Supplemental Digital Content is available in the text.
Keywords: hypertriglyceridemia, mechanical ventilation, monitoring, pancreatitis, propofol, sedation
Abstract
Objectives:
The objective of this study was to describe the incidence of propofol-induced hypertriglyceridemia and the risk factors associated with hypertriglyceridemia in mechanically ventilated ICU patients while receiving propofol.
Design:
This was a single-center case-control study.
Setting:
Brigham and Women’s Hospital, a tertiary academic medical center in Boston, MA.
Subjects:
Adult ICU patients who received continuous infusion propofol for at least 24 hours from May 1, 2019, to December 31, 2019, were included. Patients were excluded if they were diagnosed with acute pancreatitis upon admission or did not have any serum triglyceride levels evaluated during propofol administration.
Interventions:
None.
Measurements and Main Results:
The major outcome was the incidence and risk factors associated with the development of propofol-induced hypertriglyceridemia, defined as triglyceride level greater than or equal to 400 mg/dL. Minor outcomes included the prevalence of acute pancreatitis. A hybrid multivariate logistic regression analysis was used to evaluate the relation between individual risk factors and the dependent variable of hypertriglyceridemia. During the study period, 552 patients were evaluated for inclusion, of which 136 were included in the final analysis. A total of 38 patients (27.9%) developed hypertriglyceridemia with a median time to hypertriglyceridemia of 47 hours. The only significant independent risk factor for development of hypertriglyceridemia identified was the cumulative propofol dose (odds ratio, 1.04; 95% CI, 1.01–1.08; p = 0.016). Two of the 38 hypertriglyceridemia patients (5.3%) were diagnosed with acute pancreatitis.
Conclusions:
In our analysis, approximately one third of patients developed hypertriglyceridemia with cumulative propofol dose identified as a significant predictor of the development of hypertriglyceridemia. Despite a high incidence of hypertriglyceridemia, a significant number of patients continued propofol therapy, and a relatively low prevalence of pancreatitis was observed. Future analyses are warranted to further investigate these results.
Propofol is a rapid acting IV sedative-hypnotic agent used for sedation in the mechanically ventilated patient in the ICU. Although the mechanism of action is not fully clear, it is thought that propofol exerts its effects through positive modulation of the inhibitory function of the neurotransmitter gamma-aminobutyric acid (GABA) through GABAA receptors (1). Other reports also suggest that propofol decreases glutamate release through N-methyl-D-aspartate inhibition (2). The 2018 Society of Critical Care Medicine Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption (PADIS) recommend propofol as a first-line agent for sedation in mechanically ventilated patients in the ICU (3).
Propofol is highly lipophilic and formulated in a 10% oil-in-water lipid emulsion. The lipid component is based in soybean oil and contains triglycerides, phospholipids, glycerol, vitamins, and minerals. The primary lipid is linoleic acid, an omega-6 long-chain polyunsaturated fatty acid. Due to its formulation, propofol has been associated with an increased risk of developing hypertriglyceridemia (2). Although propofol-induced pancreatitis may occur independent of elevated triglyceride levels, hypertriglyceridemia is a risk factor for pancreatitis (4–6). Pancreatitis is associated with a significant increase in morbidity and mortality (5, 7, 8). Significantly elevated triglyceride levels (> 1,000 mg/dL) have been shown to increase the risk of developing acute pancreatitis (4–6).
The exact rate of occurrence of propofol-induced hypertriglyceridemia and pancreatitis is unknown; however, several studies have evaluated both endpoints along with risk factors that may increase risk (4–6, 9–13). Alternative agents, such as benzodiazepines or dexmedetomidine, may be required if patients develop significant hypertriglyceridemia or pancreatitis (3). Switching to an alternative agent may compromise optimal sedation and related outcomes in critically ill patients (3, 14).
The purpose of this study was to describe the incidence of propofol-induced hypertriglyceridemia and the risk factors associated for hypertriglyceridemia in mechanically ventilated ICU patients while receiving propofol.
METHODS
This was a single-center case-control study conducted at Brigham and Women’s Hospital, a tertiary academic medical center in Boston, MA. Partners institutional review board approval was received prior to the start of the study. The electronic health record was used to identify adult patients admitted to the ICU who received continuous infusion propofol for at least 24 hours from May 1, 2019, to December 31, 2019. Patients were excluded if they were diagnosed with acute pancreatitis upon admission, received propofol only for procedural sedation, received propofol for less than 24 hours, or did not have any serum triglyceride levels evaluated during propofol administration.
Propofol is considered a first-line sedative at our institution in mechanically ventilated patients who require continuous sedation. The maximum dose allowed in the ICU is 83 µg/kg/min, and it is recommended to monitor triglyceride levels every 48–72 hours for patients requiring prolonged infusions. The standard goal for a patient’s depth of sedation is a score of 0 to –1 on the Richmond Agitation Sedation Scale. Daily spontaneous awakening trials of all sedatives are recommended unless contraindications are present.
Pertinent baseline characteristics, past medical history, and Acute Physiology and Chronic Health Evaluation (APACHE) II score upon ICU admission were collected. The following data surrounding propofol were all collected: total daily propofol dose, cumulative propofol dose, average daily propofol dose, and total duration. The median rate of propofol administration was calculated for the entire cohort, as well as for those who developed and did not develop hypertriglyceridemia based on the total dose and duration of administration. All triglyceride and lipase levels documented during propofol administration were also collected. Additionally, ICU length of stay (LOS) and concomitant medications known to increase (atypical antipsychotics, immune suppressants, glucocorticoids, lipids, and clevidipine) or decrease (IV insulin, niacin, fibrates, and statins) triglyceride levels were collected (15, 16).
The major outcome of this analysis was to identify the incidence and risk factors associated with the development of propofol-induced hypertriglyceridemia, defined as triglyceride level greater than or equal to 400 mg/dL (10). Minor outcomes included the mean time to the development of hypertriglyceridemia, prevalence of acute pancreatitis, and mean time to development of pancreatitis. The frequency of severe hypertriglyceridemia, defined as a triglyceride level greater than or equal to 500 mg/dL according to American Heart Association/American College of Cardiology guidelines, along with the frequency of triglyceride levels greater than or equal to 1,000 mg/dL, was collected (11). Acute pancreatitis was defined as the presence of two of three following criteria: 1) abdominal pain consistent with the disease, 2) serum amylase and/or lipase greater than three times the upper limit of normal, and/or 3) characteristic findings from abdominal imaging (17).
In all patients who developed hypertriglyceridemia, median triglyceride level, peak triglyceride level, and propofol infusion rate at the time of hypertriglyceridemia were documented. In patients in which propofol was discontinued, chart documentation was reviewed to assess if discontinuation was due to hypertriglyceridemia. A full chart review was done to confirm the diagnosis of pancreatitis according to the definition above. All patients who developed pancreatitis were further evaluated for mean lipase level.
A hybrid multivariate logistic regression analysis was used to evaluate the linear relation between individual risk factors to the dependent variable of hypertriglyceridemia. Individual variables between groups, including past medical history, concomitant medications, gender, body mass index (BMI), parenteral or enteral nutrition, and propofol exposure were analyzed by a univariate analysis. All variables with a p value of less than 0.2 were then analyzed through a multivariate regression analysis. Previously identified factors for propofol-induced hypertriglyceridemia (ICU LOS, duration of propofol, and APACHE II score) were also included in the multivariate analysis (9, 10). Multicollinear variables were excluded from the multivariate analysis. Variables with a p value of less than 0.05 were identified as a positive risk factor for the development of hypertriglyceridemia while receiving propofol. Continuous data used the Student t test, whereas categorical data used the chi-square test. Data are presented as medians and interquartile ranges as well as percentages. All data was analyzed using Stata/SE statistical software Version 15.1 (StataCorp LLC).
RESULTS
During the study period, 552 patients were evaluated for inclusion, of which 416 (75.4%) were excluded from the analysis for the following reasons: 415 did not have a recorded serum triglyceride level, and one patient had acute pancreatitis upon admission. A total of 136 patients were included in the final analysis. Baseline characteristics are described in Table 1. On average, patients received 13.1 grams of propofol over a total of 72 hours. The median rate of propofol administration for all patients was 37.6 µg/kg/min. Daily triglyceride levels and propofol doses are displayed in Figure 1. The median daily triglyceride level was 188.8 mg/dL (129.3–294.6 mg/dL). The average peak triglyceride level among all patients was 217 mg/dL.
TABLE 1.
Baseline Characteristics
| Variables | All Patients (n = 136) | Developed Hypertriglyceridemia (n = 38) | Did Not Develop Hypertriglyceridemia (n = 98) | pc |
|---|---|---|---|---|
| Age (yr)a | 62 (54–72) | 57 (47.5–65.8) | 65 (57.3–73) | 0.0007 |
| Male, n (%) | 87 (63.9) | 22 (57.9) | 63 (64.3) | 0.759 |
| Weight (kg)a | 80.3 (70.2–97) | 77.8 (70.1–97.8) | 82 (70.3–96) | 0.7562 |
| Height (cm)a | 172 (163.8–180) | 168 (163.3–172.9) | 174 (165–180) | 0.0506 |
| Body mass index (kg/m2)a | 28.4 (24–2) | 28.5 (25–31.9) | 28 (23.9–31.8) | 0.3037 |
| Ethnicity, n (%) | ||||
| White | 85 (62) | 19 (50) | 66 (67.3) | 0.429 |
| African American | 13 (9.5) | 4 (10.5) | 9 (9.2) | 0.759 |
| Asian | 6 (4.4) | 1 (2.6) | 5 (5.1) | 1.00 |
| Hispanic | 10 (7.3) | 4 (10.5) | 6 (6.1) | 0.474 |
| Not reported | 19 (13.9) | 7 (18.4) | 12 (12.2) | 0.428 |
| Other | 3 (2.2) | 3 (7.9) | 0 | 0.024 |
| Comorbidities, n (%) | ||||
| Cardiovascular diseaseb | 96 (70.1) | 22 (57.9) | 74 (75.5) | 0.043 |
| Diabetes | 44 (32.1) | 10 (26.3) | 34 (13.3) | 0.349 |
| Chronic kidney disease, end-stage renal disease, or hemodialysis | 16 (11.7) | 3 (7.9) | 13 (13.3) | 0.556 |
| Acute kidney injury | 27 (19.7) | 11 (28.9) | 16 (16.3) | 0.048 |
| Hypothyroidism | 17 (12.4) | 4 (10.5) | 13 (13.3) | 0.779 |
| Alcohol Abuse | 20 (14.6) | 6 (15.8) | 14 (14.3) | 0.824 |
| Lupus | 2 (1.5) | 0 | 2 (2) | 0.629 |
| Bone marrow transplant | 3 (2.2) | 3 (7.9) | 0 | 0.021 |
| None | 17 (12.4) | 5 (13.2) | 12 (12.2) | 0.885 |
| Not assessed | 3 (2.2) | 1 (2.6) | 2 (2) | 0.833 |
| Acute Physiology and Chronic Health Evaluation II scorea | 18 (13–23.3) | 22 (15–25) | 18 (12–23) | 0.030 |
| Medical ICU, n (%) | 106 (77.9) | 31 (81.6) | 75 (76.5) | 0.299 |
| Surgical ICU, n (%) | 30 (22.1) | 7 (18.4) | 23 (23.5) | 0.258 |
| Neurosurgical | 9 (30) | 3 (42.9) | 6 (26.1) | 0.404 |
| Cardiothoracic | 8 (26.7) | 2 (28.6) | 6 (26.1) | 0.898 |
| General | 13 (43.3) | 2 (28.6) | 11 (47.8) | 0.378 |
| Baseline triglyceride levela | 84 (70–104.8) | 87 (74–104.8) | 83.5 (68.8–104.8) | 0.497 |
aData presented as median (interquartile range).
bCardiovascular disease: hypertension, coronary artery disease, heart failure, acute coronary syndrome, stroke, arrhythmia.
cComparison between patients who developed hypertriglyceridemia and those who did not.
Figure 1.
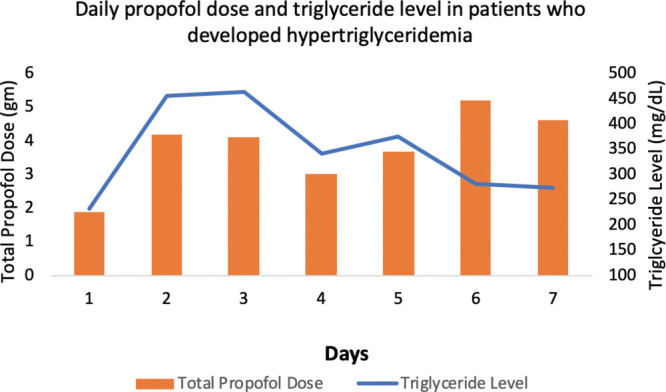
Daily propofol dose and triglyceride level in patients who developed hypertriglyceridemia.
Characteristics of patients who developed hypertriglyceridemia are compared with those who did not develop hypertriglyceridemia in Table 1. A total of 38 patients (27.9%) developed hypertriglyceridemia with a median triglyceride level of 499 mg/dL at the time. The median time to development of hypertriglyceridemia was 47 hours, with a median propofol rate of 50 µg/kg/min at the time of development of hypertriglyceridemia (Table 2). Propofol was discontinued due to hypertriglyceridemia in 24 patients (63.1%). Of the patients who developed hypertriglyceridemia, 26 (68.4%) had triglyceride greater than 500 mg/dL, and 10 patients (26.3%) had a triglyceride level greater than 1,000 mg/dL. Of the 10 patients with triglyceride levels greater than 1,000 mg/dL, the median rate of propofol was 71.5 µg/kg/min, and eight patients subsequently had propofol discontinued. Two of the 38 patients (5.3%) who developed hypertriglyceridemia were diagnosed with acute pancreatitis, including one patient with a peak triglyceride level greater than 1,000 mg/dL. Propofol was not continued in either of these patients once a triglyceride level of greater than or equal to 400 mg/dL was detected. The severity of acute pancreatitis was unknown for one patient, whereas the other patient had documented necrotizing pancreatitis. The mean lipase level in these patients was 246 U/L, and the mean time to develop pancreatitis from the start of the propofol infusion was 209 hours.
TABLE 2.
Outcomes
| Variables | Developed Hypertriglyceridemia (n = 38) | Did Not Develop Hypertriglyceridemia (n = 98) | p |
|---|---|---|---|
| Days in ICU (hr)a | 14 (8.3–25) | 8.5 (5–14) | 0.0015 |
| Duration of propofol administration (hr)a | 112.5 (72–192) | 64.5 (34–132.8) | 0.0008 |
| Total daily propofol dose (g)a | 3.5 (2.6–5.2) | 2.8 (1.5–4.2) | 0.0048 |
| Total cumulative propofol dose (g)a | 21.8 (9.3–32.4) | 12 (4.4–19.3) | 0.016 |
| Propofol discontinued due to hypertriglyceridemia, n (%) | 24 (63.2) | — | — |
| Propofol rate at the time of development hypertriglyceridemia (µg/kg/min)a | 50 (30.3–63.8) | — | — |
| Time to develop hypertriglyceridemia (hr)a | 47 (16.3–73.5) | — | — |
| Average rate during first 48 hr (µg/kg/min), n (%) | 37.1 | 28.4 | 0.264 |
| Development of pancreatitis, n (%) | 2 (5.3) | 0 | 0.083 |
| Median daily triglyceride level (mg/dL)a | 359.5 (297–549) | 145.5 (106-199) | 0.0001 |
| Patients with triglyceride level > 1,000 mg/dL, n (%) | 10 (26.3) | — | — |
| Peak triglyceride level (mg/dL)a | 605 (486–999.5) | 168.5 (108.8-239) | 0.001 |
| Triglyceride level at development of hypertriglyceridemia (mg/dL)a | 499 (438–679.8) | — | — |
| Average lipase level (U/L)a | 33 (22.5–110) | 31.5 (17.8-52.9) | 0.114 |
aData presented as median (interquartile range).
Dashes indicate no p value to compute and data point did not apply to group that did not develop hypertriglyceridemia.
Patients who developed hypertriglyceridemia were younger and had a higher severity of illness (Table 1). Patients with hypertriglyceridemia also received a higher cumulative dose (21.8 vs 12 g; p = 0.016) and duration (112.5 vs 64.5 hr, p < 0.001) of propofol. The median rate of propofol administration was 41.7 and 37.8 µg/kg/min (p = 0.655) in patients who developed or did not develop hypertriglyceridemia, respectively. There were no significant differences found between concomitant medications. Concomitant medications administered to patients can be found in the Supplementary Appendix (Supplementary Digital Content 1, http://links.lww.com/CCX/A424). After controlling for confounding variables using a multivariate regression analysis, only total propofol dose was found as an independent risk factor for the development of hypertriglyceridemia (odds ratio, 1.04; 95% CI, 1.01–1.08; p = 0.016). The results of the multivariate analysis can be found in Table 3.
TABLE 3.
Multivariate Analysis
| Variable | p | OR (95% CI) |
|---|---|---|
| Total propofol dose (g) | 0.016 | 1.04 (1.01–1.08) |
| Acute Physiology and Chronic Health Evaluation II | 0.170 | 1.05 (0.98–1.11) |
| Propofol duration (hr) | 0.624 | 1.00 (0.99–1.01) |
| Tube feed | 0.690 | 0.82 (0.31–2.16) |
| Age | 0.087 | 2.37 (0.88–6.36) |
| Acute kidney injury | 0.111 | 2.28 (0.83–6.31) |
| Cardiovascular diseasea | 0.231 | 0.57 (0.22–1.43) |
OR = odds ratio.
aCardiovascular disease: hypertension, coronary artery disease, heart failure, acute coronary syndrome, stroke, arrhythmia.
DISCUSSION
The purpose of this study was to describe the incidence of propofol-induced hypertriglyceridemia and the risk factors associated with hypertriglyceridemia while receiving propofol. We observed that 28% of patients developed triglyceride levels greater than or equal to 400 mg/dL, with a median time to development of 47 hours. Patients who developed hypertriglyceridemia had higher APACHE II scores, were more likely to have received a hematopoietic stem cell transplant, and received propofol at higher doses and for longer durations. Patients with hypertriglyceridemia also had longer ICU LOS. Pancreatitis was seen in two patients with elevated triglyceride levels. Our study is a contemporary, real-world evaluation of propofol-related hypertriglyceridemia which demonstrates higher rates of hypertriglyceridemia, but lower rates of acute pancreatitis, than previously described (9, 10).
The likelihood of critically ill patients developing hypertriglyceridemia while receiving propofol is still unknown. Many studies evaluating hypertriglyceridemia and pancreatitis secondary to propofol vary in definitions of both endpoints and are limited to case reports (5, 10, 15, 18–22). Devaud et al (9) observed that 45% of patients developed hypertriglyceridemia; however, the cutoff used for hypertriglyceridemia was greater than 2 mmol/L (~ 180 mg/dL). A study similar to ours by Devlin et al (10) found that 29 of 159 patients (18%) developed hypertriglyceridemia with a median infusion rate of 50 µg/kg/min at the time of hypertriglyceridemia development. Over 80% of patients had propofol discontinued secondary to elevated triglycerides. They found that the median time to develop hypertriglyceridemia was 54 hours with 10% of patients developing pancreatitis. Although we observed a similar time to elevated triglyceride levels, our patients experienced a higher incidence of hypertriglyceridemia. This may have been caused by several factors, including that patients in our study had a higher severity of illness and received higher doses of propofol prior to hypertriglyceridemia development. Additionally, despite a lower rate of propofol discontinuation due to hypertriglyceridemia, we observed a lower prevalence of pancreatitis. These data may support continued propofol therapy in the setting of elevated triglyceride levels, particularly in patients where transitioning to alternative sedative agents may be difficult (3, 14). In our study, 37% of patients continued propofol therapy despite triglyceride level greater than or equal to 400 mg/dL. Dose lowering strategies, such as spontaneous awakening trials and analgosedation, should be implemented to minimize triglyceride accumulation.
Many risk factors have been described previously as increasing the risk propofol-induced hypertriglyceridemia, including propofol dose, duration of administration, age, weight, BMI, severity of illness, and concomitant medications (9, 10, 15). We observed that patients who developed hypertriglyceridemia had a longer duration of propofol administration, had a higher cumulative dose, were younger, had a longer ICU LOS, and had a greater severity of illness. However, when accounting for confounding variables using a multivariate regression analysis, only the total propofol dose was identified as a significant independent risk factor.
The 2018 PADIS guidelines do not address monitoring of triglyceride or lipase levels in patients on a continuous infusion of propofol (3). Previous consensus guidelines recommend serum triglyceride monitoring after 2 consecutive days of propofol, similar to our institution’s current monitoring recommendations (23). Patients with high exposure to propofol should have consistent triglyceride monitoring. Early detection of elevated triglyceride levels may allow for earlier dose minimization strategies and monitoring for pancreatitis. Patients who continue to receive propofol despite elevated triglyceride levels should likely have daily triglyceride and lipase levels assessed. It also may be clinically warranted to collect a baseline triglyceride level in patients anticipated to be on propofol for a longer duration of mechanical ventilation.
There are several potential limitations to our study. This was a single-center retrospective observational analysis with a relatively small sample size. Because of the retrospective design, results were contingent on accurate and complete documentation in the electronic medical record. Inconsistent triglyceride monitoring created the possibility of missed events of hypertriglyceridemia. Only 42 patients (30.9%) had a baseline triglyceride level, making it difficult to capture the full effect of propofol on triglyceride levels. Last, given that our institution has specific guidelines for the administration and titration of continuous infusion propofol, the results of this analysis may not be reflective of clinical practice at other institutions.
CONCLUSIONS
We observed that approximately one third of patients who received propofol for sedation in the ICU developed hypertriglyceridemia. The cumulative propofol dose remained a significant predictor of the development of hypertriglyceridemia after logistic regression. Patients with prolonged exposure and moderate rates of continuous propofol infusions in the ICU should have early and frequent triglyceride monitoring. Despite a high incidence of hypertriglyceridemia, a significant number of patients continued propofol therapy, and a relatively low prevalence of pancreatitis was observed. Future analyses should be done to evaluate these findings.
ACKNOWLEDGMENT
We thank Leo F. Buckley III, PharmD, BCCP.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Trapani G, Altomare C, Liso G, et al. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000; 7:249–271 [DOI] [PubMed] [Google Scholar]
- 2.Kotani Y, Shimazawa M, Yoshimura S, et al. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008; 14:95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 4.Asghar MU, Cheema HA, Tanveer K, et al. Propofol infusion and acute pancreatitis: A review. Am J Ther. 2020; 27:e371–e374 [DOI] [PubMed] [Google Scholar]
- 5.Haffar S, Kaur RJ, Garg SK, et al. Acute pancreatitis associated with intravenous administration of propofol: Evaluation of causality in a systematic review of the literature. Gastroenterol Rep (Oxf). 2019; 7:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018; 6:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004; 291:2865–2868 [DOI] [PubMed] [Google Scholar]
- 8.McArthur KE. Review article: Drug-induced pancreatitis. Aliment Pharmacol Ther. 1996; 10:23–38 [DOI] [PubMed] [Google Scholar]
- 9.Devaud JC, Berger MM, Pannatier A, et al. Hypertriglyceridemia: A potential side effect of propofol sedation in critical illness. Intensive Care Med. 2012; 38:1990–1998 [DOI] [PubMed] [Google Scholar]
- 10.Devlin JW, Lau AK, Tanios MA. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: An analysis of frequency and risk factors. Pharmacotherapy. 2005; 25:1348–1352 [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen A, Savji N, Blumenthal R, et al. Hypertriglyceridemia Management According to the 2018 AHA/ACC Guideline - American College of Cardiology. Washington, DC: American College of Cardiology, 2019 [Google Scholar]
- 12.Gottardis M, Khünl-Brady KS, Koller W, et al. Effect of prolonged sedation with propofol on serum triglyceride and cholesterol concentrations. Br J Anaesth. 1989; 62:393–396 [DOI] [PubMed] [Google Scholar]
- 13.Bhukal I, Thimmarayan G, Bala I, et al. Comparison of serum triglyceride levels with propofol in long chain triglyceride and propofol in medium and long chain triglyceride after short term anesthesia in pediatric patients. Saudi J Anaesth. 2014; 8Suppl 1S53–S56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012; 307:1151–1160 [DOI] [PubMed] [Google Scholar]
- 15.Dube KM, Szumita PM, Rocchio MA, et al. The effect of concomitant sirolimus and propofol therapy on triglyceride concentrations in critically ill patients. Am J Ther. 2019; 26:e103–e109 [DOI] [PubMed] [Google Scholar]
- 16.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: Its etiology, effects and treatment. CMAJ. 2007; 176:1113–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenner S, Baillie J, DeWitt J, et al. ; American College of Gastroenterology. American college of gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013; 108:1400–15; 1416 [DOI] [PubMed] [Google Scholar]
- 18.Mateu-de Antonio J, Barrachina F. Propofol infusion and nutritional support. Am J Health-Syst Pharm. 1997; 54:2515–2516 [DOI] [PubMed] [Google Scholar]
- 19.Barrachina F, Mateu-de Antonio J. Propofol and hypertriglyceridemia: No problem? Crit Care Med. 1999; 27:224–225 [DOI] [PubMed] [Google Scholar]
- 20.Kumar AN, Schwartz DE, Lim KG. Propofol-induced pancreatitis: Recurrence of pancreatitis after rechallenge. Chest. 1999; 115:1198–1199 [DOI] [PubMed] [Google Scholar]
- 21.Metkus AP, Trabulsy PP, Schlobohm RS, et al. A firefighter with pancreatitis. Lancet. 1996; 348:1702. [DOI] [PubMed] [Google Scholar]
- 22.McLeod G, Dick J, Wallis C, et al. Propofol 2% in critically ill patients: Effect on lipids. Crit Care Med. 1997; 25:1976–1981 [DOI] [PubMed] [Google Scholar]
- 23.Jacobi J, Fraser GL, Coursin DB, et al. ; Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists (ASHP), American College of Chest Physicians. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002; 30:119–141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


