Abstract
Zika virus (ZIKV) is an emerging mosquito-borne pathogen that can cause global public health threats. In the absence of effective antiviral medications, prevention measures rely largely on reducing the number of adult mosquito vectors by targeting juvenile stages. Despite the importance of juvenile mosquito control measures in reducing adult population size, a full understanding of the effects of these measures in determining mosquito phenotypic traits and in mosquito-arbovirus interactions is poorly understood. Pyriproxyfen is a juvenile hormone analog that primarily blocks adult emergence, but does not cause mortality in larvae. This mechanism has the potential to work in combination with other juvenile sources of mortality in nature such as predation to affect mosquito populations. Here, we experimentally evaluated the effects of juvenile exposure to pyriproxyfen and predatory mosquito Toxorhynchites rutilus on Aedes aegypti phenotypes including susceptibility to ZIKV infection and transmission. We discovered that combined effects of pyriproxyfen and Tx. rutilus led to higher inhibition of adult emergence in Ae. aegypti than observed in pyriproxyfen or Tx. rutilus treatments alone. Adult body size was larger in treatments containing Tx. rutilus and in treatments mimicking the daily mortality of predation compared to control or pyriproxyfen treatments. Susceptibility to infection with ZIKV in Ae. aegypti was reduced in predator treatment relative to those exposed to pyriproxyfen. Disseminated infection, transmission, and titers of ZIKV in Ae. aegypti were similar in all treatments relative to controls. Our data suggest that the combination of pyriproxyfen and Tx. rutilus can inhibit adult Ae. aegypti emergence but may confer a fitness advantage in survivors and does not inhibit their vector competence for ZIKV relative to controls. Understanding the ultimate consequences of juvenile mosquito control measures on subsequent adults’ ability to transmit pathogens is critical to fully understand their overall impacts.
Author summary
Mosquito control approaches primarily depend on lowering the number of potential adult mosquito vectors by inhibiting juvenile stages to reduce the risk of pathogen transmission. Pyriproxyfen is a juvenile hormone analog that inhibits the emergence of adult mosquitoes by interrupting metamorphosis, but does not target larvae. This mechanism allows natural sources of mortality like predation to act in combination with pyriproxyfen to affect mosquito population size. Here, we determined the effects of juvenile exposure to pyriproxyfen and predatory mosquito Toxorhynchites rutilus on adult Aedes aegypti traits, including infection with Zika virus. Combined effects of pyriproxyfen and Tx. rutilus led to strong inhibition of adult emergence in Ae. aegypti. Treatments containing predators or those mimicking the daily mortality of predation produced larger sized adults. Susceptibility to ZIKV infection was lowest in the predator treatment and highest in the pyriproxyfen treatment. Disseminated infection, transmission, and viral titers of ZIKV were similar between treatments. Our data suggest that the combination of pyriproxyfen and predators can enhance inhibition of adult Ae. aegypti emergence, but survivors may have fitness benefits such being larger mosquitoes. Understanding the consequences of control approaches in mosquito-pathogen interactions will assist to evaluate their suitability in mosquito control programs.
Introduction
Zika virus (ZIKV) is an emerging infectious pathogen that causes public health issues in many regions of the world. Zika virus (family: Flaviviridae, genus: Flavivirus) is an enveloped, positive-sense, single-stranded RNA virus with approximately 11,000 nucleotides. First isolation of ZIKV was in 1947 from serum of a non-human primate rhesus monkey Macaca mulatta stationed in the Uganda's Zika Forest [1,2], and later ZIKV was isolated from a wild-caught mosquito Aedes africanus in 1948. Early cases of ZIKV in humans were reported in 1952 by serological surveys in eastern Nigeria and Uganda [3,4]. Although primarily a mosquito-borne agent, ZIKV may be transmitted sexually [5], through blood transfusion [6], and from mother-to-child [7,8], modalities which further complicate control strategies and ZIKV epidemiology [2,9]. Viral infection in human usually results in mild symptoms; however, ZIKV has been implicated in neurological complications resulting in Guillain-Barré syndrome (i.e., acute inflammatory polyneuropathy) and microcephaly (i.e., severe decrease in the head circumference) in newborn babies, making it a serious public health threat [10–13].
Aedes aegypti is the primary vector of ZIKV and several neglected arthropod-borne viruses (arboviruses) that affect human health, including dengue virus (DENV) and chikungunya virus (CHIKV) [14]. Originating from Africa, Ae. aegypti is now widely spread in tropical, subtropical, and some temperate areas around the world [15,16]. The invasive success of Ae. aegypti is largely attributable to its life-history characteristics which exploit human-dominated habitats. Aedes aegypti has adapted to use domestic and urban environments, where larvae dwell in man-made containers such as vases, plastic containers, tires, gutter eaves, tubs, and cisterns [17]. Females of Ae. aegypti show a preference for blood feeding on humans, a behavior which contributes to its primary role as an arbovirus vector, and gonotrophic discordance where females may ingest more than one blood meal per gonotrophic cycle, allowing for increased probability of acquiring and transmitting pathogens and associated disease incidence [18,19,20,21]. Additionally, this species exhibits skip oviposition, whereby females oviposit eggs in more than one container during a gonotrophic cycle, a hedge betting strategy [22]. Eggs of the container-dwelling Ae. aegypti are more tolerant to desiccation than other mosquitoes (e.g., Anopheles and Culex), a trait which enables eggs to resist unfavorable environmental conditions without losing their viability [23].
Since there are no effective antiviral drugs or vaccines for most arboviruses, including ZIKV, control of vectors is the primary method used to combat ZIKV transmission. Mosquito control programs have extensively relied on the use of insecticides (e.g., larvicides) against juvenile stages to suppress potential adult vectors [24,25]. Insect growth regulators (IGRs) are chemical substances that disrupt the development and growth of mosquitoes and provide an alternative to larvicides for controlling Ae. aegypti, especially among geographic populations that exhibit resistance to insecticides, particularly organophosphates and pyrethroids [24–27]. Pyriproxyfen is a synthetic analog of juvenile hormone, a natural IGR in insects, that disrupts growth and development during the juvenile stages. Pyriproxyfen primarily interferes with metamorphosis at the end of pupal development [24,28]. Pyriproxyfen and other IGRs inhibit metamorphosis to adult stages after a short time exposure at exceptionally low concentrations making them favorable options for mosquito control [29–31]. Additionally, pyriproxyfen is effective against many insects of public health importance and is recommended for controlling mosquitoes by the World Health Organization Pesticide Evaluation Scheme [32–34].
Novel strategies in how pyriproxyfen is utilized has further enhanced its potential as a tool for mosquito control. Auto-dissemination of pyriproxyfen is a promising technique for mosquito control, where pyriproxyfen can be mechanically disseminated to new oviposition sites to interrupt metamorphosis of juvenile mosquitoes by females that previously visited pyriproxyfen-treated stations or mated with contaminated males [35,36]. Pyriproxyfen may further mitigate risk of pathogen transmission through morphological and physiological aberrations among adult and juvenile mosquitoes that come in contact with pyriproxyfen [37]. For example, short exposure to pyriproxyfen induced reproductive disruption and lifespan reduction in adult mosquitoes surviving the exposure [38,39]. Also, juvenile exposure to pyriproxyfen caused damage in midgut cells of Ae. aegypti larvae compared to unexposed controls [40]. These observations suggest that exposure to pyriproxyfen during the juvenile stages may have transstadial effects and alter fitness-related traits, mosquito immunity, susceptibility to infection, and transmission of pathogens among adult survivors, as observed with other insecticides [41,42].
The mode of action of pyriproxyfen which targets pupal-adult transformation takes advantage of other natural sources of mortality among mosquito larvae, especially density-dependent mortality, a strong regulator among container inhabiting mosquitoes [43]. For instance, predation, nutrient deprivation, and intra- and interspecific competition from larval crowding may cause mortality in mosquito larvae before pyriproxyfen-induced pupal mortality and so may allow for greater inhibition of adult emergence. This assumption predicts that pyriproxyfen and other natural sources of mortality (e.g., biological control agents such as predators) act in conjunction to inhibit pathogen transmission by reducing the number of adult mosquitoes.
Biological control approaches that exploit predatory species have historically been applied to reduce pathogen transmission risk by inducing juvenile mortality and inhibiting recruitment to the adult stage [44]. The predatory mosquito larvae of Toxorhynchites rutilus have been used in biological control trials against several mosquitoes, including Ae. aegypti, with mixed success due to their voracious appetite and shared habitat with other container mosquitoes [45,46]. The use of Tx. rutilus, and other biological control agents, in combination with pyriproxyfen may exacerbate mortality in Ae. aegypti since the latter has the desirable feature of not targeting the predaceous stage (i.e., larvae) of Tx. rutilus. In addition to the direct effects of prey consumption by predators, several studies indicated that prey exposure to stress of predators can induce sublethal costs in their prey in the form of indirect effects on behavior, physiology, and morphology [47–51]. For example, exposure to predatory dragonfly nymph during the juvenile stages modified adult An. gambiae susceptibility to fungus parasites [52]. The exposure of juvenile An. coluzzii to a predatory backswimmer led to alterations in adult traits, including size and fecundity [53]. Also, exposure to predatory Tx. rutilus during juvenile stages decreased lifespan of adult survivors in Ae. aegypti, suggesting an additional benefit to mitigating arbovirus transmission risk [54]. These results suggest that the exposure to predators such as Tx. rutilus may indirectly influence phenotypic traits, including immunity, of surviving adult mosquitoes and therefore alter their susceptibility to pathogen infection.
Taken together these findings suggest that exposure to pyriproxyfen and Tx. rutilus may alter arbovirus transmission directly by reducing the number of emerged adults and indirectly by modifying traits of surviving adults and their susceptibility to pathogen infection. The purpose of our study was to assess the effects of exposure to pyriproxyfen independently or in combination with mosquito predator Tx. rutilus on inhibition of adult emergence, body size, and mosquito-arbovirus interactions. Specifically, we measured susceptibility to ZIKV infection, disseminated infection, transmission, and viral titers in Ae. aegypti following orally ingestion of ZIKV.
Methods
Ethics statement
Zika virus (Asian lineage, strain PRVABC59, GenBank number KU501215) used in this research was originally isolated from serum of an infected human patient in Puerto Rico in 2015. An isolate of ZIKV was provided by the U.S. Centers for Disease Control and Prevention (Division of Vector-Borne Diseases, Arboviral Diseases Branch). Zika virus propagation, infectious blood meal preparations, and experimental infections of adult mosquitoes were performed in an arbovirology research facility at the Florida Medical Entomology Laboratory (BSL2+ and ACL2+) in accordance with the approved protocol by the University of Florida’s Institutional Biosafety Committee and Institutional Animal Care and Use Committee.
Mosquitoes
Aedes aegypti (F2 generation) used in this research was from field collections made in Vero Beach, FL. Larvae (F2) were provided with food consisting of equal parts lactalbumin and Saccharomyces cerevisiae yeast. Newly pupated mosquitoes were transferred to plastic cups filled with water and held in adult rearing cages (30 x 30x 30 cm, BioQuip Products, Rancho Dominquez, CA) for adult emergence. Adult mosquitoes were provided with constant access to 10% sucrose solution ad libitum through cotton wicks. Mosquitoes were reared in a bioroom at controlled conditions: 60–80% relative humidity, 28°C±1°C, and 14:10-h light: dark photoperiod diurnal cycle.
Eggs of laboratory strain of predatory mosquito Tx. rutilus were obtained from Lee County Mosquito Control District in Lehigh Acres, FL. The colony of Tx. rutilus was held in a cage (65 x 37 x 50 cm) and maintained in an insectary core facility with natural light and photoperiod. Adults had constant access to 10% sucrose solution from moistened cotton and oviposition cups filled with water. Since females of Tx. rutilus lay fertile eggs autogenously, newly hatched larvae were collected from oviposition cups and transferred to small cell trays to prevent cannibalism (1 larva per cell). Larvae of Tx. rutilus were fed Ae. aegypti larvae every two days until pupation after which they were transferred into the colony cage for adult emergence.
Pyriproxyfen preparation
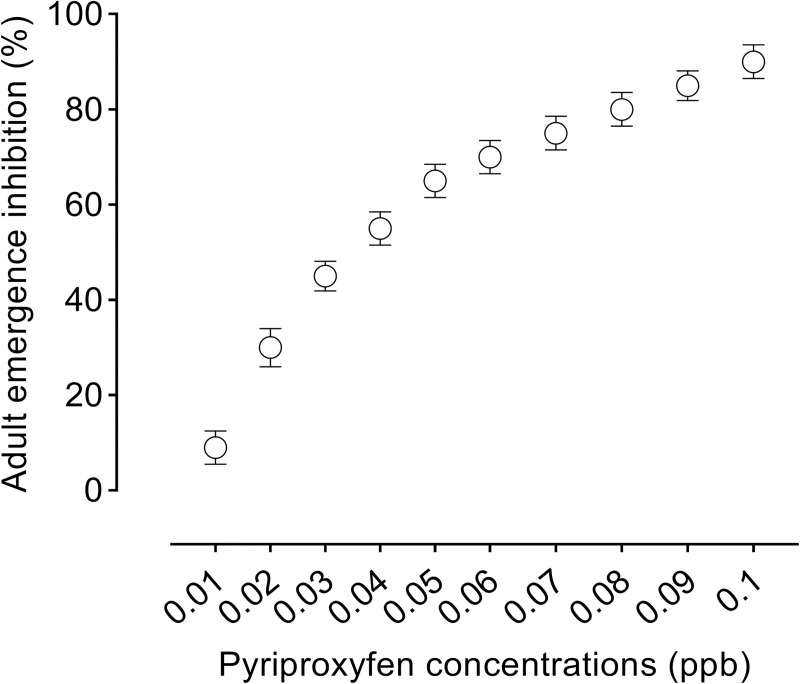
A stock solution of juvenile hormone analog pyriproxyfen (10 ppb) (2-[1-Methyl-2-(4-phenoxyphenoxy) ethoxy] pyridine—Nyguard IGR) was prepared in tap water and serial diluted to assess its toxicity to Ae. aegypti. Based on our preliminary toxicity assessment, a single low concentration of pyriproxyfen (0.022 ppb) that causes 30% inhibition of adult emergence was used in our experiments (Fig 1). Exposure to low concentration of pyriproxyfen was expected to induce direct and indirect effects on Ae. aegypti and still allow for enough survivors to assess their susceptibility to ZIKV infection, especially in treatments where mortality is anticipated to be higher than other treatments (e.g., pyriproxyfen+predator).
Fig 1. Adult emergence inhibition in Ae. aegypti in response to pyriproxyfen concentrations.
Juvenile treatment manipulation
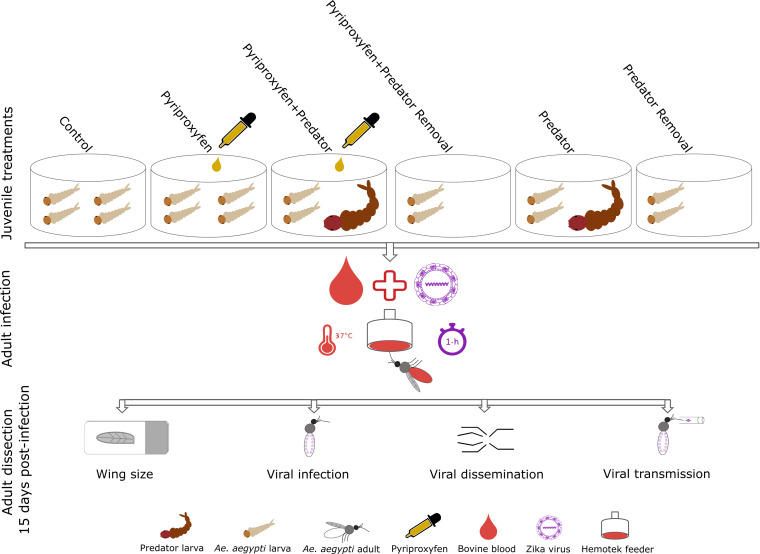
Three hundred newly hatched first-instar larvae of Ae. aegypti were placed into each experimental pan (experimental unit) containing 1.5 L of water and 0.2 g of larval food. Experimental pans were assigned to one of the following treatment groups: control, pyriproxyfen, pyriproxyfen+predator, pyriproxyfen+predator removal, predator, predator removal (Fig 2). Each of the six treatments was replicated five times for a total of 30 experimental units. After the addition of Ae. aegypti larvae to the experimental pans, a first-instar larva of Tx. rutilus was introduced to each of the replicates of pyriproxyfen+predator and predator treatments. Once Ae. aegypti developed to third-instar, a low concentration of pyriproxyfen (0.022 ppb) was applied to each of the replicates of pyriproxyfen+predator and pyriproxyfen treatments. For treatments that contain a predator (e.g., pyriproxyfen+predator and predator), total number of Ae. aegypti prey was counted daily using established methods [54,55]. On a daily basis, the number of consumed or dead Ae. aegypti prey in the pyriproxyfen+predator and predator treatments was averaged across all replicates as a measure of mortality rate and then removed from pyriproxyfen+predator removal and predator removal treatments, respectively as described elsewhere [54]. No treatment manipulations were made for the controls in which larvae were not exposed to pyriproxyfen or Tx. rutilus. Pupae of Ae. aegypti from experimental pans were collected daily and transferred to plastic cups and placed in cages, by treatment group and replicate, to capture newly emerged adults. Emergence of adults was measured for each experimental pan as the total number of adults eclosed divided by the number of larvae originally added to each pan, expressed as a percent. All experimental pans were held under controlled environmental conditions in bioroom maintained at a 14:10 light: dark photo regime, 28°C±1°C and 60–80% humidity.
Fig 2. Schematic diagram illustrating the experimental design.
Zika virus propagation
Mammalian host cells and ZIKV were cultured and maintained in growth media (HyClone, Medium 199, GE Healthcare, Logan, UT), supplemented with 10% fetal bovine serum, antibiotics (penicillin/streptomycin), and mycostatin. Confluent monolayers of African green monkey epithelial (Vero) cells (175 cm2) were inoculated with 500 μL of diluted ZIKV stock at multiplicity of infection of 0.1 and incubated at 37°C and 5% CO2 atmosphere for 1-h to initiate viral infection, after which 24-mL of media (M199) were added and incubated further until cytopathic effects observed after six days [56]. Freshly harvested media from ZIKV-infected cell cultures were combined with defibrinated bovine blood (Hemostat Laboratories, Dixon, CA) and adenosine triphosphate (0.005 M) before the start of feeding trials for the ZIKV infection study.
Zika virus infection
Seven-to ten-day-old adult females were transferred to paperboard cages (10 x 10 x 7 cm) and starved from sucrose solution but not water for 24-h prior to viral infection feeding trials. Mosquitoes were allowed to feed on ZIKV-infected blood meals using Hemotek membrane feeders (Discovery Workshops, Lancashire, UK) warmed to 37°C for 1-h. Following the feeding trials, mosquitoes were anesthetized with CO2 for sorting and fully engorged blood-fed females were returned to cages with continuous access to 10% sucrose solution via cotton pads and held at the same conditions as rearing the juvenile stages for a 15-day ZIKV incubation period. Aliquots of 1 mL of ZIKV-infected blood were taken after blood feeding trials and placed into 2 mL of cryogenic vials (MilliporeSigma, Burlington, MA) and stored at -80°C for determination of viral titers. The ZIKV titers in blood meals were 6.7±0.3 log10 plaque-forming unit equivalents per mL.
Zika virus transmission
Fifteen days post-infection, mosquitoes were anesthetized with CO2 for dissection. Legs with a single wing were removed from the rest of the body. Saliva was collected by forced salivation by inserting the proboscis of each mosquito into a microhematocrit capillary tube (Thermo Fisher Scientific, Waltham, MA) containing type B immersion oil (Cargille Laboratories, Cedar Grove, NJ). Mosquitoes were allowed to salivate for approximately 1-h, after which the saliva and oil were expelled under pressure into microcentrifuge tubes containing 300 μL of incomplete media (M199). Tests of mosquito saliva are a proxy for the ability of a mosquito to transmit virus by bite. Bodies and legs were separately placed into microcentrifuge tubes (Thermo Fisher Scientific, Waltham, MA) containing 1 mL of incomplete media (M199). All bodies, legs, and saliva samples from mosquitoes were frozen at -80°C until further processing.
Viral nucleic acid extraction and quantitative RT-PCR
Body and legs of mosquitoes were thawed then homogenized using a TissueLyser II automation system (Qiagen, Hilden, Germany) at 19.5 Hz for 3 min and centrifuged for 5 min at 13,200 rpm. Viral nucleic acids were extracted from mosquito body, legs, and saliva using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA) and eluted in 60μL of buffer according to the manufacturer's instructions. Zika virus RNA in mosquito samples was determined using the Superscript III One-Step Quantitative RT-PCR System with Platinum Taq kit (Invitrogen, Carlsbad, CA) with the C1000 Touch Thermal Cycler, CFX96 Real-Time System (Bio-Rad Laboratories, Hercules, CA). Zika virus primers and probes used in this experiment were synthesized by Integrated DNA Technologies (Coralville, IA) with the following sequences (forward primer: 5′-CTTCTTATCCACAGCCGTCTC-3′; reverse primer: 5′-CCAGGCTTCAACGTCGTTAT-3′; and probe: 5′-/56-FAM/AGAAGGAGACGAGATGCGGTACAGG/3BHQ_1/-3′). The program for quantitative RT-PCR consisted of 2 min at 94°C, 12 sec at 94°C, 30 min at 50°C, 1 min at 58°C linked to 39-cycles. Water and ZIKV RNA stock were used in each reaction run as a negative and positive control standard, respectively. To quantify viral titration of ZIKV in mosquito body, legs, and saliva, a standard curve was prepared to relate the amount of ZIKV RNA detected in mosquito samples to serial dilutions of ZIKV stock with plaque assays, expressed as plaque forming unit equivalents per mL (PFUE/mL) [57]. Infection rate was determined by the percent of females with ZIKV RNA-positive bodies from the total number that fed on the infectious blood meal. Disseminated infection and transmission rates were determined by the percent of females with infected bodies that have ZIKV RNA-positive legs and saliva, respectively [58].
Body size determination
Juvenile treatment effects on female size were determined by measuring wing length as a proxy for size of the body [59,60]. A single wing was dissected from each ZIKV-infected female and mounted on double sided tape on glass microscope slides (Cardinal Health, Dublin, OH). Wing length was measured in millimeters from axillary margin to the apical notch without considering wing fringe using computer imaging software (IMT i-Solution lit, Princeton, NJ).
Statistical analysis
Juvenile treatment manipulation effects on Ae. aegypti traits (adult emergence and female wing length) and ZIKV infection measurements (infection, disseminated infection, transmission, and viral titers) were analyzed using separate analysis of variance tests (ANOVAs) and Tukey’s multiple comparisons adjustment. Canonical-correlation analysis was performed to determine the overall relationship between Ae. aegypti traits and vector competence measurements for ZIKV [61]. A p< 0.05 was considered statistically significant. All statistical analyses were performed using SAS software [62].
Results
Adult emergence and body size
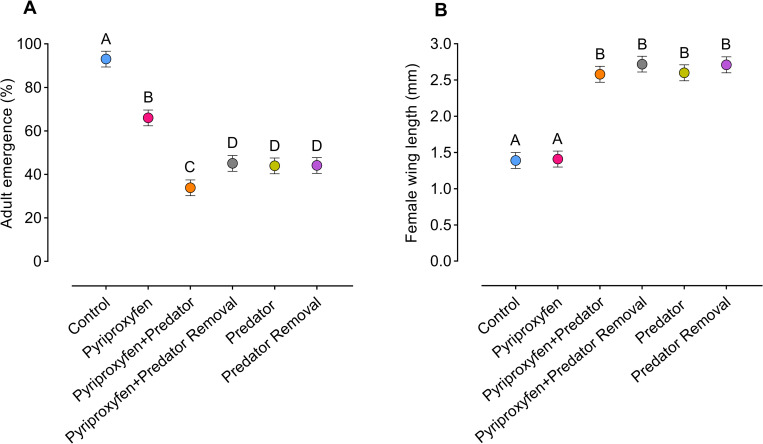
Analysis of variance showed significant juvenile treatment effects on adult emergence (F5, 24 = 557.4, p < .0001) (Fig 3A) and female wing length (an approximation of body size) (F5, 24 = 116.6, p < .0001) (Fig 3B). Control and pyriproxyfen treatments had significantly higher adult emergence (approximately 50% higher) compared to treatments involving reduced density or the presence of a predator (e.g., pyriproxyfen+predator, pyriproxyfen+predator removal, predator, predator removal treatments) (Fig 3A). Adult emergence was negatively related to wing length, indicating that treatments with high adult emergence (i.e., treatments contain high larval density) had shorter wings and treatments with low adult emergence (i.e., treatments contain low larval density) had longer wings, perhaps attributable to alterations in food and space. Wing lengths of adult females were significantly longer (approximately 45% longer) in treatments where larval density was reduced the most due to high mortality/removal (e.g., pyriproxyfen+predator, pyriproxyfen+predator removal, predator, predator removal treatments) and shorter in treatments with the highest adult emergence, including control and pyriproxyfen groups (Fig 3B).
Fig 3. Effects of juvenile treatments on Ae. aegypti traits.
(A) Adult emergence and (B) female wing length (an indicator of body size). Dots represent the means. Whiskers denote the standard error of the means. Statistical significance was determined by ANOVA. Different letters indicate significant differences (p<0.05) between juvenile treatment groups.
Zika virus infection, transmission, and viral titers in mosquitoes
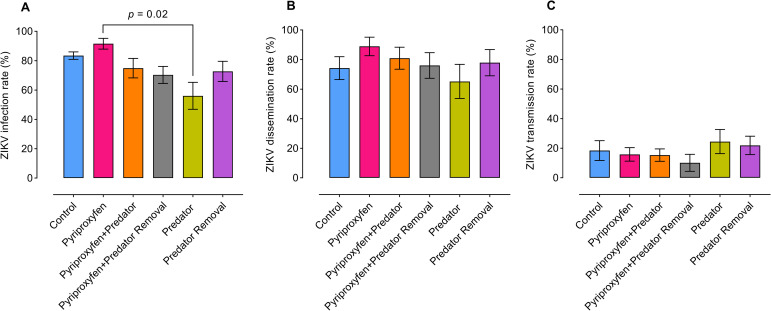
To evaluate the treatment-induced effects experienced by juvenile stages of Ae. aegypti on adult vector competence for ZIKV, a total of 353 adult females were tested for susceptibility to viral infection, disseminated infection, and saliva infection (transmission). Analysis of variance showed that treatment manipulations during juvenile stages have significant effects on susceptibility of adults to ZIKV infection, but only between pyriproxyfen and predator treatments (F5, 24 = 3.0, p = 0.02) (Fig 4A), whereas no significant effects observed between treatments in disseminated infection (F5, 24 = 0.6, p = 0.6) (Fig 4B), or transmission rates (F5, 24 = 0.7, p = 0.6) (Fig 4C). Adult mosquitoes exposed to Tx. rutilus predators during juvenile stages had lower susceptibility to ZIKV infection than other treatments. However, significant differences were observed only between predator and pyriproxyfen treatments (Fig 4A).
Fig 4. Effects of juvenile treatments on Ae. aegypti-ZIKV interactions.
(A) Zika virus infection, (B) disseminated infection, and (C) saliva infection (transmission) were determined following orally exposure to ZIKV infectious blood meals. Bars represent the means. Whiskers denote the standard error of the means. For all treatments, viral infection, disseminated infection, and transmission were estimated at 15 days post-ZIKV infection. Control (n = 80), pyriproxyfen (n = 50), pyriproxyfen+predator (n = 47), pyriproxyfen+predator removal (n = 64), predator (n = 49), and predator removal (n = 63). Statistical significance was determined by ANOVA.
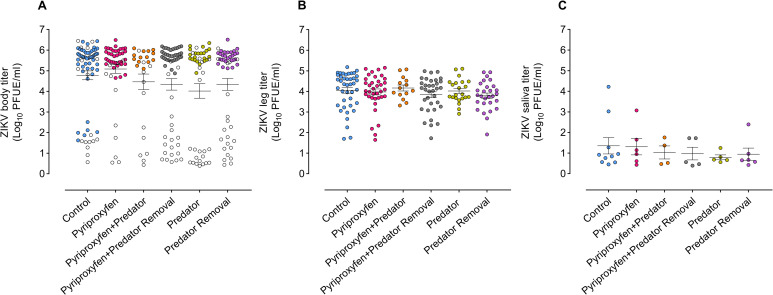
Analysis of variance showed no significant differences between treatments in viral titers in mosquitoes’ tissues, including bodies (F5, 24 = 0.5, p = 0.7) (Fig 5A), legs (F5, 24 = 0.3, p = 0.8) (Fig 5B), and saliva (F5, 24 = 0.3, p = 0.8) (Fig 5C). Canonical-correlation analysis showed no significant canonical relationship between Ae. aegypti traits (adult emergence, size) and vector competence measurements (infection, disseminated infection, transmission) (Pillai's trace 6, 25 = 0.3, p = 0.1).
Fig 5. Effects of juvenile treatments on titrations of ZIKV in Ae. aegypti tissues.
(A) Viral body, (B) leg, and (C) saliva titers (plaque forming unit equivalents/ml) of ZIKV-positive female Ae. aegypti. Horizontal lines indicate the mean of viral titers. Whiskers denote the standard error of the means. Each circle represents the titer for an individual female Ae. aegypti. Open circles in (A) represent the titers for females with non-disseminated infection of ZIKV (i.e., viral infection limited to mosquito midgut), whereas filled circles represent the titers for females with disseminated infection of ZIKV (i.e., viral dissemination from mosquito midgut epithelium). For all treatments, viral body, leg, and saliva titers were estimated at 15 days post-ZIKV infection.
Discussion
Control measures that target juvenile mosquito stages can have both direct (mortality) and indirect effects that influence phenotypic traits of adults surviving exposure to control measures which may have crucial implications for mosquito-pathogen interactions. In this study, we investigated whether Ae. aegypti exposure to pyriproxyfen and predatory mosquito Tx. rutilus during the juvenile stage influence adult traits and susceptibility to infection and transmission of ZIKV. Our study demonstrated that the combination of pyriproxyfen and predators reduced adult emergence of Ae. aegypti more than observed in pyriproxyfen or predator treatment alone. To account for density reduction attributable to predation, we mimicked the daily mortality rate by predation in removal treatments to ensure that the indirect effects on mosquito traits reflected the absence of predator stress, but included density reduction (i.e., lower competition) since the latter has been found to alter traits, including susceptibility to arbovirus infection in mosquitoes [42,63]. Individuals that emerged to adulthood in treatments associated with lower density, including pyriproxyfen+predator, predator, and removals, were larger than mosquitoes from other treatments where density was higher and predator was absent (e.g., control and pyriproxyfen). Also, the combined effect of pyriproxyfen and predator exposure may be associated with changes in other traits among survivors that relate to body size. Female mosquito body size, a density dependent variable, is an important factor that may influence critical aspects of mosquito biology such as adult lifespan, blood feeding, fecundity, and mating success [59,64–66]. Large females of Ae. aegypti have the potential for lengthened adult lifespan, ingestion of larger blood meals and associated enhanced fecundity [64,59]. Our findings emphasize the importance of considering the interaction between environmental factors during juvenile stages due to control measures in arboviral disease epidemiology.
Control of juvenile stages of mosquitoes has primarily been achieved by targeting the aquatic habitats with traditional larvicides that induce mortality in the larval stages [67,68]. Unlike traditional larvicides (e.g., temephos and Bacillus thuringiensis ssp. israelensis (Bti)), pyriproxyfen has a unique mode of action that inhibits mosquito emergence to the adult stage, but does not kill the larvae. This mechanism introduces a delay in mosquito mortality which allows Tx. rutilus to further reduce the numbers of Ae. aegypti larvae before pyriproxyfen-induced mortality at the pupal stage. In our study, Ae. aegypti emergence to adulthood had a sharper reduction in pyriproxyfen+predator treatment compared to other treatments that contained either pyriproxyfen or predator alone. This result suggests that pyriproxyfen may be an attractive compound to use in vector control programs that integrates biological control agents such as Tx. rutilus against container inhabiting mosquitoes. Our observation supports a previous study reporting higher reduction in population of Ae. aegypti following the application of malathion in combination with naturally occurring or released Tx. splendens in Florida’s urban environments [69]. In our study, we did not observe inhibition of adult emergence in Tx. rutilus predators due to the application of pyriproxyfen, suggesting that the exposure to a low concentration of pyriproxyfen has no lethal effect on Tx. rutilus. Previous studies using Toxorhynchites spp. in combination with organophosphates [69,70] or Bti [71] found that susceptibility of Toxorhynchites spp. to these toxins is lower compared to Ae. aegypti, in part attributable to the larger size of Toxorhynchites spp. Additionally, the lethal dose of Bti in Ae. aegypti appeared to have negligible effects on later instar larvae of Tx. rutilus [71]. These findings suggest that the use of multiple juvenile sources of mortality (e.g., insecticide and biological control agents) can reduce pathogen transmission by inhibiting recruitment of adult vectors and associated population size.
The size of adult mosquitoes in our experiment varied between treatments. Treatments containing a predator or numerical reductions such as removal treatments produced larger individuals compared to other treatments where the predators were absent. It is likely that larval consumption attributable to predators and low density in prey removal treatments may accelerate development and enhance growth of surviving prey as they encountered less crowded conditions (i.e., release from competition), particularly in species that show no altered behavioral responses (i.e., reduce feeding activity) in the presence of predators [51,72,73]. Our results agree with other studies that observed larger sized individuals after emergence in the presence of dipteran predators or in prey removal treatments [54,55,74]. Although the exposure to chemical compounds such as malathion [75], spinosad [76], and Bti [77] during juvenile stages was associated with enhanced size of adults that survived exposure in Ae. aegypti, in our study, however, the sizes of adult survivors in pyriproxyfen treatment were relatively similar to those in control treatments, in which there was no pyriproxyfen or Tx. rutilus. One plausible explanation for the observed discrepancy in observations between studies is that insecticides (e.g., malathion, Bti, spinosad) with the modes of action that target the larval stage of mosquitoes and result in rapid death, which may allow larvae that survived exposure to experience competitive release from resources which can allow for enhanced growth and larger adults. In contrast, IGR like pyriproxyfen does not induce deaths in larval stages which may maintain larval competition and therefore diminish their growth as a density-dependent effect.
Several studies have detected both negative and positive relationships between mosquito body size and susceptibility to arboviral infection and transmission potential [78]. An infection study using La Crosse encephalitis virus (LACV) and Ae. triseriatus found that small females emerged from nutrient-deprived larvae had higher rates of infection, dissemination, and transmission of LACV following oral exposure to infectious blood meals compared to larger adults from well-nourished larvae [79]. In the same study, authors observed that midgut morphological variations (i.e., basement membrane thickness) between small and large females were associated with changes in vector competence for LACV. Similarly, small adults of Ae. albopictus emerged from crowded larval conditions had higher susceptibility to infection and disseminated infection of arboviruses such as DENV-2 and Sindbis virus (SINV) relative to large individuals from uncrowded larval conditions [63,80]. In contrast, larger Ae. aegypti mosquitoes were more likely to become infected with DENV-2 than medium or small mosquitoes derived from larval conditions that manipulated crowding and food availability [81]. Larger-sized field-captured Ae. aegypti adults were more likely to be infected with DENV than smaller conspecifics [82]. Along the same lines, Ae. triseriatus adults derived interspecific larval competition with superior competitor Ae. albopictus were larger and were associated with higher infection and dissemination rates of LACV compared to smaller individuals of Ae. triseriatus from intraspecific treatments [83]. Collectively, these results suggest that larval conditions may play an important role in mosquito-arbovirus interactions and that size alone may not be causally connected to alterations in vector competence. In our study, we did not find an overall relationship between mosquito body size and ZIKV vector competence measurements, suggesting that mosquito size per se is not necessarily a factor influencing Ae. aegypti vector competence for ZIKV. Our findings are consistent with previous studies that found no association between mosquito size and infection and disseminated infection of SINV [75] and DENV-1 [84].
Juvenile environmental factors such as nutritional deprivation and intra- or interspecific competition are stressors that can induce changes in subsequent adult life- history traits [78], immune function [85,86], and associated susceptibility to infection with pathogens [63,78,80,87,88]. In the present study, exposure to a predator alone reduced susceptibility to ZIKV infection in subsequent adults relative to pyriproxyfen treatment, suggesting that predator stress may influence adult Ae. aegypti interactions with ZIKV, primarily acting at the initial site of infection. Although the presence of predator had no direct effect on ZIKV disseminated infection and transmission in Ae. aegypti, sublethal effects of predation stress may reduce fecundity and lifespan of adults, traits which influence pathogen transmission [53,54].
In our study, we hypothesized that larvae exposed to predation stress would show reduction in body size in comparison to their conspecific from removal treatments where predators were absent. We found that adults from larval treatments containing a predator (e.g., pyriproxyfen+predator and predator) had similar body size compared to conspecifics in removal treatments, suggesting that Ae. aegypti did not exhibit anti-predator behavior (i.e., reduction in feeding activity and longer development time which can produce smaller adults) [54,72,89]. In contrast, adults of malaria mosquito An. coluzzii showed smaller body sizes (shorter wings) at metamorphosis in the presence of a predatory backswimmer [53]. Similarly, a field study showed smaller sized Ae. triseriatus mosquitoes from tires with Tx. rutilus compared to containers without Tx. rutilus, perhaps attributable to reduced movement, food intake and size at metamorphosis [90]. Collectively, these results suggest that species-specific anti-predator behavior among mosquitoes likely influences net growth.
Different responses to viral pathogen infection following exposure to insecticides have been observed in mosquito vectors [78,91]. Interestingly, mosquitoes exposed to pyriproxyfen during juvenile stages, in our study, had enhanced susceptibility to ZIKV infection, but not disseminated infection or transmission, only when compared to the predator treatment. The reason for the lack of significant differences among other treatments is unclear, but it may possibly reflect differences in immune responses early during infection attributable to the juvenile environment. Previous studies showed that juvenile mosquito exposure to Bti did not significantly affect the adult vector competence for arboviruses, including DENV-1 and CHIKV [79,91]. Additionally, adult mosquito exposure to pyrethroid bifenthrin incorporated with sugar solution showed no alterations in ZIKV infection, disseminated infection, or viral titers after 14 days post-infection [92]. These outcomes suggest that insecticide-induced stress experienced during either juvenile or adult stages may have minimal influence in altering mosquito vector competence for arboviruses. On the contrary, enhancement in susceptibility of mosquitoes to infection with arboviruses, including DENV and SINV was previously observed following insecticide exposure [41,75,93]. Taken together these observations suggest that exposure to insecticides may cause enhancement or cause no effect on mosquito susceptibility to infection with pathogens. These contrasting consequences underline the need to continue assessing the sublethal effects of insecticides exposure to understand their subtle effects on mosquito-pathogen interactions rather than just focus on their lethal mortality. Future studies should consider the influence of sublethal exposure of insecticides and predators on mosquito immune responses to pathogens and additional phenotypic traits of adults that are likely to contribute to vectorial capacity, including host seeking and blood feeding behaviors, reproduction, and adult lifespan.
Our data demonstrate that combined effects of pyriproxyfen and predator Tx. rutilus have potential to reduce ZIKV transmission through high level of adult Ae. aegypti emergence inhibition which can lead to greater reduction in mosquito population size. However, surviving individuals may have altered traits such as greater net growth and size at emergence, potentially enhancing fitness. Our study underscores the importance of measuring the consequences of abiotic and biotic interactions occurs during juvenile stages due to mosquito control practices on mosquito-pathogen interactions as related to public health protection.
Acknowledgments
We thank the U.S. Centers for Disease Control and Prevention (CDC-Fort Collins, CO, USA) for providing an isolate of Zika virus (strain PRVABC59). We also thank the Lee County Mosquito Control District (LCMCD-Lehigh Acres, FL, USA) for providing eggs of Tx. rutilus and Robert L. Aldridge for assistance with the experiment.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Dick GW. Epidemiological notes on some viruses isolated in Uganda (yellow fever, Rift Valley fever, Bwamba fever, West Nile, Mengo, Semliki Forast, Bunyamwera, Ntaya, Uganda S and Zika viruses). Trans R Soc Trop Med Hyg. 1953;47(1):13–43. 10.1016/0035-9203(53)90021-2 . [DOI] [PubMed] [Google Scholar]
- 2.Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80. 10.1016/j.antiviral.2016.03.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48(2):139–145. 10.1016/0035-9203(54)90006-1 . [DOI] [PubMed] [Google Scholar]
- 4.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15(9):1347 10.3201/eid1509.090442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mead PS, Hills SL, Brooks JT. Zika virus as a sexually transmitted pathogen. Curr Opin Infect Dis. 2018;31(1):39–44. 10.1097/QCO.0000000000000414 . [DOI] [PubMed] [Google Scholar]
- 6.Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19(14):20761 10.2807/1560-7917.es2014.19.14.20761 . [DOI] [PubMed] [Google Scholar]
- 7.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Possible association between Zika virus infection and microcephaly-Brazil, 2015. Morb Mortal Wkly Rep. 2016;65(3):59–62. 10.15585/mmwr.mm6503e2 . [DOI] [PubMed] [Google Scholar]
- 8.Blohm GM, Lednicky JA, Márquez M, White SK, Loeb JC, Pacheco CA, et al. Evidence for mother-to-child transmission of Zika virus through breast milk. Clin Infect Dis. 2018;66(7):1120–1121. 10.1093/cid/cix968 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foy BD, Kobylinski KC, Foy JL, Blitvich BJ, da Rosa AT, Haddow AD, et al. Probable non–vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880 10.3201/eid1705.101939 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuevas EL, Tong VT, Rozo N, Valencia D, Pacheco O, Gilboa SM, et al. Preliminary report of microcephaly potentially associated with Zika virus infection during pregnancy-Colombia, January-November 2016. Morb Mortal Wkly Rep. 2016;65(49):1409–1413. 10.15585/mmwr.mm6549e1 . [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects-reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987. 10.1056/NEJMsr1604338 . [DOI] [PubMed] [Google Scholar]
- 12.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly. N Engl J Med. 2016;375(1):1–4. 10.1056/NEJMp1605367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. J Am Mosq Control Assoc. 2017;317(1):59–68. 10.1001/jama.2016.19006 . [DOI] [PubMed] [Google Scholar]
- 14.LaBeaud AD. Why arboviruses can be neglected tropical diseases. PLoS Negl Trop Dis. 2008;2(6):e246 10.1371/journal.pntd.0000246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabachnick WJ. Evolutionary genetics and arthropod-borne disease: the yellow fever mosquito. Am Entomol. 1991;37(1):14–26. [Google Scholar]
- 16.Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti-a review. Mem Inst Oswaldo Cruz. 2013;108:11–7. 10.1590/0074-0276130395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5(3):e68 10.1371/journal.pmed.0050068 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42(5):844–849. 10.1093/jmedent/42.5.844 . [DOI] [PubMed] [Google Scholar]
- 19.Crawford JE, Alves JM, Palmer WJ, Day JP, Sylla M, Ramasamy R, et al. Population genomics reveals that an anthropophilic population of Aedes aegypti mosquitoes in West Africa recently gave rise to American and Asian populations of this major disease vector. BMC Biol. 2017;15(1):16 10.1186/s12915-017-0351-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott TW, Clark GG, Lorenz LH, Amerasinghe PH, Reiter P, Edman JD. Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. J Med Entomol. 1993;30(1):94–99. 10.1093/jmedent/30.1.94 . [DOI] [PubMed] [Google Scholar]
- 21.Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, Lorenz LH, et al. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J Med Entomol. 1993;30(5):922–927. 10.1093/jmedent/30.5.922 . [DOI] [PubMed] [Google Scholar]
- 22.Colton YM, Chadee DD, Severson DW. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med Vet Entomol. 2003;17(2):195–204. 10.1046/j.1365-2915.2003.00424.x . [DOI] [PubMed] [Google Scholar]
- 23.Suman DS, Wang Y, Bilgrami AL, Gaugler R. Ovicidal activity of three insect growth regulators against Aedes and Culex mosquitoes. Acta Trop. 2013;128(1):103–9. 10.1016/j.actatropica.2013.06.025 . [DOI] [PubMed] [Google Scholar]
- 24.Mulla MS. The future of insect growth regulators in vector control. J Am Mosq Control Assoc. 1995;11:269–273. . [PubMed] [Google Scholar]
- 25.Floore TG. Mosquito larval control practices: past and present. J Am Mosq Control Assoc. 2006;22(3):527–533. 10.2987/8756-971X(2006)22[527:MLCPPA]2.0.CO;2 . [DOI] [PubMed] [Google Scholar]
- 26.Staal GB. Insect growth regulators with juvenile hormone activity. Annu Rev Entomol. 1975;20(1):417–460. 10.1146/annurev.en.20.010175.002221 . [DOI] [PubMed] [Google Scholar]
- 27.Goindin D, Delannay C, Gelasse A, Ramdini C, Gaude T, Faucon F, et al. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect Dis Poverty. 2017;6(1):38 10.1186/s40249-017-0254-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jindra M, Bittova L. The juvenile hormone receptor as a target of juvenoid “insect growth regulators”. Arch Insect Biochem Physiol. 2020;103(3):e21615 10.1002/arch.21615 . [DOI] [PubMed] [Google Scholar]
- 29.Vasuki V, Rajavel AR. Influence of short time exposure to an insect growth regulator, hexaflumuron, on mortality and adult emergence of vector mosquitoes. Mem. Inst Oswaldo Cruz. 1992;275–283. 10.1590/s0074-02761992000200016 . [DOI] [PubMed] [Google Scholar]
- 30.Sihuincha M, Zamora-Perea E, Orellana-Rios W, Stancil JD, Lopez-Sifuentes V, Vidal-Ore C, et al. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J Med Entomol. 2005;42(4):620–630. 10.1093/jmedent/42.4.620 . [DOI] [PubMed] [Google Scholar]
- 31.Darriet F, Corbel V. Laboratory evaluation of pyriproxyfen and spinosad, alone and in combination, against Aedes aegypti larvae. J Med Entomol. 2006;43(6):1190–1194. 10.1603/0022-2585(2006)43[1190:leopas]2.0.co;2 . [DOI] [PubMed] [Google Scholar]
- 32.Hirano M. Pyriproxyfen and other juvenile hormone analogues. Rev Toxicol. 1998;2:357–394. [Google Scholar]
- 33.World Health Organization. Review of insect growth regulator pyriproxyfen GR. In: report of the 4th WHOPES working group meeting; 2000 Dec. 50–67 p.
- 34.Paul A, Harrington LC, Scott JG. Evaluation of novel insecticides for control of dengue vector Aedes aegypti (Diptera: Culicidae). J Med Entomol. 2006;43(1):55–60. 10.1603/0022-2585(2006)043[0055:EONIFC]2.0.CO;2 . [DOI] [PubMed] [Google Scholar]
- 35.Devine G. Auto-dissemination of pyriproxyfen for the control of container-inhabiting mosquitoes-a progress review. Outlooks Pest Manag. 2016;27(4):164–167. [Google Scholar]
- 36.Gaugler R, Suman D, Wang Y. An autodissemination station for the transfer of an insect growth regulator to mosquito oviposition sites. Med Vet Entomol. 2012;26(1):37–45. 10.1111/j.1365-2915.2011.00970.x . [DOI] [PubMed] [Google Scholar]
- 37.Maoz D, Ward T, Samuel M, Mueller P, Runge-Ranzinger S, Toledo J, et al. Community effectiveness of pyriproxyfen as a dengue vector control method: a systematic review. PLoS Negl Trop Dis. 2017;11(7):e0005651 10.1371/journal.pntd.0005651 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohashi K, Nakada K, Ishiwatari T, Miyaguchi JI, Shono Y, Lucas JR, et al. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). J Med Entomol. 2014;49(5):1052–1058. 10.1603/me12006 . [DOI] [PubMed] [Google Scholar]
- 39.Yadav K, Dhiman S, Acharya BN, Ghorpade RR, Sukumaran D. Pyriproxyfen treated surface exposure exhibits reproductive disruption in dengue vector Aedes aegypti. PLoS Negl Trop Dis. 2019;13(11):e0007842 10.1371/journal.pntd.0007842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiaz M, Martínez LC, Plata-Rueda A, Gonçalves WG, de Souza DL, Cossolin JF, et al. Pyriproxyfen, a juvenile hormone analog, damages midgut cells and interferes with behaviors of Aedes aegypti larvae. PeerJ. 2019;7:e7489 10.7717/peerj.7489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muturi EJ, Kim CH, Alto BW, Berenbaum MR, Schuler MA. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health. 2011;16(8):955–964. 10.1111/j.1365-3156.2011.02796.x . [DOI] [PubMed] [Google Scholar]
- 42.Muturi EJ, Costanzo K, Kesavaraju B, Alto BW. Can pesticides and larval competition alter susceptibility of Aedes mosquitoes (Diptera: Culicidae) to arbovirus infection? J Med Entomol. 2011;48(2):429–436. 10.1603/me10213 . [DOI] [PubMed] [Google Scholar]
- 43.Juliano SA. Population dynamics. J Am Mosq Control Assoc. 2007; 23(2):265 10.2987/8756-971X(2007)23[265:PD]2.0.CO;2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YJ, Higgs S, Vanlandingham DL. Biological control strategies for mosquito vectors of arboviruses. Insects. 2017;8(1):21 10.3390/insects8010021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins LE, Blackwell A. The biology of Toxorhynchites mosquitoes and their potential as biocontrol agents. Biocontrol News and Information. 2000;21(4):105N–16N. [Google Scholar]
- 46.Schiller A, Allen M, Coffey J, Fike A, Carballo F. Updated methods for the production of Toxorhynchites rutilus septentrionalis (Diptera, Culicidae) for use as biocontrol agent against container breeding pest mosquitoes in Harris County, Texas. J Insect Sci. 2019;19(2):8 10.1093/jisesa/iez011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werner EE. Nonlethal effects of a predator on competitive interactions between two anuran larvae. Ecology. 1991;72(5):1709–1720. [Google Scholar]
- 48.Relyea RA. Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology. 2000;81(8):2278–2289. [Google Scholar]
- 49.Stoks R, Block MD, Slos S, Doorslaer WV, Rolff J. Time constraints mediate predator-induced plasticity in immune function, condition, and life history. Ecology. 2006;87(4):809–815. 10.1890/0012-9658(2006)87[809:tcmppi]2.0.co;2 . [DOI] [PubMed] [Google Scholar]
- 50.Creel S, Christianson D, Liley S, Winnie JA. Predation risk affects reproductive physiology and demography of elk. Science. 2007;315(5814):960 10.1126/science.1135918 . [DOI] [PubMed] [Google Scholar]
- 51.Relyea RA, Rosenberger D. Predator effects on metamorphosis: the effects of scaring versus thinning at high prey densities. Copeia. 2018;106(3):457–467. [Google Scholar]
- 52.Ong’wen F, Onyango PO, Bukhari T. Direct and indirect effects of predation and parasitism on the Anopheles gambiae mosquito. Parasit Vectors. 2020;13:43 10.1186/s13071-020-3915-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roux O, Vantaux A, Roche B, Yameogo KB, Dabiré KR, Diabaté A, et al. Evidence for carry-over effects of predator exposure on pathogen transmission potential. Proc Biol Sci. 2015;282(1821):20152430 10.1098/rspb.2015.2430 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellamy SK, Alto BW. Mosquito responses to trait-and density-mediated interactions of predation. Oecologia. 2018;187(1):233–243. 10.1007/s00442-018-4107-5 . [DOI] [PubMed] [Google Scholar]
- 55.Alto BW, Malicoate J, Elliott SM, Taylor J. Demographic consequences of predators on prey: trait and density mediated effects on mosquito larvae in containers. PLoS One. 2012;7(11):e45785 10.1371/journal.pone.0045785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimler RA, Alto BW. Florida Aedes aegypti (Diptera: Culicidae) and Aedes albopictus Vector Competency for Zika Virus. J Med Entomol. 2019;56(2):341–346. 10.1093/jme/tjy231 . [DOI] [PubMed] [Google Scholar]
- 57.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25(2):169–193. 10.1677/jme.0.0250169 . [DOI] [PubMed] [Google Scholar]
- 58.Turell MJ, Gargan TP II, Bailey CL. Replication and dissemination of Rift Valley fever virus in Culex pipiens. Am J Trop Med Hyg. 1984;33(1):176–181. 10.4269/ajtmh.1984.33.176 . [DOI] [PubMed] [Google Scholar]
- 59.Nasci RS. The size of emerging and host-seeking Aedes aegypti and the relation of size to blood-feeding success in the field. J Am Mosq Control Assoc. 1986;2(1):61–62. . [PubMed] [Google Scholar]
- 60.Van Handel EM, Day JF. Correlation between wing length and protein content of mosquitoes. J Am Mosq Control Assoc. 1989;5:180–182. . [PubMed] [Google Scholar]
- 61.Sherry A, Henson RK. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess. 2005;84(1):37–48. 10.1207/s15327752jpa8401_09 . [DOI] [PubMed] [Google Scholar]
- 62.SAS Institute Inc. SAS Stat® User’s Guide. SAS Institute Inc; Cary, NC, USA: 2011. [Google Scholar]
- 63.Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;86(12):3279–3288. 10.1890/05-0209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinwascher K. Egg size variation in Aedes aegypti: relationship to body size and other variables. Am Midl Nat. 1984;76–84. [Google Scholar]
- 65.Tsunoda T, Fukuchi A, Nanbara S, Takagi M. Effect of body size and sugar meals on oviposition of the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae). J Vector Ecol. 2010;35(1):56–60. 10.1111/j.1948-7134.2010.00028.x . [DOI] [PubMed] [Google Scholar]
- 66.Dieng H, Abang F, Ahmad AH, Abd Ghani I, Satho T, Miake F, et al. Physical characteristics and reproductive performance in Aedes (Diptera: Culicidae). J Entomol Acarol Res. 2016;48(3):323–331. [Google Scholar]
- 67.Biber PA, Dueñas JR, Almeida FL, Gardenal CN, Almirón WR. Laboratory evaluation of susceptibility of natural subpopulations of Aedes aegypti larvae to temephos. J Am Mosq Control Assoc. 2006;22(3):408–411. 10.2987/8756-971X(2006)22[408:LEOSON]2.0.CO;2 . [DOI] [PubMed] [Google Scholar]
- 68.Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc. 2007;23:133–163. 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2 . [DOI] [PubMed] [Google Scholar]
- 69.Tietze NS, Schreiber ET, Hester PG, Hallmon CF, Olson MA, Shaffer KR. Susceptibility of first instar Toxorhynchites splendens to malathion, naled and resmethrin. J Am Mosq Control Assoc. 1993;9:97–99. . [PubMed] [Google Scholar]
- 70.Rawlins SC, Ragoonansingh R. Comparative organophosphorus insecticide susceptibility in Caribbean populations of Aedes aegypti and Toxorhynchites moctezuma. J Am Mosq Control Assoc. 1990;6(2):315–317. . [PubMed] [Google Scholar]
- 71.Lacey LA, Dame DA. The effect of Bacillus thuringiensis var. israelensis on Toxorhynchites rutilus (Diptera: Culicidae) in the presence and absence of prey. J Med Entomol. 1982;19(5):593–596. 10.1093/jmedent/19.5.593 . [DOI] [PubMed] [Google Scholar]
- 72.Grill CP, Juliano SA. Predicting species interactions based on behaviour: predation and competition in container-dwelling mosquitoes. J Anim Ecol. 1996;65(1): 63–76. [Google Scholar]
- 73.LaFiandra EM, Babbitt KJ. Predator induced phenotypic plasticity in the pinewoods tree frog, Hyla femoralis: necessary cues and the cost of development. Oecologia. 2004;138(3):350–359. 10.1007/s00442-003-1412-3 . [DOI] [PubMed] [Google Scholar]
- 74.Costanzo KS, Muturi EJ, Alto BW. Trait-mediated effects of predation across life-history stages in container mosquitoes. Ecol Entomol. 36:605–615. [Google Scholar]
- 75.Muturi EJ, Alto BW. Larval environmental temperature and insecticide exposure alter Aedes aegypti competence for arboviruses. Vector Borne Zoonotic Dis. 2011; 11(8):1157–1163. 10.1089/vbz.2010.0209 . [DOI] [PubMed] [Google Scholar]
- 76.Antonio GE, Sanchez D, Williams T, Marina CF. Paradoxical effects of sublethal exposure to the naturally derived insecticide spinosad in the dengue vector mosquito, Aedes aegypti. Pest Manag Sci. 2009;65(3):323–326. 10.1002/ps.1683 . [DOI] [PubMed] [Google Scholar]
- 77.Alto BW, Lord CC. Transstadial effects of Bti on traits of Aedes aegypti and infection with dengue virus. PLoS Negl Trop Dis. 2016;10(2): e0004370 10.1371/journal.pntd.0004370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alto BW, Lounibos LP. Vector competence for arboviruses in relation to the larval environment of mosquitoes. In: Takken W, Koenraadt S, editors. Ecology of parasite-vector interactions. Springer; 2013. p. 81–101 [Google Scholar]
- 79.Grimstad PR, Walker ED. Aedes triseriatus (Diptera: Culicidae) and LaCrosse virus. IV. nutritional deprivation of larvae affects the adult barriers to infection and transmission. J Med Entomol. 1991;28:378–386. 10.1093/jmedent/28.3.378 . [DOI] [PubMed] [Google Scholar]
- 80.Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc R Soc B. 2008;275(1633):463–471. 10.1098/rspb.2007.1497 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sumanochitrapon W, Strickman D, Sithiprasasna R, Kittayapong P, Innis BL. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. Am J Trop Med Hyg. 1998;58(3):283–286. 10.4269/ajtmh.1998.58.283 . [DOI] [PubMed] [Google Scholar]
- 82.Juliano SA, Ribeiro GS, Maciel-de-Freitas R, Castro MG, Codeço C, Lourenço-de-Oliveira R, et al. She’s a femme fatale: low-density larval development produces good disease vectors. Mem Inst Oswaldo Cruz. 2014;109(8):1070–1077. 10.1590/0074-02760140455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biol Invasions. 2008;10(7):1109–1117. [Google Scholar]
- 84.Zirbel KE, Alto BW. Maternal and paternal nutrition in a mosquito influences offspring life histories but not infection with an arbovirus. Ecosphere. 2018;9(10):e02469. [Google Scholar]
- 85.Suwanchaichinda C, Paskewitz SM. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J Med Entomol. 1998;35(2):157–161. 10.1093/jmedent/35.2.157 . [DOI] [PubMed] [Google Scholar]
- 86.Telang A, Qayum AA, Parker A, Sacchetta BR, Byrnes GR. Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti). Med Vet Entomol. 2012;26(3):271–281. 10.1111/j.1365-2915.2011.00993.x . [DOI] [PubMed] [Google Scholar]
- 87.Breaux JA, Schumacher MK, Juliano SA. What does not kill them makes them stronger: larval environment and infectious dose alter mosquito potential to transmit filarial worms. Proc R Soc B Biol Sci. 2014;281(1786):20140459 10.1098/rspb.2014.0459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vantaux A, Lefèvre T, Cohuet A, Dabiré KR, Roche B, Roux O. Larval nutritional stress affects vector life history traits and human malaria transmission. Sci Rep. 2016;6:36778 10.1038/srep36778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuharah WF, Fadzly N, Yusof NA, Dieng H. Risky behaviors: effects of Toxorhynchites splendens (Diptera: Culicidae) predator on the behavior of three mosquito species. J Insect Sci. 2015;15(1). 10.1093/jisesa/iev115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito: influences of food type and predation. Oikos. 1993:114–118. [Google Scholar]
- 91.Richards SL, White AV, Balanay JA. Potential for sublethal insecticide exposure to impact vector competence of Aedes albopictus (Diptera: Culicidae) for dengue and Zika viruses. Res Rep Trop Med. 2017;8:53 10.2147/RRTM.S133411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knecht H, Richards SL, Balanay JA, White AV. Impact of mosquito age and insecticide exposure on susceptibility of Aedes albopictus (Diptera: Culicidae) to infection with Zika virus. Pathogens. 2018;7(3):67 10.3390/pathogens7030067 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moltini-Conclois I, Stalinski R, Tetreau G, Després L, Lambrechts L. Larval exposure to the bacterial insecticide Bti enhances dengue virus susceptibility of adult Aedes aegypti mosquitoes. Insects. 2018;9(4):193 10.3390/insects9040193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.