Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinico-neuroradiological entity that is manifested by characteristic magnetic resonance imaging (MRI) depictions of subcortical/cortical hyperintensities in the parieto-occipital lobes. Paroxysmal hypertension, headache, and palpitation are the most common clinical manifestations of pheochromocytoma, which are catecholamine-secreting enterochromaffin tumors. PRES is a rare complication of pheochromocytoma. Herein, we describe a 44-year-old woman who presented with postoperative confusion and headache. MRI images showed multiple asymmetrical hyperintensities with surrounding edema and contrast enhancement, predominantly in the right parietal lobe, left cerebellar hemisphere, and dentate nuclei, in favor of hemorrhagic metastases. The results of further investigations, including abdominopelvic computed tomography and the 24-hour urine test for metanephrine and normetanephrine, were in favor of a pheochromocytoma. The patient was scheduled for adrenalectomy and histopathologic examination of the tissue, which confirmed the diagnosis. Surprisingly, her symptoms and neuroimaging abnormalities improved significantly without any treatment during the follow-up period. Based on these findings, the diagnosis of PRES was considered, and the patient was followed. She was symptom-free at 3 years’ follow-up. The literature contains only four case reports of PRES as a complication of pheochromocytoma; however, all these cases had bilateral symmetrical hemispheric involvement and occurred during childhood and adolescence
Keywords: Pheochromocytoma , Posterior leukoencephalopathy syndrome , Adult , Headache , Neuroimaging
What’s Known
Pheochromocytomas are rare neuroendocrine tumors, and only four cases of pheochromocytoma and posterior reversible encephalopathy syndrome (PRES) have been reported in the literature.
PRES may be manifested with atypical features in neuroimaging findings.
What’s New
This is the first report of pheochromocytoma and PRES where all the atypical neuroimaging features present simultaneously in one case and result in a diagnostic challenge.
Investigation for pheochromocytomas in the diagnostic workup of PRES is necessary, especially in cases with an undetermined etiology but without a history of hypertension.
Introduction
Pheochromocytomas are rare neuroendocrine tumors that mostly arise from the chromaffin tissue in the adrenal medulla. 1 Posterior reversible encephalopathy syndrome (PRES) is characterized clinically by headache, confusion, seizure, and visual disturbance with imaging findings of bilateral posterior cortical and subcortical edema. PRES can occur in relationships with different conditions. However, regardless of the underlying etiology, the main abnormality is cerebral vasogenic edema. 2 PRES in association with pheochromocytoma is extremely rare, and only a few cases of pheochromocytoma with central nervous system (CNS) complications have been reported. 3 - 6 Herein, we present a case of PRES with atypical features in a patient suffering from pheochromocytoma, for whom the initial diagnosis was brain metastases.
Case Report
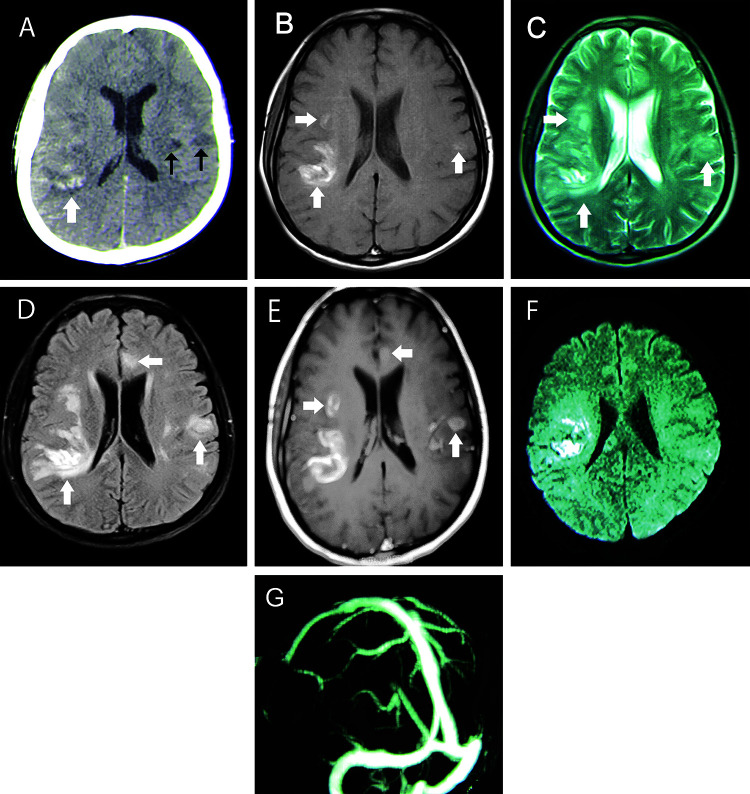
In June 2012, a 44-year-old woman was referred to the Emergency Department of Shariati Hospital, affiliated to Tehran University of Medical Sciences, Tehran, Iran. The patient complained of postoperative headache and confusion. Three days prior to the present admission, she underwent a transabdominal hysterectomy and left salpingo-oophorectomy (TAH-LSO), due to abnormal uterine bleeding in another center, and postoperatively developed decreased levels of consciousness with a Glasgow coma scale (GCS) of 12 out of 15 and severe headache. Owing to her confusional state, spiral brain computed tomography (CT) was performed, which showed multiple hemorrhagic lesions with surrounding edema (figure 1A). Therefore, she was referred to our tertiary center for further workup. In our center, on the first day of admission, she achieved normal levels of consciousness, and her headache was completely resolved. She only complained of paresthesia and numbness on the left side of her face and body. Her previous medical and drug history was unremarkable. On admission, she was afebrile and her blood pressure was 110/70 mm Hg and remained within the normal range during the hospitalization period. She was completely oriented, and her GCS was 15. Neurological examination revealed decreased pinprick and touch sensations on the left side of her body; it was otherwise unremarkable. Brain magnetic resonance imaging (MRI) showed multiple hyperintense lesions in T1- and T2-weighted sequences in the supra- and infratentorial regions with surrounding edema. The lesions were depicted as hyperintensities in T1-weighted sequences, which suggested the presence of hemorrhage (figure 1B). T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI showed hyperintense signals in the frontal and parietal lobes with asymmetrical patterns of involvement (figure 1C & D). Regarding the hyperintensities of lesions in both T1- and T2-weighted sequences, the hemorrhagic lesions were in the late subacute phase. The hemorrhagic lesions were dominantly located in the subcortical white matter. A ring enhancement pattern was noted after contrast injection (figure 1E). Diffusion-weighted imaging demonstrated diffusion restriction in some of the lesions (figure 1F). Additionally, brain magnetic resonance venography was requested for her, which was normal (figure 1G). Blood tests, including the erythrocyte sedimentation rate, C-reactive protein, the thyroid function test, the liver function test, blood urea nitrogen, and creatinine, were normal. Vasculitis markers, the extractable nuclear antigen profile, blood culture, anti-toxoplasma antibodies, and viral markers were negative. On abdominopelvic CT, one large heterogeneous mixed solid cystic mass (60×40 mm) in the left adrenal gland was detected (figure 2). Following this finding, the 24-hour urine levels of metanephrine and normetanephrine were monitored. The urine levels of metanephrine and normetanephrine were found to be higher than 2000 and 3000 µg/d, respectively, which was significantly higher than the normal values. 131-I metaiodobenzylguanidine (MIBG) scintigraphy revealed a marked increase in MIBG uptake in the left adrenal gland. Electrocardiography and transesophageal echocardiography were normal. The results of other laboratory tests were insignificant. Ultimately, the patient underwent radical left adrenalectomy following medical treatment, first with alpha-blockers and then beta-blockers. Histologic section of the adrenal gland demonstrated the zellballen pattern (figure 3) with no capsular and vascular invasion, consistent with the diagnosis of a benign pheochromocytoma. Another brain MRI was done two months later, which showed a significant resolution of the lesions (figure 4). In addition, the 24-hour urine test for metanephrine and normetanephrine was normal at two months’ follow-up. We retrospectively reviewed the patient’s images and established diagnosis of PRES with atypical features. The patient showed no new symptoms during three years of clinical follow-up. Written informed consent was obtained from the patient for the publication of her information in this case report on the condition of anonymity.
Figure 1.
(A) Non-enhanced spiral brain computed tomography (CT) scan shows hemorrhagic lesions with surrounding edema in the right parietal lobe (white arrow) and two hypodensities in the left parietal lobe (black arrows). (B) T1-weighted and (C) T2-weighted magnetic resonance (MR) images show multiple hyperintense lesions with surrounding edema in the right and left parietal lobes predominantly on the right side (white arrows). (D) Fluid-attenuated inversion recovery (FLAIR) MR images show multiple hyperintensities in the frontoparietal lobes, especially on the right side, and a mild mass effect on the right lateral ventricle (white arrows). (E) Contrast-enhanced T1-weighted MR image shows multiple ring-enhancing lesions in the frontoparietal lobes, especially on the right side (white arrows). (F) Diffusion-restriction of the right parietal lobe lesions is seen in the diffusion-weighted MR image. (G) Brain MR venography is normal.
Figure 2.
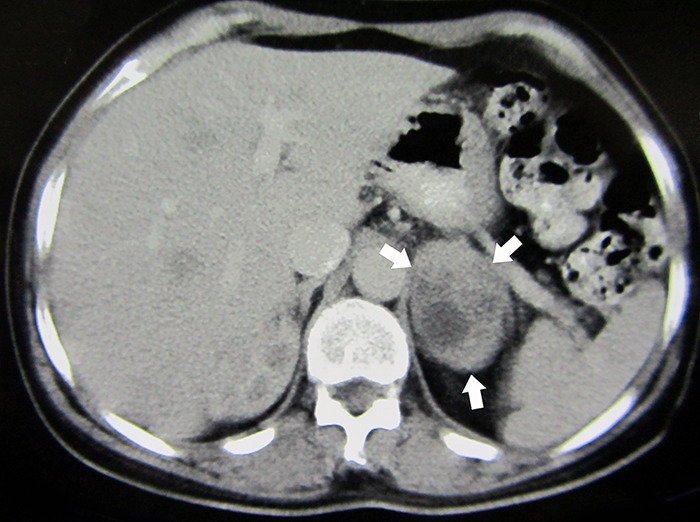
Abdominopelvic computed tomography (CT) shows a large heterogeneous solid cystic mass (60×40 mm) in the left adrenal gland (white arrows).
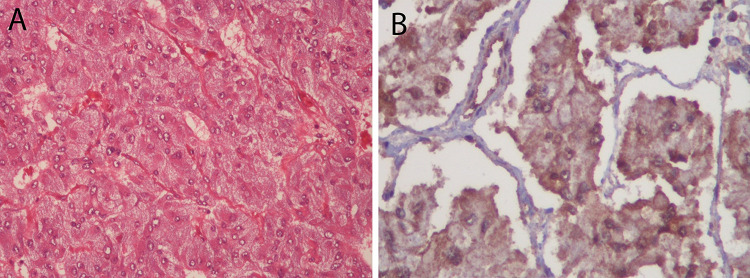
Figure 3.
(A) Histological examination shows epithelioid tumor cells with eosinophilic cytoplasm arranged in a nesting pattern (H&E stain ×200). (B) Positive cytoplasmic reaction of the tumor cells for SDHB (succinate dehydrogenase) is seen in the immunohistochemistry stain.
Figure 4.
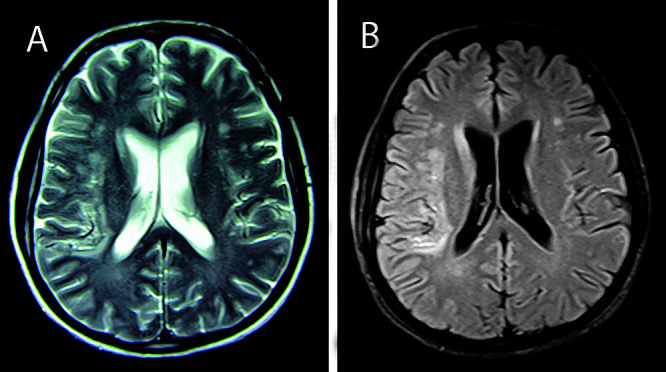
(A) T2-weighted and (B) FLAIR MR images acquired at two months post-treatment show a significant resolution of the hyperintensities in the right and left frontoparietal lobes.
Discussion
In the present case, given the brain CT and MRI findings, our differential diagnosis initially included multiple hemorrhagic metastases; CNS vasculitis; cerebral venous thrombosis, and less likely embolic hemorrhagic infarct; infectious encephalitis including viral, fungal, bacterial, and parasitic infections (e.g. toxoplasmosis); and PRES. Furthermore, on account of the amount and site of the patient’s lesions in the brain MRI, normal brain magnetic resonance venography, negative vasculitis markers, normal electrocardiography and transesophageal echocardiography, and improvements in the level of consciousness without any treatment, we ruled out the possibility of cerebral venous thrombosis, CNS vasculitis, embolic stroke, and infectious encephalitis. Our other differential diagnosis was multiple hemorrhagic brain metastases, and sure enough, we found a pheochromocytoma in our workup. Our initial suspicion was a malignant pheochromocytoma with metastasis to the brain, but the histological examination of the left adrenal gland and significant resolutions of the brain lesions in follow-up MRI guided us toward the diagnosis of a benign pheochromocytoma with PRES.
Pheochromocytomas are catecholamine-releasing tumors that are derived from the chromaffin cells. The annual incidence of pheochromocytomas is one to four per million persons. 1 Solitary tumors occur more frequently on the right side; 5 however, in our patient, the tumor originated from the left adrenal gland. While pheochromocytomas are usually benign tumors, in about 10% of cases, they exhibit malignant behaviors and can involve extra-adrenal sites. 7 There are various potential triggers of pheochromocytoma crisis that can lead to hemodynamic instability, including physical activity, stress, blood loss, some drugs, surgery, and anesthesia. 1
PRES is a clinico-radiological entity characterized by headache, confusion, seizure, visual disturbance, and radiological findings of edema in the cortical and subcortical areas of the brain, which are mainly perfused by the posterior circulation. 2 The etiologies of PRES are variable, but the most common are hypertension, uremic state, rheumatologic diseases such as systemic lupus erythematosus, and immunosuppressive drugs. 4 The pathophysiology of PRES remains controversial. There are two possible mechanisms regarding the pathogenesis of PRES. One entails the breakdown of cerebral autoregulation, which leads to increased permeability in the blood-brain barrier and results in vasogenic edema. This theory is called “cerebral hyperperfusion”. The autoregulatory system is mediated through sympathetic innervations, which are less extensive in the vertebrobasilar circulation than in the anterior circulation. Therefore, vasogenic edema is expected to be witnessed dominantly in the parieto-occipital cortex and the subcortical white matter. 2 , 8 The other hypothesis is termed “the toxic/immunogenic theory”, which focuses on endothelial dysfunction as a result of exogenous (e.g. chemotherapy or immunosuppressive drugs) or endogenous (e.g. sepsis or (pre) eclampsia) toxins. The result of both theories is blood-brain barrier dysfunction with cerebral vasogenic edema. 2 , 9
Four cases of pheochromocytomas presenting with PRES have been reported so far in the literature. 3 - 6 All the previously reported cases showed relatively symmetrical hyperintensities on neuroimaging. PRES was manifested in childhood and adolescence in three of the earlier presented cases, 4 - 6 and in adulthood in the other one. 3 Only the patient reported by Serter and others demonstrated PRES with atypical features, including hemorrhagic transformation in the bilateral basal ganglia. 6 In contrast to all the previously reported cases, 3 - 6 the pattern of involvement in our patient was asymmetrical and unilateral in some areas, which is extremely rare according to the report by Saad and others. 10 In our patient, we detected deep white matter hematoma, which, according to McKinney and others, is a rare presentation of PRES. 11 Saad and others and Kastrup and colleagues described subcortical contrast enhancement and diffusion restriction as unusual presentations of PRES and reported their occurrence in a minority of their patients. 10 , 12 We detected all of the atypical manifestations, including deep parenchymal hematoma, subcortical contrast enhancement, and diffusion restriction, in our patient.
Our presented case implies that all of the unusual manifestations of PRES may occur simultaneously in one case, and thus, render the diagnosis challenging. Accordingly, a thorough knowledge of these diverse clinical and radiological presentations of PRES is required for an accurate diagnosis. Given that encephalopathic conditions and imaging abnormalities in PRES are reversible if suitable treatment is initiated promptly, particular attention to early and precise diagnosis is warranted. Although PRES is usually a reversible condition, it can result in irreversible damage with chronic neurological deficit and even death, if timely treatment is not initiated. 2 Further, pheochromocytoma requires urgent management with fluid resuscitation and antihypertensive therapy, including alpha- and beta-blocker drugs, followed by the surgical resection of the tumor. 1
Conclusion
Thorough knowledge of the different clinical and radiological presentations of PRES is mandated. PRES may arise as the first presentation of pheochromocytomas; these tumors should, thus, be considered and investigated in the diagnostic workup of adult patients presenting with PRES, even though the past medical history or presenting features is not suggestive of pheochromocytoma.
Acknowledgement
We express our thanks to Dr. H. Saffar at the Pathology Department of Tehran University of Medical Sciences for pathology reports.
Conflict of Interest: None declared.
References
- 1.Pourian M, Mostafazadeh DB, Soltani A. Does this patient have pheochromocytoma? A systematic review of clinical signs and symptoms. J Diabetes Metab Disord. 2015;15:11. doi: 10.1186/s40200-016-0230-1. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–25. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 3.Jung YJ, Kwon Y, Heo SH, Hwang KJ, Chang D-I. Progressive Posterior Encephalopathy Syndrome Related with Drug-refractory Hypertension in a Patient with Pheochromocytoma. Journal of Neurocritical Care. 2014;7:141–4. doi: 10.18700/jnc.2014.7.2.141. [DOI] [Google Scholar]
- 4.Moorthy S, Subramaniam T, Prabhu N, Sree KK, Nair R. Posterior reversible encephalopathy syndrome in a child with pheochromocytoma. Indian Journal of Radiology and Imaging. 2002;12:321. [Google Scholar]
- 5.Sanjay KM, Partha PC. The posterior reversible encephalopathy syndrome. Indian J Pediatr. 2008;75:953–5. doi: 10.1007/s12098-008-0168-5. [DOI] [PubMed] [Google Scholar]
- 6.Serter A, Alkan A, Aralasmak A, Kocakoc E. Severe posterior reversible encephalopathy in pheochromocytoma: importance of susceptibility-weighted MRI. Korean J Radiol. 2013;14:849–53. doi: 10.3348/kjr.2013.14.5.849. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–36. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 8.Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017;264:1608–16. doi: 10.1007/s00415-016-8377-8. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179–90. [PMC free article] [PubMed] [Google Scholar]
- 10.Saad AF, Chaudhari R, Wintermark M. Imaging of Atypical and Complicated Posterior Reversible Encephalopathy Syndrome. Front Neurol. 2019;10:964. doi: 10.3389/fneur.2019.00964. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:896–903. doi: 10.3174/ajnr.A2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastrup O, Schlamann M, Moenninghoff C, Forsting M, Goericke S. Posterior Reversible Encephalopathy Syndrome: The Spectrum of MR Imaging Patterns. Clin Neuroradiol. 2015;25:161–71. doi: 10.1007/s00062-014-0293-7. [DOI] [PubMed] [Google Scholar]