization in unresectable hepatocellular carcinoma: an update systematic review and meta-analysis. Front Pharmacol. 2016;7:70. doi: 10.3389/fphar.2016.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie H, Yu H, Tian S, et al. What is the best combination treatment with transarterial chemoembolization of unresectable hepatocellular carcinoma? A systematic review and network meta-analysis. Oncotarget. 2017;8(59):100508–100523. doi: 10.18632/oncotarget.20119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin S, Cheng Y, Liang J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study. Oncologist. 2014;19(11):1169–1178. doi: 10.1634/theoncologist.2014-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin S, Zhang X, Guo W, et al. Prognostic nomogram for advanced hepatocellular carcinoma treated with FOLFOX 4. Asian Pac J Cancer Prev. 2017;18(5):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013;58(1):81–88. doi: 10.1016/j.jhep.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 52.Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer. 2007;109(7):1384–1390. doi: 10.1002/cncr.22532 [DOI] [PubMed] [Google Scholar]
- 53.Taïeb J, Bonyhay L, Golli L, et al. Gemcitabine plus oxaliplatin for patients with advanced hepatocellular carcinoma using two different schedules. Cancer. 2003;98(12):2664–2670. doi: 10.1002/cncr.11869 [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Zheng YH, Han L, Qin SK. Efficacy and safety of the oxaliplatin-based chemotherapy in the treatment of advanced primary hepatocellular carcinoma: a meta-analysis of prospective studies. Medicine. 2016;95(40):e4993. doi: 10.1097/MD.0000000000004993 [DOI] [PMC free article] [PubMed] [Google Scholar]