1. Introduction
1.1. Purpose and Scope:
The purpose and scope of this document is to update the 2009 Society for Vascular Nursing’s (SVN) Endovascular Abdominal Aortic Aneurysm (AAA) Repair Practice Guideline [1] to reflect current evidence-based changes.
1.2. Assessment of Scientific Evidence
A comprehensive literature search of studies published in the English language from 1991–2019 was conducted. Earlier studies were included if clinically relevant. Search terms were: Abdominal Aortic Aneurysm, Endovascular Abdominal Aortic Aneurysm Repair (EVAR), Percutaneous Endovascular Aneurysm Repair (pEVAR), Fenestrated EVAR (FEVAR), Aortic Aneurysm Surgery, Stent Graft, Endograft and combinations thereof. The databases searched: CINAHL, The Cochrane Library, Elsevier Science Direct, Ovid, MEDLINE, PubMed, BMJ Clinical Evidence, National Guidelines Clearinghouse, MD Consult, and Nursing Consult. Textbooks and review articles were also included. Recommendations for nursing practice were based upon the data grading system proposed by Melnyk and Finout-Overholt. [2]
The SVN clinical practice guideline uses the following classification:
Class I: Randomized control trial without significant limitations, systematic reviews or meta-analysis
Class II: Randomized control trial with important limitations (e.g., methodological flaws or inconsistent results), observational studies (e.g., cohort or case-control)
Class III: Qualitative studies, case study, or series
Class IV: Evidence from reports of expert committees and/or expert opinion of the guideline panel, standards of care and clinical protocols, animal studies
1.3. History of Endovascular AAA Repair
In the 1960s and American radiologist, Dr. Charles Dotter invented balloon angioplasty. [3] In 1978, Dr. Palmaz (a vascular radiologist) used this technique to develop the first balloon mounted arterial stent. [4] In 1990, Dr. Juan Parodi (a vascular surgeon) along with Dr. Palmaz performed elective repair of five patients with AAA using a custom-made Dacron tube endoprosthesis fixed with a balloon-expandable stent inserted transfemorally. [5] Since 1991, endovascular repair for AAA has become widely accepted and available due in part to advances in stent-graft design to accommodate various anatomic features. [6] Bifurcated endografts were initially implanted in 1994. [7] Aorto-uni-iliac or aorto-uni-femoral grafts, reported in 1997, required occlusion of the contralateral common iliac artery and femoral-femoral bypass grafting. [8, 9] For ruptured AAA, endovascular treatment[10, 11] is considered the best option. The first reported endovascular treatment of a ruptured AAA was in 1994 by Yusuf, et al. [12] Four years later, the modular design of the current endografts was first developed by May, et al, 1998. [13]
1.4. Rationale for Guideline:
Ruptured AAA is ranked as the 13th leading cause of mortality in the United States, which is approximately 15,000 deaths per year. The incidence of AAA rupture, ranging from 1 to 21 cases per 100,000 persons, is on the rise despite increased AAA diagnosis. [14] Men have a 2–6 times greater frequency of AAA than women, and AAA are found more frequently in Caucasians compared to non-Caucasians. [15] There is no known treatment to induce regression once the AAA has formed. Most current therapies are aimed at limiting further aneurysmal expansion.
Therefore, the primary goal is to diagnose and treat AAA prior to rupture as the mortality of ruptured AAA is 80–90%. Thirty to 50% of individuals with ruptured AAA die before reaching a hospital and 30–40% of those with ruptured AAA die after reaching the hospital. [16]
1.5. Clinical Practice Guideline Purpose and Goals
This evidence-based clinical practice guideline was developed by vascular clinical nurse experts who are members of the Society for Vascular Nursing Practice and Research Committee. The purpose of this guideline is to assist nurses in delivering the optimal evidence-based care for the patient undergoing Endovascular AAA Repair (including discussion regarding surgical cut down for EVAR, Percutaneous access for pEVAR, and Fenestrated endovascular repair for FEVAR). This document should be tailored to the needs of the practice setting as well as the values and preferences of the patient.
The goals of care for this patient population are:
Ensure optimal nursing care is based on recommended clinical practice guidelines.
Provide a safe and caring environment throughout each phase of the patient’s experience before, during and after endovascular AAA repair.
Assess, plan, implement, and evaluate individualized patient care effectively.
1.6. Review of Abdominal Aorta Anatomy and Physiology
The aorta is the major blood vessel in the body that carries blood from the heart to the other major bodily organs. The aorta is divided into thoracic and abdominal sections. The abdominal aorta begins at the diaphragm and ends at the top of the bifurcation into the iliac arteries. The aorta lies in the posterior wall of the abdomen, anterior to the vertebral column. It follows the curvature of the lumbar vertebrae, causing it to convex anteriorly. The aorta runs parallel to the inferior vena cava (IVC). The abdominal aorta supplies blood to the intestines (via the superior mesenteric artery (SMA), celiac artery and the inferior mesenteric artery (IMA), abdominal organs, kidneys (via renal arteries), lumbar region, pelvis and legs. [17]
All arteries have three distinct tissue layers: tunica intima (also known as the endothelium), tunica media and tunica adventitia. The tunica intima is the innermost arterial layer that is in contact with the blood. The tunica media, which is the middle layer, consists of smooth muscle cells surrounded by elastin, collagen and proteoglycans that provide elastic properties of the artery. The tunica adventitia is the outermost layer and consists mainly of collagen but also contains fibroblasts, immunomodulatory cells, and adrenergic nerves and acts as a support. [18]
Typically, the diameter of the aorta decreases from its thoracic section to the abdominal and infrarenal (below the renal artery) portions. In a normal aorta the medial elastin layers, elastin and collagen content decrease from the thoracic to the abdominal portion. The elastic fibers in the aorta contract following each heartbeat to help propel blood away from the heart and toward organs and tissues.
1.7. Pathogenesis of Abdominal Aortic Aneurysm
An AAA is a focal dilation 50% greater than the normal abdominal aortic diameter of 2 cm, and by definition is greater than 3 cm in diameter. The infrarenal abdominal aorta is the most common site of true arterial aneurysmal formation. [19] Risk factors associated with the development of AAA include advanced age, male gender, Caucasian race, a positive family history (15–25% have a first-degree relative with the same type of aneurysm), smoking, and the presence of other large vessel aneurysms. Atherosclerosis has also been associated with AAA. [11, 20]
AAAs tend to progressively dilate over time. Expansion rates for AAA vary, however large aneurysms generally expand at a faster rate than small aneurysms. [11, 20] The main risk factors associated with expansion and rupture of AAA are somewhat different from those that contribute to the development of AAA and include large aneurysm diameter, rapid expansion, smoking, hypertension, elevated peak wall stress, a history of cardiac or renal transplant, decreased forced expiratory volume, and female gender. [11, 20]
The mechanisms for the development, expansion, and rupture of AAA have been validated in animal models. Aneurysmal degeneration of the abdominal aorta is a multifactorial systemic process due to alterations in vascular wall biology thought to be caused by inflammation, smooth muscle cell apoptosis, and extracellular matrix degradation. AAAs are characterized by transmural inflammatory changes in the tunica media, causing loss of elastin and smooth muscle cells and abnormal collagen remodeling and cross-linking resulting in progressive thinning and weakening of the aortic wall with enlargement of the aortic diameter. [21]
Aneurysm location/classification definitions [22]:
Suprarenal (visceral) – The aneurysm originates above the renal arteries.
Pararenal – The aneurysm involves the aorta at the level of the renal arteries, i.e., the renal artery originates from an aneurysmal aorta.
Juxtarenal – The aneurysm originates at the level of the renal arteries but the aorta at the renal arteries is normal.
Infrarenal – The aneurysm originates below the renal arteries.
Most AAAs are infrarenal, however approximately 15% are juxtarenal. [23]
Peripheral Artery Disease (PAD) has been associated with AAA. According to the 2016 PAD Guidelines, the prevalence of AAA was higher in patients with symptomatic PAD than in the general population and in patients with atherosclerotic risk factors. The prevalence of AAA in patients with PAD increased with age, beginning in patients ≥55 years of age, and was highest in patients ≥75 years of age. To date, there are no data on AAA screening in patients with asymptomatic PAD. Screening duplex ultrasound for AAA is a class IIA recommendation for patients with symptomatic PAD. [10]
1.8. Aneurysm Screening:
Screening for AAA is associated with a reduction in AAA rupture, mortality and emergency surgery. Screening men ages 65–75 years is uniformly agreed upon in clinical practice guidelines, while screening women is controversial as data is lacking. The European Society for Vascular Surgery [24] does not recommend screening for women and the United States Preventative Services Task Force [25] recommends reimbursement for screening men only. However, screening women may be more cost-effective. Though the prevalence of AAA in non-smoking women is low, AAA prevalence is higher in women who smoke and the rate of AAA rupture in general is higher. [11, 26]
The following recommendations are from the Society for Vascular Surgery [11]:
One-time abdominal ultrasound screening for AAA in patients 65–75 years with a history of smoking. [11]
Abdominal ultrasound screening for AAA in first degree relatives of patients with a AAA between age 65–75
Abdominal aortic aneurysms >2.5-<3 cm should have AAA duplex every 10 years
Abdominal aortic aneurysms 3.0–3.9 cm should have AAA duplex every 3 years
Abdominal aortic aneurysms 4.0–4.9 cm should have AAA duplex every year
Abdominal aortic aneurysms 5.0–5.4 cm should have AAA duplex every 6 months
CT scan in patients with known AAA with recent onset abdominal or back pain in the presence of an abdominal pulsatile mass. [11]
1.9. Selection Criteria for Endovascular AAA Repair and Summary of Evidence
1.9.1
Nonruptured AAA:
Most AAAs are asymptomatic and often diagnosed incidentally. Aneurysm rupture is the most feared complication, and is associated with high morbidity and mortality (refer to 1.4). The AAA transverse diameter is the best predictor of rupture risk; the bigger the aneurysm, the more likely it is to rupture. Symptoms suggesting acute AAA growth include back or abdominal pain which is steady, gnawing in quality, and not affected by movement. Other rare complications of untreated AAA include aneurysm thromboembolism and thrombosis, which can lead to acute limb ischemia. [27]
Elective AAA repair is not advised until the risk of rupture exceeds the risks associated with repair. Repair is recommended for AAA diameter exceeding 5.5 cm, [11, 28] symptomatic patients, patients who have evidence of embolization or rupture, or when the AAA that has expanded by more than 0.5 cm within a six-month interval or >10 mm over a year. [28] Rapid expansion of an AAA is thought to increase the risk for rupture. Women have a higher rupture rate and mortality (3 times higher) than men since current evidence that suggests that AAA in women may grow at a faster rate and may rupture at a smaller size (4.5–5.0 cm). [29]
Elective repair is recommended for low or acceptable procedural risk patients with a fusiform (spindle-like shape that is wide in the middle and tapers at both ends) AAA that is ≥5.5cm. [11]
Elective repair in women with AAA between 5.0 and 5.4 cm is recommended. [11]
Procedural selection criteria rely on careful assessment of factors that influence rupture risk, procedural mortality and life expectancy. Other factors such as the patient’s age, rate of aneurysm expansion, and the presence of PAD or peripheral aneurysm are also important to consider when determining when to proceed with elective AAA repair. [30]
When elective AAA repair is indicated, the choice between open surgical and endovascular AAA repair is debatable.
Elective EVAR repair is associated with lower rates of perioperative (30-day) morbidity and mortality compared with elective open repair (<2% versus approximately 5 %), long-term outcomes are similar to open AAA repair (Chaikof 2018). Of those undergoing EVAR, pEVAR has shown less morbidity and mortality, shorter hospital stay and lower cost than surgical cut down EVAR. [31, 32]
Open or endovascular repair of infrarenal AAA and/or common iliac aneurysms is indicated in patients who are good surgical candidates (Class I). [28]
Open AAA repair is reasonable to perform in patients who are good surgical candidates but who cannot comply with the periodic long-term surveillance required after endovascular repair (Class III). [28]
Endovascular repair of infrarenal aortic aneurysm in patients who are high risk from a surgical or anesthetic perspective, as determined by the presence of coexisting severe cardiac, pulmonary, and/or renal disease is of uncertain effectiveness (Class II).[28]
1.9.2
Ruptured AAA (rAAA):
Rupture of an abdominal aortic aneurysm (AAA) occurs in approximately 15,000 patients per year in the United States. Ruptured AAA is nearly always fatal without repair. When a ruptured AAA is identified, repair should be undertaken emergently to give the patient the best chance for survival. Timeliness of rAAA repair affects outcomes and the goal should be for intervention in less than 90 minutes employing the 30-30-30-minute framework (namely 30 minutes to evaluate and diagnose, 30 minutes to transfer from rural to tertiary facility, and 30 minutes to intervention).[11]
When anatomically feasible, EVAR is recommended over open repair for ruptured AAA. [11]
Emergency EVAR was associated with a significantly reduced perioperative (30-day) mortality risk relative to open repair (pooled odds ratio [OR] 0.62, 95% CI 0.52–0.75). However, not all institutions are equipped to treat ruptured AAAs using minimally-invasive technology. Although 70% of patients may be candidates for EVAR, ruptured AAA is more often repaired with open surgical techniques since there are limited number of centers available to perform emergency EVAR. The patients who are anatomically suited to EVAR, but high risk for open repair (systolic blood pressure <80 mmHg, advanced age (>80 years), cardiac arrest/myocardial infarction, loss of consciousness, creatinine >1.3mg/dL on admission, female sex, and hemoglobin <9.0, dialysis dependence, colonic ischemia), should be considered for transfer to a vascular center suited for emergency EVAR if the patient is hemodynamically stable. [11]
1.9.3
Specific anatomical requirements/considerations for endovascular AAA repair [30]:
Adequate length of normal aorta below the renal arteries for device attachment
Shape and angulation of AAA neck that does not prohibit device fixation
Characteristics of the iliac arteries to allow catheter and device access (i.e., need adequate diameter and length, minimal calcification and tortuosity)
Associated renal and visceral artery involvement
Patient comorbidities, body habitus, age
1.9.4
Advantages/Disadvantages of EVAR
Advantages of Endovascular AAA Repair [30]:
Lower short-term rates of death and complications
Decreased mortality especially among the elderly population
Abdominal incision avoided for high risk for patients who have severe cardiopulmonary disease, advanced age, morbid obesity, or hostile abdomen
Reduction in cardiopulmonary complications
Reduced length of hospital stay
Eliminates sequelae of laparotomy such as hernia or bowel obstruction
Disadvantages of Endovascular AAA Repair [33]:
Renal complications related to contrast dye or graft related ischemia
Proximal or distal attachment failure, graft migration or endovascular stent graft leaks (endoleak) (refer to 4.3.1)
Need for long-term surveillance imaging
1.9.5
Estimation of Operative Risk:
Operative risk and life expectancy can be estimated using the Vascular Quality Initiative (VQI) Risk Calculator [34] is used to assess preoperative mortality [11]
Since EVAR does not require intrathoracic or intraabdominal exposure of the aorta, or aortic cross-clamping, perioperative morbidity and mortality are reduced compared with open repair. Also, EVAR has made treatment possible for some patients with comorbidities who might not otherwise be candidates for aortic repair. [35]
Unlike open AAA repair, EVAR and pEVAR can be performed under local anesthesia which carries a reduced risk of surgical morbidity and mortality. [36]
1.10. Simplified Procedural Technique for Endovascular AAA Repair
1.10.1
EVAR is a multimodal procedure. It may be performed by a vascular surgeon, cardiothoracic surgeon, vascular and interventional radiologist or interventional cardiologist or combination thereof. The procedural location depends upon the treating physician and may be done in a standard operating room or specialized hybrid room. Other locations include the Interventional Radiology (IR) or Cardiac Catheterization Laboratory. The use of high-quality fluoroscopy and angiographic equipment is required to assist in optimal stent-graft deployment. [37] If the procedure is being performed in the IR or Cardiac Catheterization Lab, the assisting nursing personnel should have additional education specific to the interventional laboratory, as well as training in moderate sedation administration, and Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) (Class IV). [38]
1.10.2
Stent-graft selection is dependent upon physician preference and patient anatomic characteristics as determined by pre-procedure imaging results. There are no studies available that evaluate direct comparisons of different aortic stent-graft types. [39] General EVAR stent graft characteristics include full support throughout the graft and all are self-expanding. In addition, all have a method of fixation that is designed to prevent graft migration after deployment. [40] Most aortic stent-grafts are modular with several components, and typically includes a main body, contralateral and ipsilateral limbs. [41] Figure D depicts an example of a modular aortic stent-graft.
FIGURE D.
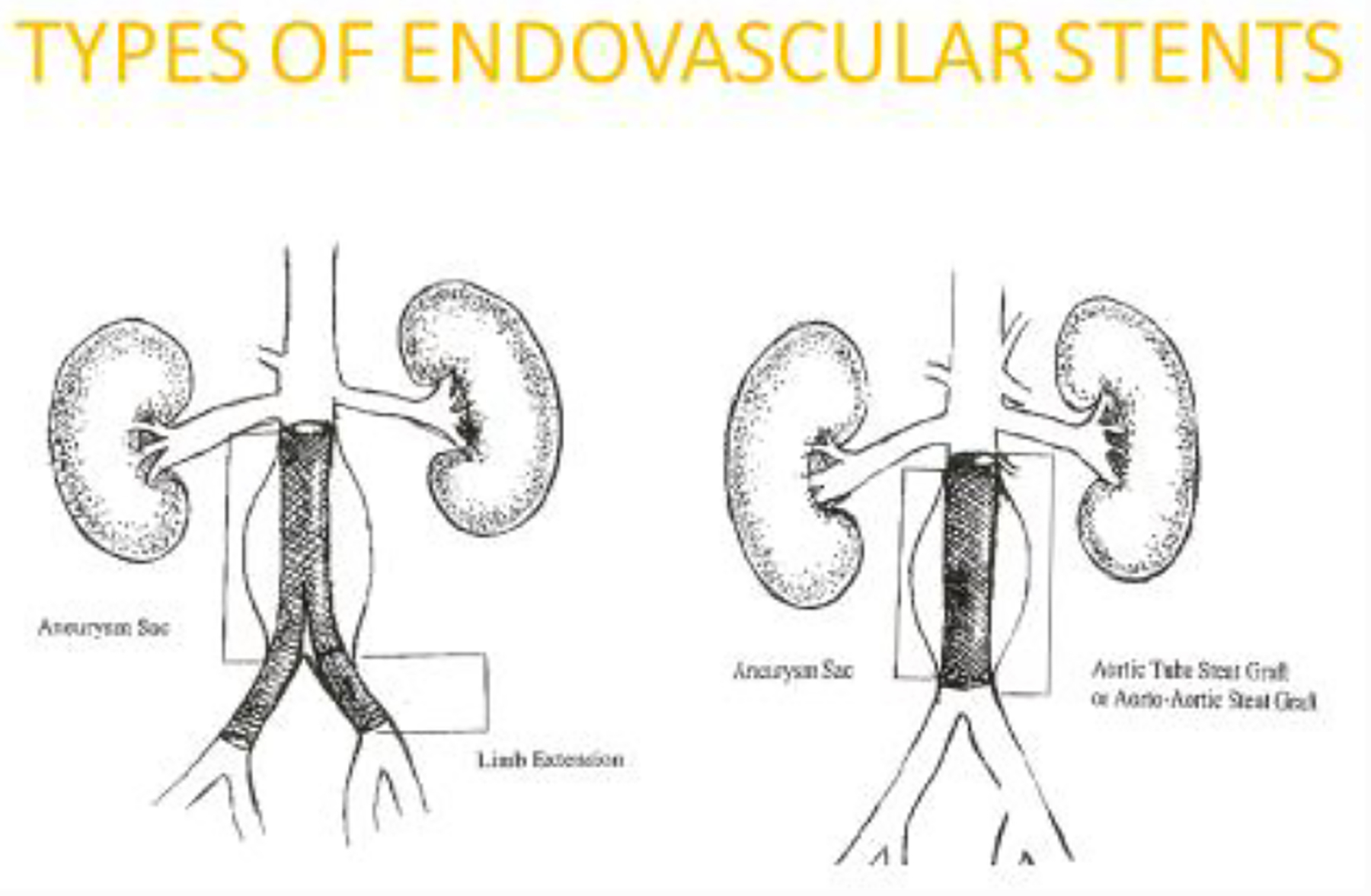
Modular Aortic Stent Graft
1.10.3
Anesthesia options include local, regional or general anesthesia as determined by physician preference, patient co-morbidities and body habitus. Regional techniques may include paravertebral, spinal, continuous spinal and epidural. If local or regional anesthesia is used, the concomitant administration of intravenous sedation is included. (Class I). [42] Locoregional anesthesia has been shown to reduce operative time, postoperative complications, hospital stay, and reduced need for intensive care. [36]
1.10.4
Bilateral common femoral artery access for the EVAR delivery system may be obtained via an open (surgical cut down) or pEVAR with a suture-mediated closure device. [41, 43] Ultrasound guided access is recommended to optimize arterial access and reduce access site complications. [44] In addition to reducing operative time and hospital stay, pEVAR has been shown to reduce wound complications compared to surgical cut down EVAR. [32] If an open cut down approach is chosen, transverse incisions are typically used to expose the femoral arteries. This is associated with a lower rate of wound complications in comparison to vertical incisions. [45] However, if significant femoral disease is present, a vertical incision allows for additional femoral exposure. [37] Post-procedure wound infections are minimized by keeping the incision above the femoral crease. [37] The patient is heparinized. The iliofemoral artery introducer sheath size ranges from 6F to 20F. The selected introducer size is dependent upon the femoral and external iliac artery diameter, stent graft selected, and the presence of iliofemoral occlusive disease. If the external iliac artery is small, retroperitoneal exposure of the iliac artery may be necessary and a synthetic graft sutured onto the common iliac artery. The EVAR delivery system is deployed through this conduit. [42]
1.10.5
After bilateral femoral artery access has been attained, a guidewire is advanced retrograde through the contralateral femoral artery up to the proximal thoracic aorta under fluoroscopy. A marker pigtail catheter is then inserted over the guidewire to the level of L1-L2. The main body of the stent graft is advanced over a stiff wire through the ipsilateral femoral artery with proximal placement below the renal arteries. The position of the contralateral graft opening (known as the gate) on the main body is verified through fluoroscopy before insertion and during device advancement. Once the main body of the endograft is positioned, proximal deployment begins. [37] When the proximal end of the stent graft main body is placed, deployment of the remaining stent graft body continues until the contralateral gate is in place.
1.10.6
From the contralateral femoral artery, a guide wire is advanced over the marker pigtail catheter. This catheter assists in verifying appropriate cannulation of the contralateral gate. Retrograde cannulation of the contralateral gate of the stent graft is undertaken to deploy the contralateral iliac graft limb. The deployment is performed so there is overlap with the limb and the main body contralateral gate. Ipsilateral iliac limb placement follows the same approach as described above. A completion arteriogram is done for placement verification, patency and to assess for endoleaks (refer to 4.3.1). [37, 41] The catheters/guidewires are removed. Arteriotomy closure is via cardiovascular sutures. Deep and superficial tissues are re-approximated with sutures of the physician choice. If a percutaneous approach is utilized, a vascular closure device may be included. [46]
1.11. Endovascular Techniques Used for Complex Abdominal Aortic Aneurysms (aka Parallel Graft-Endovascular Aneurysm Repair (pg-EVAR)
1.11.1
Although not the focus of this guideline, a brief overview of endovascular techniques for complex AAAs is included here. Thirty to 40% of patients have unsuitable anatomy for standard EVAR repair often due to short aortic neck (normal aorta below the renal arteries). [47] Advanced endovascular techniques have been developed for some complex aneurysm patients with the benefit of avoiding an open aortic repair in high-risk patients. Due to their complexity, these techniques are often associated with higher doses of contrast material and radiation exposure compared to standard EVAR. Physicians also report a significant learning curve with these newer techniques. [48]
Ullery [47] reports the two most common methods are fenestrated EVAR (FEVAR) and the snorkel/chimney EVAR technique (sometimes called branched or parallel grafts). Use of these practices allows for cranial extension of the proximal seal zone to the suprarenal aorta with preservation of visceral and renal vessel patency.
Patients with hostile visceral segments (e.g., calcified visceral segments or severe neck angulations) are not candidates.
Although all successful EVAR insertions require planning, Eagleton [49] cites the need for additional rigor in these complex cases. These include expert knowledge of device construction and ancillary tools/devices, the ability to obtain, interpret, and manipulate three-dimensional work stations.
These procedures are much more challenging for the physician and misalignment of branch vessel grafts or fenestrations can lead to target vessel occlusion.
A National Surgical Quality Improvement (NSQIP) database analysis from 2005–2012 comparing EVAR to FEVAR, showed that FEVAR was associated with longer median operative time and a significant increase in postoperative transfusions and complications; these included a significant increase in procedural site infection, and a trend toward increased cardiac complications, postoperative dialysis, and longer hospital stay. [50]
Like any repair of the paravisceral aorta, renal failure is one of the most frequent complications and complex endovascular repair is no exception. Although a major FEVAR study reported a 0 % rate of acute renal injury at 30 days (despite a high rate of renal infarction), a more recent study of FEVAR/branched grafts noted an acute renal failure rate as high as 25%. [49]
Most large series report favorable technical success rates of >95% for complex aortic aneurysm repair using the two techniques described. [49] Like traditional EVAR, secondary interventions are required to maintain graft patency and address endoleak (refer to 4.3.1) development. Long-term durability of these techniques is not yet known. [47]
1.11.2
Fenestrated Stent Grafts:
In 2012, the Zenith Fenestrated stent graft was commercially approved for endovascular repair of short necked AAAs in the United States. [51]
A FEVAR procedure typically requires larger sheath sizes and more catheter and wire exchanges than EVAR. [50]
Fenestrations are circular or elliptical holes intentionally designed within the proximal main body and are usually customized for each patient. [47] Scallops or U-shaped gaps are commonly employed in the same proximal main body to allow perfusion to renal or visceral vessels. See Figure F.
The Zenith device consists of three modular parts that are inserted retrograde via the femoral arteries. Rarely, the left brachial approach is also required in cases of difficult vessel catheterization.
Small fenestrations are usually stented to allow for proper alignment of the visceral segment. [47]
Since most fenestrated grafts are custom made, there is a delay of 4–6 weeks in obtaining the graft thus prohibiting use in urgent situations. Some off the shelf fenestrated grafts are now being manufactured and may be suitable for up to 70% of FEVAR candidates. [49]
Figure F.
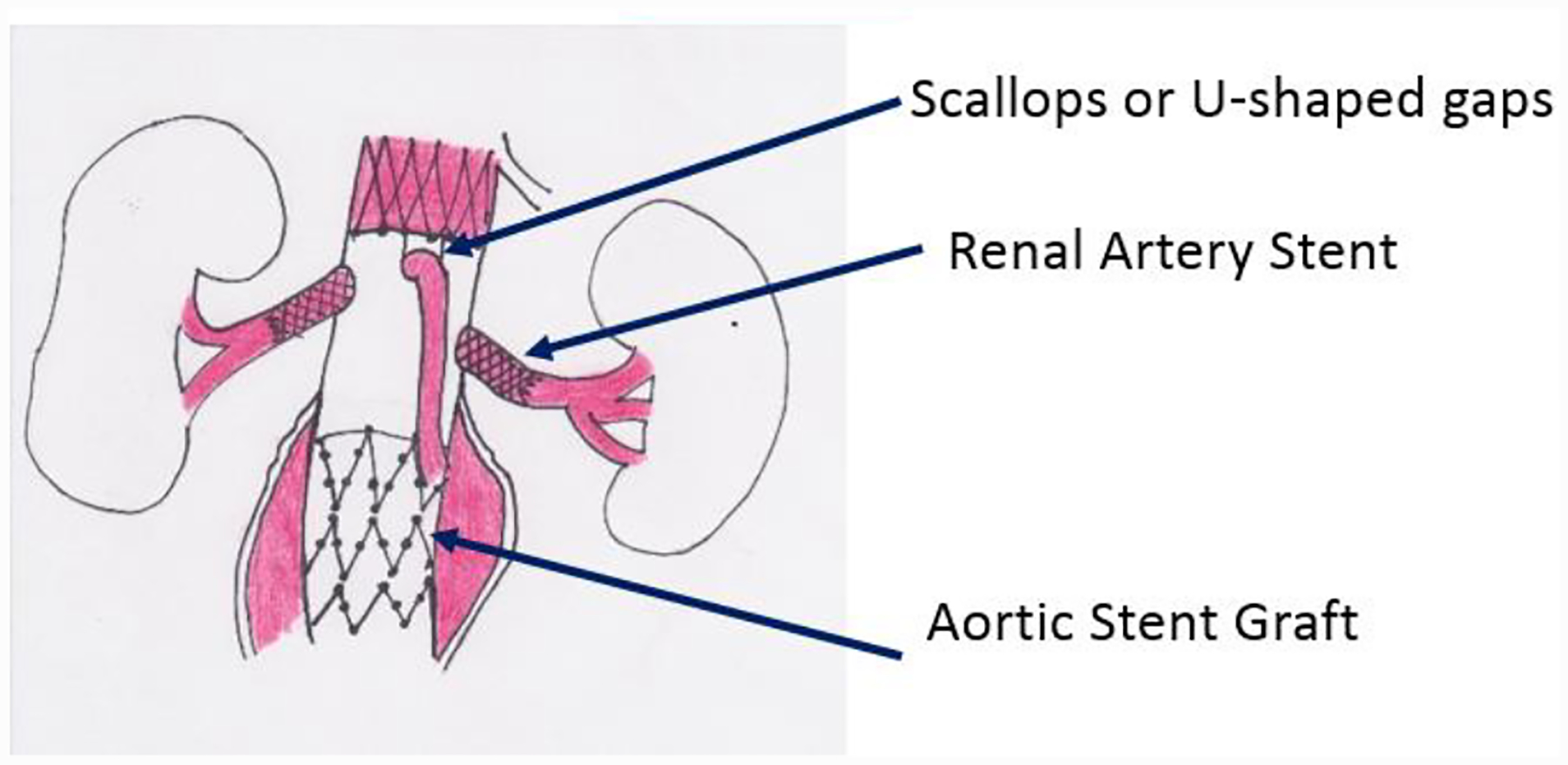
Diagram of Fenestrated Endograft
1.11.3
Chimney/Snorkel Configuration:
In contrast, the chimney or snorkel configuration utilizes an antegrade approach (usually the brachial artery) to facilitate placement of a covered stent into one or more branch vessels in a parallel course outside the main aortic stent graft (latter placed retrograde via transfemoral route). [47]
The proximal portion of the chimney/snorkel stent(s) extends beyond the proximal edge of the main aortic stent graft, thereby extending the proximal seal zone. See Figure G. This type of configuration is associated with a high rate of Type 1 endoleaks (refer to 4.3.1). This endovascular method was first described in 1999, remains off-label, and can be used in urgent cases including patients with ruptured AAA. [49]
The technical requirements of this approach are less demanding than the fenestrated approach and may be associated with less fluoroscopic time.
Chimney stents may become kinked, compressed, or occluded. [52]
Axillo-subclavian disease can prohibit safe passage of delivery devices to the renal or visceral vessels. [47]
The hypogastric snorkel technique is another advanced endovascular method which can be employed for persons with common iliac artery aneurysms or when distal common iliac seal zones are short. [52] This method involves placement of a covered stent parallel to an iliac limb device and is used to reduce the risks of buttock claudication, buttock necrosis, and bowel ischemia in the aforementioned patients.
FIGURE G.
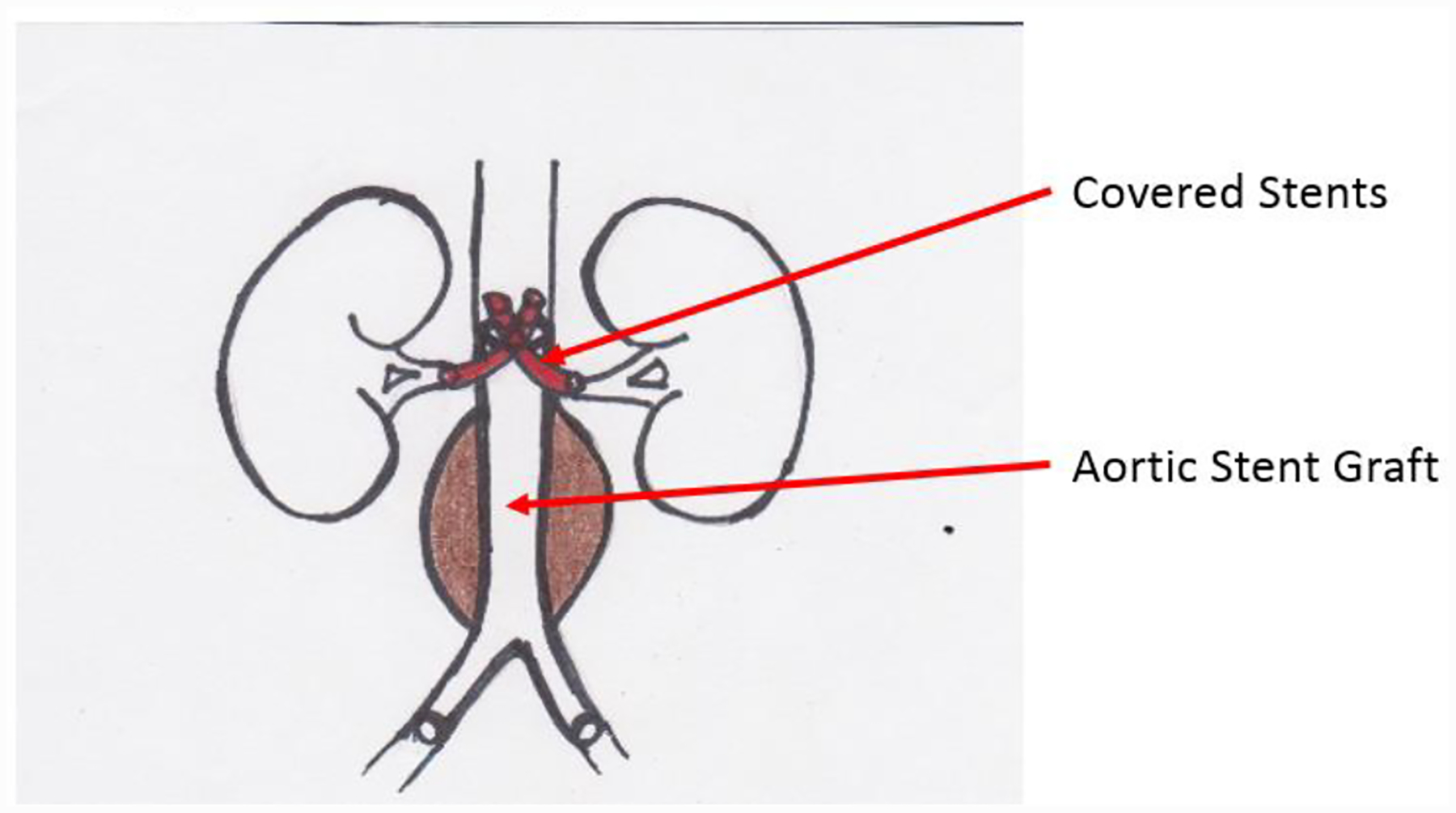
Diagram of Chimnay/Snorkel Graft
1.11.4
Iliac Branch Endoprothesis (IBE)
Another complex segment of EVAR is an inadequate distal landing zone and the involvement of the iliac arteries. Often in these patients, one or both of the iliac arteries are excluded extending the stent graft into the external iliac artery. The risk of this technique include thigh or buttock claudication, ischemic colitis, spinal cord injury, erectile dysfunction, gluteal/perineal necrosis and acute limb ischemia. The use of the iliac branch device can minimize these complications by incorporating one or both of the iliac arteries using modular stent grafts. [53]
2. Pre-Procedural Nursing Care
2.1. Nursing Assessments
Patients undergoing vascular procedures often have comorbid diseases, all of which should be assessed and, if possible, optimized before the vascular procedure. Due to the systemic nature of atherosclerosis, patients with vascular disease frequently have arterial disease affecting multiple vascular areas. Coronary Artery Disease (CAD) is the leading cause of perioperative mortality at the time of vascular procedure, and long-term survival after vascular procedures is significantly limited by the occurrence of morbid cardiac events. [42]
2.1.1
Assess for co-morbid conditions and risk factors for atherosclerosis such as renal disease, hypertension, tobacco use, dyslipidemia, diabetes, obesity, substance abuse, prior vascular procedure.
2.1.2
Obtain a list of current medications including over-the-counter drugs. Assess for allergies or hypersensitivities to medications such as intravenous (IV) contrast, foods or latex. [54, 55]
2.1.3
Obtain baseline vital signs to include apical/radial pulse, respirations, pulse oximetry, temperature and blood pressure. Use a standard technique for obtaining the blood pressure including the appropriate cuff size and supporting the arm at heart level (Class IV). [56, 57] Include bilateral upper extremity blood pressures. Unequal upper extremity blood pressures greater than 15mmHg difference in systolic blood pressure (SBP) may indicate subclavian artery stenosis. [57] [58] Inform the physician if this SBP difference is noted. Notify the surgeon/interventionalist and anesthesiologist if the systolic blood pressure is greater than 180mmHg or diastolic blood pressure is greater than 110mmHg within two (2) hours of the procedure start time. The treating physician may consider postponement due to an increased risk of stroke from these pressures or place the patient on additional antihypertensive medications. [59]
2.1.4
Perform a targeted physical assessment. Notify the physician of any abnormal findings. Clinical practice recommendations include general appearance, status of the skin (integrity, presence of lesions/edema, nails), head/neck, neurologic status, chest including breath sounds/heart sounds, abdominal assessment (distention/bowel sounds/genitals), bilateral extremity motor strength and movement, temperature, color, sensation, pulses and capillary refill. [60] If no institutional protocol in place, assess pedal pulses, mark location and rate as follows: 0, absent; 1, diminished; 2, normal; or 3, bounding. [10] If pulses are not palpable, perform Doppler examination documenting presence or absence of posterior tibial and dorsalis pedis signals and document location on the foot. [61]
2.2. Abdominal Aortic Aneurysm Diagnostic Studies
2.2.1
AAA Duplex
AAA Duplex has been widely used for screening and surveillance of abdominal aortic aneurysms. It offers the advantage of decreased cost, increased availability, and lack of radiation exposure or nephrotoxicity. The reliability of duplex ultrasound scanning for routine surveillance of AAA is well accepted. [62] Instruct patient to have nothing by mouth (NPO) at least six hours before the scheduled exam. [63]
2.2.2
Computed Tomography Arteriography (CTA)
CTA is the cornerstone of pre-procedural imaging for EVAR. The maximum recommended slice diameter is 2.5 mm for standard devices. Fenestrated or branched devices requires 1 mm or less cuts. Intravenous contrast should be routine unless the patient has severe renal insufficiency or contrast allergy. The axial, coronal, sagittal, and three-dimensional reconstructions should all be reviewed. [64, 65]
CTA utilizes non-contrast technique or IV contrast to identify the anatomy of the aorta. CTA uses ionizing radiation when taking x-ray pictures in the form of slices of the aorta. Contrast media may be injected to enable visualization of blood vessels and to identify area(s) of arterial stenosis. [64, 65]
Preparation:
Pending institutional practices, instruct patient regarding timing of solid food/fluids before the scheduled exam
Complete a thorough history of allergies and specific types of reaction to previous contrast media
Baseline renal function tests of blood urea nitrogen (BUN) and creatinine are completed to ensure safety of contrast administration.
Premedication and IV hydration may be given if contrast allergy or renal insufficiency develops.
Metformin should be held as noted below (refer also to 2.6.4).
Instruct patient to wear comfortable clothing but patients may be asked to change into a gown and remove hairpins, hearing aids, glasses, metal dental work, necklaces, earrings, and any body piercing. [65]
Advantages of CTA:
High resolution CT images that can be evaluated in multiple planes
Fast testing modality
Can visualize multiple organs simultaneously
Detects concomitant pathology
Disadvantages of CTA:
More expensive than AAA duplex
IV contrast and ionizing radiation exposure
Intravascular calcium burden can affect visualization, and assessment of plaque structure. [37]
Patients with significant renal insufficiency present challenges in pre-procedural imaging before EVAR.
Non-contrast CT scanning may be reasonable when planning a standard EVAR as diameter and length measurements can be made when anatomy is uncomplicated and there is no clinical suspicion for associated occlusive disease. [37] However, without contrast, potentially important anatomic information can be missed including: (1) presence of laminated thrombus in the aortic neck (2) patency of important side branches, such as the hypogastric arteries, and (3) occlusive disease in the common or external iliac arteries. Significant calcification in the iliac arteries, particularly the external iliac arteries, should make the operator suspicious. [37]
Alternative Imaging:
In patients with severe renal insufficiency who need better definition of anatomy prior to performing an EVAR, intravascular ultrasound (IVUS) can be used to size the aortic and iliac seal zones, evaluate for potential aortic neck eccentric thrombus, and interrogate the external iliac arteries for potential occlusive disease. [66]
Direct angiographic imaging can be obtained using carbon dioxide as the contrast agent with relatively good visualization. [37]
2.2.3
Magnetic Resonance Angiogram (MRA)
MRA uses radio waves and magnetic fields to create detailed images showing blood flow inside the vessels. Gadolinium, a non-iodinated contrast media, may be used to improve the test’s accuracy by making the arteries more visible. Gadolinium is not without consequence as it can trigger nephrogenic systemic fibrosis in those with a history of renal disorders. [67] MRA can precisely define the dimensions and extent of aortic arch aneurysms, dissections, vascular tumors and periaortic abscesses. It also can identify the structure of atherosclerotic plaque. [68]
Preparation for MRA:
History of contrast allergies
History of any metal implants in their body. These include pacemakers or implantable cardioverter defibrillator (ICD); surgical metal rods, screws, plates, stents, clips, pins, staples and wires (some may not be exclusionary), intravascular embolization coils, cochlear implants, metal heart valves, shrapnel or bullets, implantable ports and nerve stimulators, penile implants. [69]
Removable dental work and body piercings must be removed. [70]
Advantages of MRA:
Noninvasive, with no ionizing radiation or iodinated contrast.
Can visualize arteries beyond coverage area for AAA Duplex and is helpful in providing preoperative information including the type and localization of an aneurysm, its diameter and cranial caudal extent, the aneurysmal wall structures and periaortic space, the relationship to mesenteric and renal arteries and the presence of iliac aneurysm or stenosis. [71]
Disadvantages of MRA:
Possible over-estimation of the degree of stenosis due to arterial calcification.
Difficult to perform if patient is claustrophobic or uncooperative. [72]
2.2.4
Abdominal Aortic Aneurysm Contrast Angiography
Standard digital subtraction arteriography (DSA) has little utility in the preoperative evaluation for EVAR (as it only delineates aortic lumen) and should not be routinely employed. With rare exception, all anatomic details can be obtained with CTA scanning. [37]
2.2.5
Ankle-Brachial Index (ABI)
PAD is an independent risk factor for increased Major Adverse Cardiovascular and Cerebrovascular event (MACCE) and stroke [73, 74] in patients with AAA undergoing EVAR. Thus, patients with AAA should be screened for PAD. The Ankle-Brachial Index (ABI) is the most cost-effective screening tool used to diagnose lower extremity PAD. Patients with the history or physical exam suggestive of PAD or at risk for PAD should undergo an ABI. The following individuals are at risk for PAD [10]:
Age 65 years or older.
50–54 years old with atherosclerotic risk factors of hypertension (HTN), diabetes mellitus (DM), dyslipidemia, history of smoking or a family history of PAD.
Under 50 years of age with DM and one other atherosclerotic risk factor and known atherosclerosis in another vascular bed (such as carotid, coronary, subclavian, renal, mesenteric arteries or abdominal aortic aneurysm).
Patients with left ventricular dysfunction or heart failure. [75]
2.3. Diagnostic Laboratory Testing
Healthcare facilities have developed protocols and algorithms to incorporate evidence-based practice as opposed to a clinician “style.”[76] Patients with signs or symptoms of active cardiovascular disease should be evaluated with appropriate testing, regardless of their pre-procedural status. [77]
- Verify that ordered pre-procedural laboratory tests have been done within the pre-established time period (usually within 7 days of the procedure or as specified by the institution). General recommendations are described by Fleisher, et.al, 2014. Typical baseline laboratory tests include a complete blood count, blood urea nitrogen, serum creatinine, electrolytes, partial thromboplastin time, prothrombin time with International Normalized Ratio (INR). [78, 79] Include measurement of blood glucose in diabetic patients. Patients should be typed and screened preoperatively. [80]
- Patients with Hemoglobin <7g/dl are recommended for blood transfusion
- Platelet count <150,000/uL should have a hematology evaluation preoperatively. [11]
Perform a 12-lead Electrocardiography (ECG) within 30 days of EVAR. [11, 79] ECG is recommended for patients undergoing high-risk vascular procedure and those undergoing intermediate-risk surgeries who have additional risk factors. [77] Further cardiac testing may be warranted based on patient’s risk factors and past medical history.
Perform additional ancillary diagnostic studies based upon the institutional protocol as well as patient factors including age, gender, and presence of severe obesity or comorbidities (e.g. pregnancy test, pulmonary function studies, stress test, and chest x-ray). [79, 81] Notify physician of any abnormal results.
2.4. Anesthesia Evaluation
An anesthesia evaluation is not always needed pending institutional protocol. When anesthesia evaluation is needed, confirm that an anesthesia assessment has been scheduled before the procedure.
Goals of pre-procedural clinical assessment and evaluation:
2.5. Pre-Procedural Patient Preparation/Education
2.5.1
Remove jewelry/body piercings [84]
2.5.2
Clip any hair on the patient at the selected procedural site with electric clippers as ordered [85, 86] (Class I). Clipping instead of shaving reduces the incidence of procedural/surgical site infections (SSI).
2.5.3
Cleanse patient[85, 86] pre-procedurally using a chlorhexidine shower or disposable wipes to reduce bacterial skin flora and the risk of SSI. [87]
2.5.4
Establish peripheral intravenous (IV) access; depending upon institutional protocol, two IV sites may be preferred. Hypnotic agents and other medications are administered intravenously during the procedure. [83]
2.5.5
Maintain NPO status based upon surgeon/interventionalist/anesthesiologist preference and type of food ingested. This can range from 8 hours (fried/fatty foods) to 2 hours (non-alcoholic clear liquids). NPO status reduces the potential for aspiration due to anesthesia (Class IV). [83]
2.5.6
Provide additional patient education regarding procedure. This may include handouts, videos and internet resources. This process should continue through preadmission testing and be completed on the day of the procedure. Pre -procedural teaching decreases anxiety regarding the surgical/procedural experience. [88]
2.5.7
Smoking Cessation
Smoking cessation is recommended for at least 2 weeks prior to aneurysm repair. [11]
2.5.8
Infection Prophylaxis
Any infection or potential infection that could lead to sepsis (such as a dental abscess) should be should be eliminated 2 weeks prior to EVAR. [11]
2.6. Pre-Procedural Management of Medications
EVAR has also been associated with a significant risk of implant and procedure-related complications, such as graft thrombosis and cardiovascular events, including death, necessitating interventional and pharmaceutical management. To reduce the overall risk for cardiovascular events, the implantation of an endovascular aortic graft necessitates the use of at least one antiplatelet agent postoperatively and this treatment is in-line with the medical management of coronary artery disease which also includes antiplatelet and statin therapy. [89]
Continue most medications that have withdrawal potential and hold non-essential medications the day of EVAR.[90] Refer to additional sources for a broader overview of chronic medications in the pre-procedural setting. [45, 90, 91]
2.6.1
Antiplatelet Agents
Continue aspirin in patients with prior coronary percutaneous interventions. However, when applicable, P2Y12 platelet inhibitors should be stopped at the most 10 days prior to procedure, however this is done in consultation with the patient’s cardiologist. [11]
The antiplatelet agents that are more frequently prescribed include aspirin and clopidogrel.
Aspirin irreversibly inhibits platelet cyclo-oxygenase, blocking thromboxane A2 production in platelets and therefore decreases platelet aggregation- a P2Y12 platelet inhibitor.
Clopidogrel is a thienopyridine derivative that inhibits platelets by selectively and irreversibly binding to the P2Y12 receptor and, therefore, blocks the adenosinediphosphate (ADP) dependent pathway of platelet activation.
In patients taking a combination of two different antiplatelet agents, known as dual antiplatelet therapy (DAPT), strong considerations must be given to the risk of excess bleeding. [89]
2.6.2
Lipid-Lowering Therapy
-
Administer statin medications in patients with PAD (Class I). [10]
Although statins are known for cholesterol lowering effects, the theorized reduction in vascular inflammation and stabilization of plaque supports their pre-procedural administration. [92] A study by the Vascular Study Group of New England showed reduced 30-day mortality and 18% improvement in 5-year survival after various vascular surgeries when antiplatelet and statin medication were utilized pre-procedurally and at discharge. [93, 94] Hold non-statin lipid lowering medications such as bile acid sequestrants (cholestyramine and colestipol) which may interfere with bowel absorption of peri-procedural medications. [95]
2.6.3
Antihypertensive Medications
Administer previously prescribed calcium channel blockers, alpha agonists, vasodilators, and nitrates the morning of the procedure with a sip of water. [59]
Hold angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) the day of the procedure [11] due to the potential of intravenous (IV) contrast worsening renal function.
Hold any diuretic medication the day of the procedure (Class II). [79, 90] Loop and thiazide diuretics can cause hypovolemia and hypokalemia and ca[91]n intensify the known hypotensive effects of anesthesia induction. [45, 90]
Administer previously prescribed beta-blocker (Class IV). [78] If not already on a beta blocker, when indicated, start well in advance of the procedure to ensure tolerance and safety. [11]
2.6.4
Diabetic Medications
Hold metformin in patients with an estimated glomerular filtration rate (eGFR) of <60ml/min or up to 48 hours before administration of contrast if eGFR < 45ml/min. [11]
Hold other oral hypoglycemic agents the morning of the procedure. [96]
Adjust any prescribed long-acting insulin based upon the healthcare facility’s protocol. Supplemental short-acting insulin may be administered during this period to maintain a target glucose level between 140–180 mg/dl in the critically-ill patient (Class II). [97]
2.6.5
Chronic Pain Medications
Stop all non-steroidal anti-inflammatory medications at least 3 days prior to procedure or as directed by the physician. [90, 98] These medications reduce platelet aggregation and increase the risk of bleeding by a decreased production of thromboxane. [98]
Continue chronic opioid medications the day of procedure to reduce irritability, diaphoresis, insomnia, nausea and other symptoms of withdrawal. [59]
2.6.6
Pulmonary Medications
Administer chronic bronchodilators for at least 2 weeks prior to aneurysm repair. [11]
Continue pulmonary inhalers on the day of procedure to reduce the incidence of post-procedural complications. [98, 99]
Hold theophylline the evening before procedure as it may precipitate arrhythmias and interact with anesthetic medications. [59, 98]
Continue glucocorticoids (used for pulmonary or other indications) the day of the procedure as to avoid symptoms of adrenal insufficiency; consider stress (supplemental) dose steroid if a patient has received oral corticosteroids for 3 consecutive weeks or more. [59]
2.7. Management of Antithrombotic Medications and Dietary Supplements
Inform patients regarding the risks and benefits of a temporary interruption of their anti-thrombotic therapy. [100] If the patient agrees, the anticoagulation manager and operating physician need to develop a patient specific plan. [100, 101]
2.7.1
Heparin
Withhold any non-bridged unfractionated heparin or low-molecular weight heparin based upon the healthcare facility’s protocol
2.7.2
Vitamin K Antagonists
Withhold Vitamin K antagonists (VKA’s) such as warfarin for approximately 5 days before the procedure (Class IV). [102] The International Normalized Ratio (INR) should be normal or near normal (less than 1.5).
If bridging therapy is required (e.g., patient with chronic atrial fibrillation or mechanical heart valve patient who is at high risk for venous thromboembolism), administer a therapeutic dose of a low-molecular weight heparin (LMWH) such as enoxaparin. The last LMWH dose should be administered approximately 24 hours before the procedure (Class IV). [102]
If the patient is receiving therapeutic-dosed unfractionated heparin (UFH) as the bridging medication, the last dose should be stopped within 4 to 6 hours of the procedure (Class IV). [102] In the chronic atrial fibrillation or mechanical heart valve patient who is at a low risk for VTE; no bridging therapy is recommended (Class IV). [102]
2.7.3
Other Oral Antithrombotic Agents
Withhold Dabigatran for 1 to 2 days before the procedure in patients with a creatinine clearance ≥50ml/minute. If the creatinine clearance is < 50ml/minute, withhold medication for 3 to 5 days. A longer withholding time may be considered in patients where total hemostasis is necessary. [103]
Stop Rivaroxaban, at least 24 hours before the procedure. [104]
Hold Apixaban at least 24 hours in elective procedures that are considered to be low-risk for clinically significant bleeding. But since AAA surgery is considered moderate to high risk for clinically significant bleeding, apixaban should be withheld for 48 hours before the procedure. [105]
Hold Endoxaban 24 hours before the procedure. [106]
2.7.4
Herbal/Dietary Supplements
Instruct the patient not to take any herbal or dietary supplements for the specified number of days prior to the procedure, based upon the institution’s protocol.
There is an increased potential for bleeding and alteration in the effects of medications such as warfarin (Class IV). [107, 108]
Ginkgo, Vitamin E and garlic (allium sativum) demonstrate antiplatelet activity and increase bleeding time (Class I). [107, 108]
Case reports have shown an interaction between herbal supplements St. John’s Wort (hypericum perforatum) and American ginseng that decrease the INR (Class IV). [107, 108] Dan Shen (salvia miltiorrhiza) increases the INR (Class IV). [107, 108]
2.8. Prevention of Adverse Contrast Media Hypersensitivity Reaction
2.8.1
For patients allergic to contrast media:
Administer a combination of glucocorticoids and antihistamine medication 13 hours prior to the procedure. Prednisone 50mg (adults) dose is given orally 13 hours, 7 hours and 1 hour pre procedure.[109]
Inject methylprednisolone 40mg (adult) intravenously if patient cannot take oral medications; interval dosing at 13, 7 and 1-hour pre-procedure.
Diphenhydramine 50 mg (adult dose) is administered either orally or IV an hour before procedure. [109]
2.8.2
Risk factors for contrast media hypersensitivity [109]
Acute or chronic kidney disease
Diabetes mellitus
Myeloma,
Dehydration
Cardiopulmonary disease
Repeat contrast administration
Female gender
Use of ACE inhibitors, beta blockers or proton pump inhibitors
2.9. Prevention of Contrast-Induced Nephropathy
Contrast–Induced Nephropathy (CIN) is defined as an increase in serum creatinine levels > 25% or 44.2 μmol/L (0.5 mg/dL) within 3 days of intravenous contrast; incidence 7–11%. There are currently no recommended protocols to prevent CIN.
2.9.1
Administer IV hydration with normal saline or 5% dextrose/sodium bicarbonate for patients at increased risk of CIN undergoing EVAR both before and after procedure. [11]
2.9.2
No benefit was found using IV sodium bicarbonate over sodium chloride or oral acetylcysteine over placebo for the prevention of kidney injury or death. [110]
2.9.3
The greatest reduction in CIN risk was found the administration of statins plus N-acetylcysteine and intravenous saline compared with the use of N-acetylcysteine and saline alone. [11, 111]
2.9.4
Risk Factors for CIN: chronic kidney disease, diabetes mellitus, hypertension, and being elderly.
2.9.5
A linear relationship exists between the development of CIN and contrast infused; for every 100 mL of contrast material administered, there is a 12% increase in the risk for CIN. [11]
3. Peri-Procedural Nursing Care
3.1. Before Patient Enters the Procedural/Operating Room:
3.1.1
Verify correct patient identification using two identifiers. This is done every time the care responsibility of a patient is transferred to another person in the peri-procedural course to avoid wrong-site procedure utilizing a standardized checklist. (Class I). [112–114]
3.1.2
Ascertain that the patient has signed the procedural consent and anesthesia consent. Confirm the operating physician or interventionalist has signed the procedural and blood consents and the anesthesiologist or nurse anesthetist has signed the anesthesia consent.
3.2. Intra-Procedural Care
3.2.1
Assist with attachment of ECG monitor leads, non-invasive blood pressure cuff and pulse oximetry probe once patient is on the procedural/operating room table. If indicated, obtain baseline blood pressure, heart rate, respirations, SpO2 and ECG rhythm.
Monitor blood pressure and ECG rhythm as indicated with anesthesia. Notify anesthesia and the operating physician immediately if hypotension or hypertension occurs. Blood pressure fluctuations are due to multifactorial causes that may include prior history of hypertension, pain-induced sympathetic nervous system stimulation and anesthesia induction. [115] Treatment is dependent upon the physician preference and includes the following options [115, 116]:
Hypotension (in suspected hypovolemia): cautious volume expansion using isotonic crystalloid or colloid per physician choice.
Hypotension (in normovolemia): IV phenylephrine
Hypertension: IV labetalol, IV nitroglycerin
3.2.2
Maintain core body temperature at or above 36 degrees C during the aneurysm repair. [11]
3.2.3
Assist with setup and insertion of an arterial line if directed.
3.2.4
Insert a urinary catheter if indicated by institutional protocol.
3.2.5
Conduct a procedural time-out before the procedure is started to avoid making a mistake. (Class I) [113]
3.2.6
Administer, the ordered intravenous (IV) antibiotic prophylaxis within one (1) hour of surgical incision at the recommended infusion rate to reduce the incidence of SSI. [117] (Class I)
Antibiotic Prophylaxis:
First-generation cephalosporin or vancomycin (if allergic to penicillin) should be given intravenously 30 minutes before EVAR.
Antibiotics should not be continued for more than 24 hours. [11]
3.2.7
If general anesthesia is utilized, observe for the development of malignant hyperthermia (MH) which is a genetic disorder that is precipitated by use of volatile anesthetic agents (halothane, enflurane, isoflurane, desflurane, and sevoflurane) or the muscle relaxant succinylcholine, or both. [118] It is characterized by life-threatening, extremely high fever (greater than 110 degrees Fahrenheit), skeletal muscle spasms, hypotension, change in level of consciousness, tachypnea, and tachycardia. Acidosis, hyperkalemia, cardiac arrhythmias, and rhabdomyolysis may occur. [119] Early recognition is integral to preventing mortality. Immediate treatment consists of switching to a non-inducing agent, administering dantrolene 2 mg/kg IV, and repeating until the cardiovascular symptoms stabilize to a maximum of 10mg/kg (Class IV). [119, 120] In addition, hyperventilation with 100% oxygen, application of external cooling devices and administration of chilled IV normal saline are used (Class IV).[119, 120]
3.2.8
Monitor for intra-procedural complications and assist as indicated in stabilization:
-
Conversion to open AAA repair.
The overall incidence of conversion (early or late) is approximately 2%. Risk factors include female gender, non-Caucasian ethnicity and large aneurysm size. [121] Despite the newer endovascular salvage techniques for complications post EVAR, some patients may require conversion to open repair. Elective open surgical conversion following failed endovascular repair may be associated with increased morbidity and mortality, particularly if the patient was deemed at high risk for open repair. [33]
Vascular access bleeding, hematoma, thromboembolism
Respiratory depression (if local anesthesia with sedation)
3.2.9
Assist with the application of physician-determined type of sterile dressing (including topical sprays) over the procedural incision site. There is no clear evidence that supports one type of dressing over another to prevent surgical site infection (SSI). The choice of dressing should be based on cost and symptom management goals (Class I). [123]
3.2.10
When general anesthesia is used, assist with ECG-monitored transport to the Post-Anesthesia Care Unit or other designated unit according to institutional protocol.
3.2.11
Confirm pedal pulses (see 2.1.4) with physician prior to leaving the operating room
4. Post-Procedural Nursing Care
After procedural recovery has occurred, the patient is transferred to a secondary nursing unit until discharge. Depending upon institutional protocol, this may be an intensive care unit, or a specialized cardiovascular unit. Patients are instructed to lay flat for 1 to 4 hours after sheath removal with closure device, 4 to 8 hours after sheath removal with manual compression. [124] Length of stay is typically 1–3 days. [125]
4.1. Assessments
4.1.1.
Obtain vital signs (VS) to include blood pressure (BP), apical pulse, respirations, peripheral capillary oxygen saturation (SpO2). Additionally, check and document level of consciousness, pain and sedation level at the specified intervals during recovery and progress thereafter according to institutional protocol. If the patient is transferred to a Post Anesthesia Care Unit (PACU), then use of a PACU scoring system is required (Class IV). [126] Maintain the systolic BP greater than 90mmHg and less than 180mmHg to avoid post-procedure complications of hypotension, hematoma formation. [115] Respirations should include rate, depth, effort and symmetry. If an arterial line is present, maintain system according to institutional policy.
There is a lack of evidence regarding the frequency in obtaining VS in the immediate post-procedure period. A single randomized controlled trial of 189 patients compared an experimental protocol (VS every 1 hour for 2 hours then every 4 hours for 24 hours) to standard practice (VS every 1 hour for 4 hours then every 4 hours for 24 hours) for post-operative patient monitoring. There were no significant differences observed between the two groups at 4 or 24 hours. [127] The authors recommend that clinician judgment should be used in monitoring VS frequency. The American Society of PeriAnesthesia Nurses (ASPAN) advises that VS frequency should be determined by each individual facility and pain should be assessed frequently. [126, 128] The ASPAN website reports that expert opinion states VS should be taken every 5 to 15 minutes during the initial stabilization and more frequently if clinically indicated. [126]
Blood pressure should be monitored post procedure to prevent cardiac or renal complications.
Hypotension. The treatment is the same as described under 3.2.1.
Hypertension. The treatment, blood pressure parameters and pathophysiological mechanisms behind the development of hypertension are the same as delineated under 3.2.1.
4.1.2.
Pain and sedation levels should be assessed using a standardized scoring system. Sedation scale options include the Richmond-Agitation Sedation, Motor Activity, Sedation-Agitation or the Ramsey. [129] Pain is typically self-reported using a numeric or Faces scale. [128]
4.1.3.
Cardiac Monitoring
4.1.4.
Administer oxygen via nasal cannula at flow rates as ordered. The goal is to maintain a target oxygen saturation of at least 94% in acutely ill patients and 92% in those patients at risk for hypercapneic respiratory failure unless otherwise specified (Class IV). [130]
4.1.5.
Maintain a patent IV site and continuous ECG monitoring throughout the hospital stay or as specified by the physician. The potential for cardiac arrhythmias, coronary ischemia and other hemodynamic complications may develop in the post-operative period). [11, 131]
4.1.6.
Post-procedural assessment following EVAR should include a thorough lower extremity pulse exam or ankle-brachial index (ABI). [11] See also Section 2.1.4.
Assess circulation, motion, and sensation (CMS), color, and capillary refill. Ensure patient has bilateral pedal pulses. The location of pedal pulses should be marked. Assess blanching in the toe nails, ability to wiggle toes or dorsi/plantar flex. Assess for new numbness and tingling.
4.1.7
Institute ordered venous thromboembolism (VTE) prophylaxis measures to avoid venous stasis and thrombus development
The use of intermittent pneumatic compression and early ambulation is recommended for all patients undergoing EVAR. [11]
VTE risk factors include procedure length > 45 minutes, advanced age, and often the presence of obesity. [11]
Patients at low risk for bleeding should receive either low molecular weight heparin (e.g., enoxaparin 40 mg subcutaneous daily) or unfractionated heparin (e.g., heparin 5000 IU subcutaneous two to three times a day); it should be initiated within 24 hours and as per the judgment of the surgeon/interventionalist. [11]
Consider postoperative anticoagulation for EVAR patients at high risk for DVT (prior history of DVT or pulmonary embolus, persons who are overweight (BMI >25), have limited mobility, have a malignancy or a hypercoagulable state. [11]
4.1.8
Maintain head of bed below 45 degrees to prevent flexion at graft site. [132]
4.1.9
Monitor urine output (0.5–2.0 ml/kg/hour) to ensure adequate kidney perfusion. [133, 134]
4.2. Incisional/Access Site Care
4.2.1
Remove any dressings if present, at the time interval specified. The timing of dressing removal (within 48 hours or after 48 hours) does not appear to have a detrimental effect on outcomes (Class I). [135]
4.2.2
Keep the incision site dry for the first 48 hours after the procedure. Avoid use of topical antimicrobial agents (Class IV). [136, 137]
Once groin dressings are removed, advise patient to keep groin wound clean and dry and monitor for evidence of infection.
4.3. Assessment for Post-Procedural Complications
Monitor for post-procedural complications, notifying physician if any are present
EVAR has also been associated with a risk of implant and procedure-related complications, such as graft thrombosis and cardiovascular events, necessitating interventional and pharmaceutical management. [138] The overall complication rate related to EVAR ranges from 16 to 30%. [33]
Risk factors for late mortality in patients with AAA who have undergone repair are: age≥80, female gender, heart failure, ischemic heart disease, COPD, chronic kidney disease, and diabetes. [73] Thus, patients with these risk factors should be monitored more closely for post-procedural complications.
Nursing care of patients undergoing EVAR with complex fenestrated or branched devices have not been established but will be directed by institutional protocols. Usual EVAR care may be supplemented since these grafts incorporate the visceral and renal vessels into the repair (and in some cases the hypogastric arteries) and target vessel occlusion or embolus can compromise the blood flow to the end organs. Nursing measures may include more frequent monitoring and reporting of intake and output, abdominal assessments, blood pressure monitoring, and laboratory testing. When the brachial artery is utilized (e.g., for chimney/snorkel cases and some FEVAR cases), nurses will need to monitor the upper extremity for access site complications such as hematoma, thrombosis, atheroemboli, and infection. [47] Ideal pharmacologic adjuncts have not yet been established for these advanced endograft techniques. [45]
4.3.1
Endograft-related complications
The incidence of endovascular complications is 11–30 % such as vascular injury, stent fracture/kinking or endoleaks. [33]
Endoleak- described as incomplete exclusion of the aneurysm sac by the graft. There is persistent blood flow in the aneurysm sac following stent grafting. [37, 40, 138] An endoleak occurs when there is blood flow outside the stent graft but within the aneurysm sac (Picel 2014). [139]
Endoleak types:
Type I Endoleak: Persistent blood flow in aneurysmal sack following stent grafting that typically occurs during the EVAR procedure. Occurs at the stent graft attachment sites due to an incomplete attachment site of the proximal or distal graft to the aortic lumen. Predisposing factors include tortuous configuration, atherosclerotic burden at attachments sites, and advanced techniques that preclude apposition of the graft to the vessel lumen.
Type II Endoleak: The most common type of Endoleak. Results from leakage of collateral vessels into the excluded sac most commonly the IMA or lumbar arteries. Type II endoleaks are generally not treated unless there is an increase in the sac size (Sternbergh 2014, Tefera 2014, Walker 2010). [37, 40, 138] Risk factors include a larger IMA ostium, a greater number of lumbar arteries, and lack of pre-embolization of the IMA or internal iliac arteries. [140]The best indicator of hemodynamic significance in type II endoleaks is the change in size of the aneurysm sac. If the sac is increasing in size, there is a higher risk of long-term rupture. If the sac is stable or decreasing in size, the risk is likely to be less. Patients who present with symptoms suggestive of sac pressurization/expansion such as nonspecific abdominal or back pain that is otherwise unexplained by the patient’s history associated with an increase in aneurysm sac diameter should be considered for treatment.
Type III Endoleak: Graft mechanical failure: graft rupture or leakage between the graft components. Requires urgent repair.
Type IV Endoleak: Occurs due to flow directly through the fabric of the graft. Rarely seen in the newer grafts.
Type V Endoleak: Is usually diagnosed when all other types of endoleak have been excluded. It refers to increase in the sac size without endoleak.
Graft migration occurs in up to 3% of patients. [139] Migration of greater than 10 mm is considered significant. [140] There is risk for graft thrombosis with significant migration. Migration can result in endoleak, limb occlusion and rupture. It is usually repaired with a graft limb extension. [139] Graft kinking results from migration or decreasing aneurysm sac diameter which can lead to limb thrombosis. Treatment options include aorto-uni-iliac graft to avoid thrombosis, thrombectomy and stent placement to salvage a thrombosed limb, or femoral to femoral artery bypass. Surveillance is recommended to evaluate for limb thrombosis which may result in buttock claudication especially in the presence of bilateral internal iliac artery exclusions. [37, 40, 138]
4.3.2
Infection/Groin Complications
Incidence of access site complications is 9–16%. [33]
Signs of infection including redness at the incision site, fever, abnormal laboratory values, abnormal tenderness or distention, purulent drainage, or ill-defined pain should be reported to physician. [11]
Retrospective studies have identified age >80, use of an intraortic balloon pump, procedural hypotension, female gender and excessive anticoagulation as predictors of retroperitoneal bleeding. [141]
4.3.3
General anesthesia-related complications
If the procedure is performed under general anesthesia, the initial primary focus is on airway and breathing assessments. Airway and oxygenation are supported as indicated with airway positioning, oxygen as indicated, monitoring respiratory rate, depth and effort along with oxygen saturation, nebulizer treatments, medications, and oropharyngeal suctioning. Additional complications are treated per institutional protocol. The following complications from general anesthesia may be observed: [142–144]
Upper airway obstruction from loss of pharyngeal muscle tone, edema, laryngospasm, neuromuscular blockade or history of obstructive sleep apnea.
Hypoxemia from alveolar hypoventilation or pulmonary edema.
Hemodynamic instability (hypotension/hypertension)
Nausea and/or vomiting
Shivering
Delirium
4.3.4
Intestinal Ischemia- Bowel ischemia occurs in 1 to 3 percent of patients following endovascular aneurysm repair, likely due to atheroembolism or thromboembolism from manipulation of wires and catheters in the suprarenal aorta. Suspect ischemic bowel if patient has abdominal pain/tenderness, post-procedural diarrhea, especially if bloody, nausea and vomiting, fever, and/or change in mental status. [33, 145]
Abdominal Compartment Syndrome- has been reported following EVAR
The risk is higher in patients with ruptured AAA due to the extent of fluid resuscitation and the volume effect of the retroperitoneal hematoma.
One study reported up to a 10% incidence of this in patients following EVAR of ruptured abdominal aortic aneurysm. [30]
Abdominal compartment syndrome can theoretically occur following elective EVAR. [30]
Symptoms of Abdominal Compartment Syndrome or intra-abdominal hypertension (IAH), may initially be shortness of breath and reduced urine output, followed by abdominal pain, increase in abdominal girth, melena, nausea and vomiting. Complications from abdominal compartment syndrome include decreased cardiac output, atelectasis, acute kidney injury and mesenteric ischemia. [146]
4.3.5
Lower Extremity Ischemia / Acute Limb Ischemia (ALI)
Incidence of endograft limb occlusion leading to ischemia is up to 7%. [33]
The third most common reason for readmission post EVAR; 60% occurring within 6 months of EVAR (Wang, 2017). Risk factors for limb occlusion: small or tortuous iliac artery, and significant thrombus load within the abdominal aneurysm sac. [147]
ALI involves an abrupt hypoperfusion of the limb, usually occurring in less than two weeks post EVAR, caused by thrombus, embolus or trauma. The following symptoms (“6 Ps”) can occur in ALI: pain, pulselessness, paresthesias, pallor, poikilothermia (cold sensation) and paralysis. [10, 17, 24, 148]
Evaluate for graft limb occlusion if patient develops new-onset of lower extremity ischemia, claudication or reduction in femoral pulse or ABI post EVAR. [11]
Observe for Blue Toe Syndrome- characterized by tissue ischemia due to cholesterol or atherothromboli embolization that can be dislodged when EVAR device is inserted, leading to the occlusion of small vessels and causing a mottling or cyanosis of the digits. [149]
Compartment Syndrome-results from edema within the osteofascial compartments of the limb causing intracompartmental pressure resulting in compression of vascular structures leading to limb ischemia. [17]
4.3.6
Aortoenteric Fistula (AEF)
Incidence of endograft infection is 0.4–3% and is associated with a mortality rate of 25–50%. [33]
Usually occurs later, most commonly involves the duodenum but can be any part of large or small intestines. Signs include upper gastrointestinal (GI) bleeding or herald bleeding. Any GI bleed should prompt further investigation to rule out AEF (Class 1). [11]
4.3.7
Post Implantation Syndrome (PIS) [11, 150]
Symptoms: fever >100.4 degrees Fahrenheit for > one day and leukocytosis (WBC >12,000 with negative blood cultures). Patient should report fevers of this magnitude/duration to the EVAR physician
Systemic inflammation may be demonstrated by elevated levels of inflammatory markers such as high sensitivity C-reactive protein (hs-CRP) and interleukin 6 (IL-6).
Occurs in approximately 35% of EVAR patients, may increase major adverse cardiac events (MACE), leading to prolonged hospitalization
May be associated with polyester stent grafts, heart failure, preoperative leukocytosis, and new thrombus formation within the excluded aneurysm sac
Data regarding the exact frequency, effect on adverse events rates and treatment are lacking
4.3.8
Spinal Cord Ischemia
Assess for neuromotor deficits: check lower extremity strength/mobility and quality of bladder control.
Spinal cord ischemia (SCI) can occur as a result of microthromboemboli or if the spinal arteries are occluded by the stent graft. [131] There is greater risk for SCI if the EVAR patient has had prior TEVAR. [151]
4.3.9
Acute Kidney Injury
4.3.10
Cardiopulmonary Complications
Incidence of MI or respiratory compromise is 3–12 % post EVAR. [33]
4.4. Diabetic Patient Management
Maintain hospitalized patient glucose between 140–180. [97]
In diabetic patients, provide glucose monitoring through a point-of-care device at intervals as specified per institutional protocol.
For patients with diabetes or hyperglycemia (serum glucose >140 mg/dL) perform an A1C before the procedure if not performed in the prior 3 months. [97]
Though not defined in EVAR literature, elevated blood sugars >180 mg/dL in the first 48 hours is an independent predictor of SSI. [137, 153]
Administer insulin protocols allowing for predefined adjustments in the insulin dosage based on glycemic fluctuation
Initiate insulin therapy for persistent hyperglycemia starting at a threshold >180 mg/dL (10.0 mmol/L). [97]
Use of insulin sliding scale alone is discouraged. [97]
Noncritically ill patients, who have good nutritional intake, should be given basal plus bolus correction insulin; short acting, scheduled meal time insulin may be indicated as well. [97]
Adjust insulin regimens if peri-procedural glucocorticoid therapy is utilized (e.g., patient pre-medicated for contrast allergy or patient on chronic therapy). [97]
Home Medications:
Metformin is held 48 hours after the procedure due to risk of peri-procedural contrast induced nephropathy (CIN), nausea, vomiting, dehydration and lactic acidosis [131, 154]as long as renal function remains stable (<25% increase in creatinine) and GFR does not drop below 30/ml/min/1.73 m2. [11]
Sodium–glucose cotransporter 2 (SGLT2) inhibitors cannot be recommended for hospital use as they may cause hypotension, diabetic ketoacidosis (even without significant hyperglycemia), urinary tract infection and more; use of GLP 1 inhibitors are also avoided since they are known for unfavorable gastrointestinal symptoms. [97]
Patients with hyperglycemia or diabetes, who are discharged with new medication regimens, should see their diabetes provider within 1–2 weeks’ after discharge; otherwise, 1 month follow up advised. [96]
4.5. Patient Positioning and Activity Level
4.5.1
Provide VTE Prophylaxis (Refer to section 4.1.7)
4.5.2
Encourage incentive spirometry to minimize post-procedural pulmonary complications; respiratory failure is not common after EVAR but a significant number (2.9%) still experience respiratory compromise. [155]
4.5.3
Sit the patient up in bed several hours after return to the unit the same evening following a routine morning EVAR, via open femoral exposure. [131]
4.5.4
Discontinue as ordered invasive lines and monitors on the first post-procedural day. [131]
4.5.5
Ambulation/Activity:
Maintain bedrest for 4 to 8 hours after sheath removal with manual compression. [124] If an open cut down approach is utilized for graft deployment; bedrest time is dependent upon the physician preference.
- Regardless of femoral cut down or percutaneous access, duration of bedrest restriction and time to ambulation will vary by provider and institution
- There are no research studies specifying the precise bedrest time following EVAR when performed via cut down or percutaneous approach. Riles [156] indicates a bedrest requirement of 2–4 hours following use of a percutaneous closure device while Naidu [124] notes that ambulation can occur 4 to 8 hours after manual compression
- The clinician should be mindful of patient factors that may delay ambulation (and a subsequent early discharge); these may include anesthetic levels, need for anticoagulation, administered antiplatelet or thrombolytic therapy, unstable cardiovascular status, hematoma or bleeding at the closure site, hypotension, pain while walking, and other comorbid conditions requiring observation. [157]
- The Perclose device allows for immediate repeat vascular access should it be necessary (although it has not been studied). [158]
- For the occasional EVAR patient requiring Intensive Care Unit (ICU) care, use of vasoactive medications is not an absolute contraindication to active mobilization (e.g., dangling at edge of bed or walking to chair); rather, changes in condition, direction of overall patient trend and vital signs can be factored into this decision. [159]
- Patients are instructed to avoid strenuous activity for one week and not to drive until pain free and off analgesic medications. Most patients are discharged by post-procedural day two and feel fully recovered within 1 to 2 weeks. [131]
4.6. Post Procedure Medications
4.6.1
Home Medications:
Most oral medications (non-diabetic) can be resumed several hours after the procedure once oral intake is tolerated. [91, 160]
Give a parenteral formulation of an oral beta blocker if it was prescribed prior to EVAR until the oral regimen can be resumed; abrupt discontinuation of this class of medication can have potential adverse effects including myocardial ischemia, rebound tachycardia and uncontrolled hypertension.
Continue alpha-2 agonists if previously prescribed (e.g., clonidine) in the perioperative period as several trials have reported a reduced incidence of cardiac events in patients who undergo vascular procedures with known CAD. [79]
Restart angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) postoperatively once euvolemia is achieved. [11]
4.6.2
Other Post Procedural Medications:
Administer appropriate interventions to control pain, anxiety, hypoxia and hypothermia. These are fundamental factors in maintaining normotension. [91]
- Hypertension:
- Parenteral antihypertensive alternatives include enalapril, nicardipine, or verapamil. Labetalol, nitroglycerin, and nitroprusside are also appropriate medications for persistent mean BP >90mmHg [161]
- Some recommend maintaining a MAP of 75 to 90 mm Hg in the initial forty-eight hours post EVAR as this resulted in a lower incidence of Type II endoleaks 7 days postoperative and improved AAA sac shrinkage at twelve months. [162]
- Intravenous hydralazine should be avoided in most patients with ischemic heart disease because it can cause reflex tachycardia and cardiac ischemia.
- Hypotension:
- Post-procedural hypotension can occur as a result of blood or fluid loss and the effects of vasoactive anesthetic medications. However, unlike open AAA repair, EVAR patients rarely require large amounts of IV fluids and blood products. [131]
- Hypotension should prompt evaluation for occult bleeding by performing CT scan. See also section 4.3.3
A bolus of intravenous calcium may be required if patient is hypo-calcemic (e.g., from use of heparin or many blood products preserved with citrate. [163]
4.6.4
VTE Prophylaxis: refer to 4.1.7
4.6.5
Anti-Coagulants:
Vitamin K antagonists (VKA’s)-Resume 12 to 24 hours after the procedure; these agents take several days for their anticoagulant effect to develop (Class IV). [102]
Resume dual anticoagulants (DOACs) such as dabigatran, rivaroxaban, apixaban, and edoxapan postoperatively when there is complete hemostasis and when oral therapy is tolerated.
DOACs have shorter half- lives and carry an increased risk of bleeding when therapeutic anticoagulation is utilized in the first 48–72 hours after a procedure.
Dabigatran, eliquis and rivaroxaban now have reversal agents should major bleeding occur with one of these newer therapies. DOAC dose adjustments may be necessary if the EVAR procedure is complicated by AKI and rivaroxaban may need to be discontinued if a patient develops hepatic impairment. [101]
4.6.6
Pain Management:
Provide adequate relief of pain post-procedure
Multimodal analgesia is recommended for acute postoperative pain. [164] This method combines pain relievers from two or more different drug classes or anesthesia techniques for analgesia and often allows for lower opioid dosing.
Effective analgesia is important as it may reduce the incidence of myocardial ischemia. [165]
In addition, poor postoperative analgesia can lead to poor patient outcomes, increased risk of readmission, increased health care costs, and patient dissatisfaction. [166]
- Nonsteroidal Anti-Inflammatory Drugs:
- Chaer [30] briefly states that nonsteroidal anti-inflammatory drugs (NSAIDS) and/or opioids are mainly used to control postoperative EVAR pain. However, these agents need to be used judiciously, especially in the early post-procedural period.
- Naughton [167] advises withholding NSAIDS for at least 24–48 hours before and after any contrast dye administration and monitoring renal function 24–48 hours post procedure. Patient risk factors for drug induced nephrotoxicity are age >60 years, underlying renal insufficiency, volume depletion, diabetes, heart failure, and sepsis.
- Perazella [168] also indicates an increased risk of nephrotoxicity when NSAIDS are concurrently administered with radiocontrast.
Groin incisions generally cause minimal discomfort. Assess pain location, quality, intensity, effects on function and aggravating/alleviating factors. Institute pain management strategies. Assess response to pain medication. [17]
-
Opioid Administration:
Administer opioid medications for severe post-procedural pain- When acute pain is severe enough for opioid administration, the lowest effective dose of an immediate release opioid should be prescribed and three or fewer days is generally adequate; rarely will more days be necessary for control of any acute pain. [166]
- Refer to the Washington State Interagency Guideline on Opioid Prescribing [169] and the updated Practice Guidelines by the American Society of Anesthesiologists Task Force on Acute Pain Management [170] for further guidance relating to opioids. Important points include checking your state’s PDMP (Prescription Drug Monitoring Program), assessing for preexisting pain or anxiety conditions, setting reasonable patient expectations regarding pain, teaching the patient about safe keeping of opioids, and more. One investigator [171] described an average opioid consumption that was 2.64 times greater in patients undergoing open AAA repair versus EVAR.
Administer an around the clock regimen of a non-opioid agent such as acetaminophen. [170] Initially, consider a patient-controlled analgesia (PCA) device for the EVAR patient with open femoral exposure as to avoid delays in pain medication administration; basal rates are not recommended. [164]
Consider employing non-drug nursing techniques as well, including ice (wrapped in a towel for up to 20 minutes at a time), guided imagery, repositioning, and distraction, to name a few.
4.6.7
Risk Factor Modification
Mitigating risk factors for atherosclerosis should be continued post EVAR to reduce cardiovascular risk.
4.6.7.1
Smoking Cessation Medications:
Smoking cessation is indicated for all patients and they should be asked about smoking status at every visit; if current tobacco use is confirmed, advise client to quit and offer behavioral and pharmacotherapy treatment and/or referral to a smoking cessation program. [173] Smoking cessation medications include varenicline, bupropion, and nicotine replacement therapy.
Patients who smoke can benefit from transdermal nicotine replacement to alleviate symptoms of withdrawal.
4.6.7.2
Dyslipidemia Medications:
Administer moderate to high intensity statin medications in patient s with PAD, including those patients with an AAA since patients with AAA have risk factors for cardiovascular disease to include dyslipidemia. [10, 174]
The exceptions to this recommendation are patients > 80 years, or who are maintained on hemodialysis, or who have New York Heart Association (NYHA) class II to IV heart failure. [174, 175]
4.6.7.3
Antiplatelet therapy:
4.6.7.4
Hypertension Management:
Administer hypertensive medications as ordered. See also 4.6.2. In patients with established cardiovascular disease, maintain the blood pressure according to the American College of Cardiology/American Heart Association pharmacologic guidelines when systolic blood pressure (SBP) is ≥ 130 mm Hg or diastolic blood pressure (DBP) is ≥ 80 mm Hg. [161]
Utilize appropriate blood pressure (BP) measurement techniques which include using proper sized cuff, and automated oscillometric blood pressure device, and having the patient sit for at least five minutes before measurement. [56, 57]
Immediate postoperative EVAR BP parameters will be determined by the surgeon/interventionalist.
4.6.7.5
Diabetes Management:
Restart Metformin 48 hours post EVAR (Refer to sections 2.6.4 & 4.4)
4.7. Nutrition and Elimination
Post-procedural nutrition and elimination requirements are dependent upon whether AAA was ruptured or not and the healthcare facility protocol. Routine nasogastric decompression is not recommended. [11]
4.7.1
Non-Ruptured AAA:
- Start ice chips once the patient has recovered from anesthesia. Diet is advanced to the preoperative diet either the evening of the procedure or the next day per order once complaints of nausea and vomiting have subsided.
- EVAR patients typically do not have an ileus due to absence of visceral manipulation. [11]
Monitor intake and output every 8 hours.
Discontinue the urinary catheter if present no later than the second postoperative day on an elective repair as to prevent a catheter-associated urinary tract infection (CAUTI), counting the procedure day as day zero (Class IV). [134]
4.7.2.
Ruptured AAA:
- Clarify with the physician when to restart diet.
- This may be slower due to physician preference and risk of abdominal compartment syndrome
Monitor intake and output at least initially every 8 hours.
Discontinue the urinary catheter once risk for abdominal compartment syndrome resolves.
4.7.3
Constipation
Constipation is defined as absence of bowel movements for 3, 6 or 9 days and requiring treatment with laxatives or enemas. [178]
Administer prescribed stool softeners and/or laxatives for complaints of constipation.
Once pre-operative diet is resumed, encourage fluid intake and fiber-rich food. Promote activities including getting out of bed into a chair and ambulation when ordered.
Typically, constipation is not present during hospitalization as the EVAR post-procedure length of stay is often 2 days or less. If constipation occurs, it is the result of pre-existing conditions, general anesthesia, alterations in diet and fluid intake, opioids and inactivity, all which contribute to a decrease in peristalsis. [85, 179]
4.8. Post-Procedural Diagnostic Studies
Obtain ordered diagnostic studies per physician preference.
4.8.1
Surveillance:
Performing short and long-term EVAR image surveillance is needed due to an overall complication rate of up to 30% and an annual rate of aneurysm rupture up to 1%. [40, 139]
Post procedure Diagnostic Studies: Long-term follow-up is recommended after EVAR for early detection and management of complications; these include endoleak, graft translocation, thrombosis and infection.
Periodic long-term surveillance imaging should be performed to monitor for endoleak, confirm graft position, document shrinkage or stability of the excluded aneurysm sac, and determine the need for further intervention in patients who have undergone endovascular repair of infrarenal aortic aneurysms (Class 1). [28]
Perform CT scan at 1 month, 6 months and then annually if no endoleak or sac size increase is detected.
Baseline CT scanning is recommended 1 month, 6 months and 12 months post EVAR, consider more frequent imaging if endoleak detected at 1 month. [180]
AAA duplex as an alternate imaging modality at 12 month intervals after EVAR if no if endoleak or sac enlargement on diagnostic studies in first year. [180]
AAA duplex and non-contrast CT scanning as an alternate imaging modality in patients with contraindications to iodinated contrast agents to evaluate aneurysm sac and stent fracture. These modalities can often evaluate the aneurysm sac and detect stent fracture. [180]
If Type II endoleak is detected at 1-month surveillance, recommend both abdominal duplex and contrast-enhanced CT. [11]
If Type II endoleak is associated with a stable or shrinking aneurysm sac, abdominal duplex is recommended at 6-month intervals for 24 months, then annually thereafter. [11]
Noncontrast-enhanced CT is recommended of the entire aorta at 5-year intervals after EVAR. [11, 180]
4.8.2
Alternate Surveillance:
Perform alternate surveillance measures when there is concern for radiation exposure and the use of nephrotoxic agents.
- Abdominal Duplex:
- If no sac expansion or endoleak is seen during the first year, follow-up with ultrasound (duplex) scanning may be reasonable.
- Combined with a non-contrast CT scan, ultrasound detection of endoleak may be a very attractive approach in patients with renal insufficiency
- Ultrasound limitations include results dependent upon the skill of the technologist; study is difficult in persons with a large body habitus; and graft integrity, migration and kinking are poorly evaluated with this modality. [139]
- MR Angiogram:
- MRA with gadolinium contrast can also be used to detect graft patency, thrombosis, endoleak, and aortic rupture. MRA can be used in patients with Zenith grafts with 1.5 T magnets. MRA is contraindicated in persons with GFR < 30 mL/min due to risk of nephrogenic system fibrosis.
- Angiography:
- Angiography can be useful in identifying an endoleak, which is not seen on CTA or other diagnostic studies. Angiography is the means by which endoleaks can be treated with stent grafts, glue and coils.
4.9. Post-Procedural Patient Education
Provide discharge patient education regarding the following as indicated by institutional protocol and physician preference. Educate patients/families about need for life-long AAA surveillance as EVAR complications can be treated with minimally invasive procedures to prevent rupture. A patient education tool for EVAR is available [131]. Patient education also includes:
4.9.1
Continue prescribed medications with an emphasis on compliance as a means to reduce complications and disease progression.
4.9.2
Activity and incisional care to include the following
May shower after discharge at the time specified by the physician. Avoid use of lotions or ointments on the incision. Avoid rubbing the incision. Inspect the incision daily for signs of redness or drainage. Avoid clothing that rubs against the incision until it heals. [181, 182] There is currently no evidence supporting or refuting benefits or harms from early versus delayed bathing in development of surgical/procedural site infections (Class I). [135]
Limiting the amount of weight, the patient may lift is dependent upon physician preference. Clinically, patients are often cautioned against lifting anything heavier than 10 pounds for 48 hours after discharge. [183]
4.9.3
Signs and symptoms to report to the physician [131] :
Cardiopulmonary: dyspnea, chest pain, peripheral edema, productive sputum
Signs of infection: fever, incisional changes
4.9.4
Management of modifiable risk factors:
Smoking cessation counseling/adjunctive options. Refer to section 4.6.4
- Glycemic control
- In patients with diabetes as measured by the glycosylated hemoglobin level (A1c) to be maintained below or around 7% (Class IV). [154] A high A1c level (>7%) may contribute to intimal hyperplasia as it was found to be an independent predictor leading to coronary artery restenosis after stenting. [184] Refer to section 4.4.
To reduce cardiovascular risk as an ourpatient, consideration may be given to add SGLT-2 (sodium-glucose cotransporter-2) inhibitors which impede the SGLT-2 proteins in the renal tubules from reabsorbing glucose back into the bloodstream) or a GLP-1R (glucagon-like peptide-1 receptor) agonist to improve glycemic control (Class IIB). [185]
- Hypertension management:
- Refer to sections 4.6.3 and 4.6.10.
- Provide education regarding lifestyle modifications: smoking cessation, weight reduction, limit sodium intake, avoid use of illicit drugs (cocaine, methamphetamines), limit alcohol consumption, increase physical activity level, and stress reduction (Class IV). [186]
- Cholesterol reduction through dietary modifications, disease control and medication compliance to reduce the risk of atherosclerotic disease progression and stenosis. [174] Refer to section 4.6.8
4.9.5
Prevention of Infection-Antimicrobial Administration:
Provide antibiotic prophylaxis prior to any dental procedure involving the manipulation of the gingival or periapical region of teeth or perforation of the oral mucosa, including scaling and root canal procedures to prevent postoperative graft infection regardless of whether done by EVAR or Open Surgical Repair (OSR). [11]
Antibiotic prophylaxis is suggested before respiratory tract procedures, gastrointestinal or genitourinary procedures, and dermatologic or musculoskeletal procedures for any patient with an aortic prosthesis if the potential for infection exists or the patient is immunocompromised. [11]
5.0. Outcomes Reporting
Assist in data collection and reporting of outcomes as indicated based on the institutional quality improvement requirements.
Utilize a standardized registry such as the Vascular Quality Initiative from the Society for Vascular Surgery. [187] Utilize the Vascular Quality Initiative (VQI) Risk Calculator to assess preoperative mortality [11] (see also 1.9.5). This will allow for assessment of risk-adjusted outcomes, more efficient use of resources [188] and benchmark institutional metrics against national results.
Figure A.
Arterial Anatomy
FIGURE B.
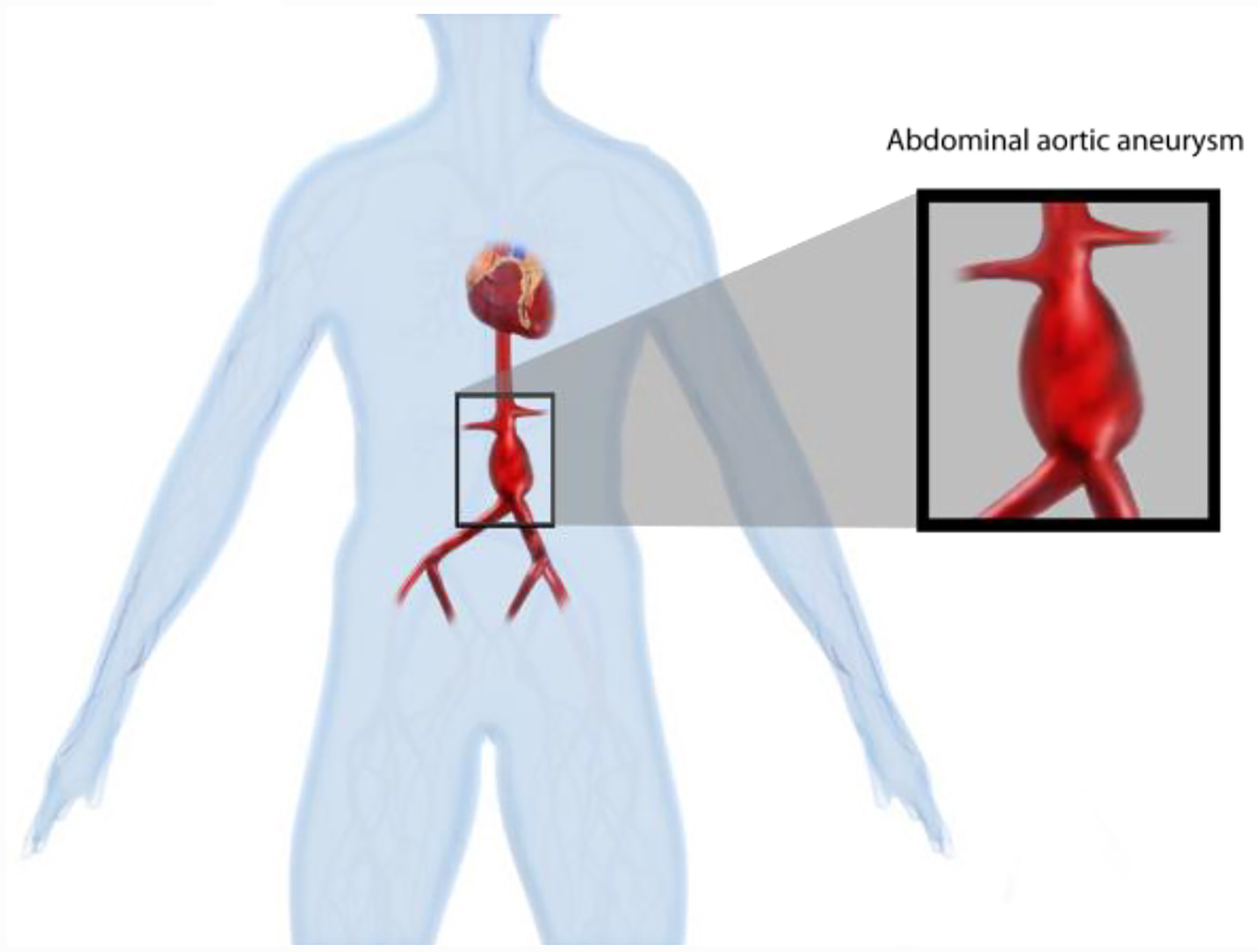
Infrarenal Abdominal Aortic Aneurysm
Figure C.
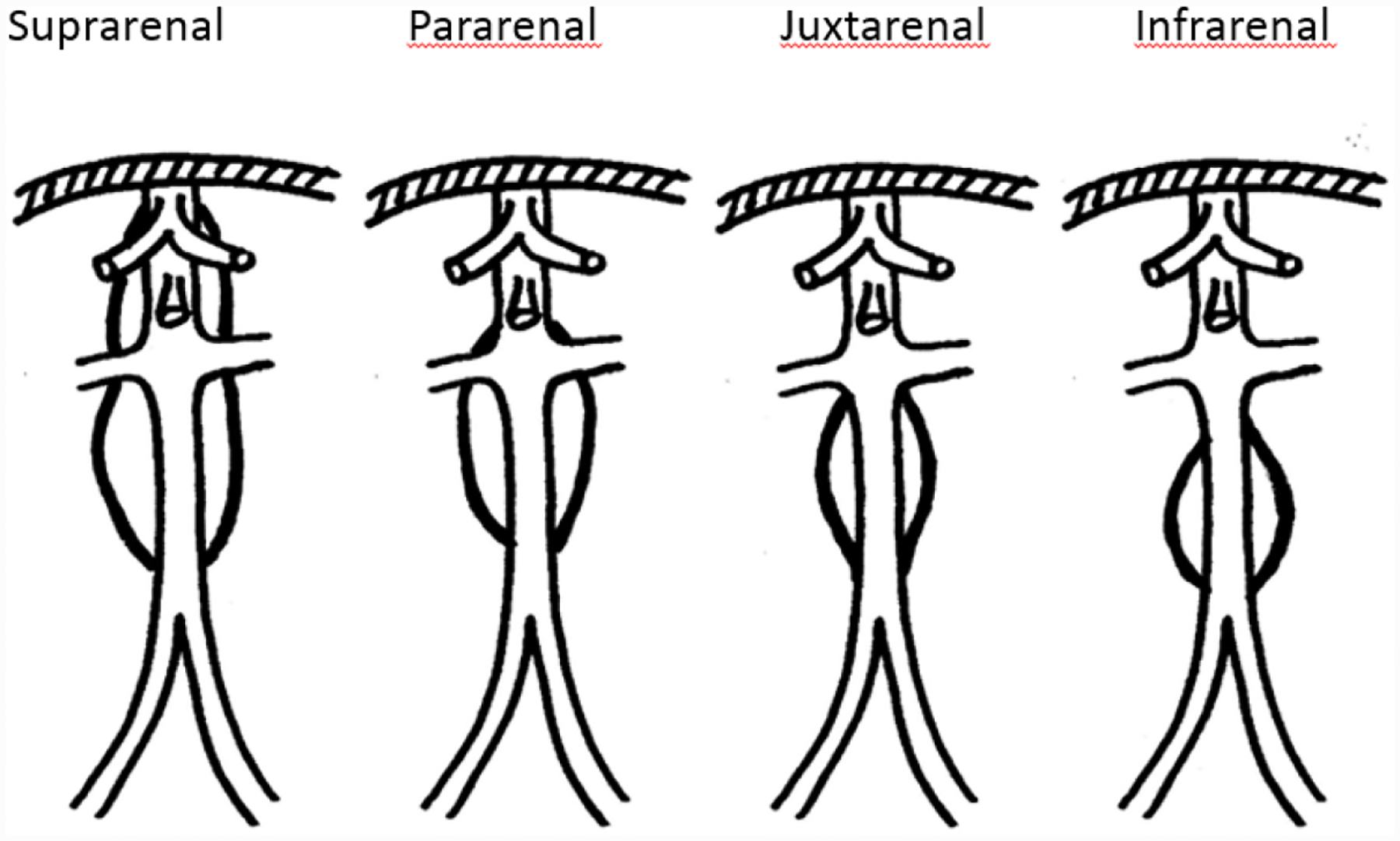
Classification of abdominal aortic aneurysms.
FIGURE E.
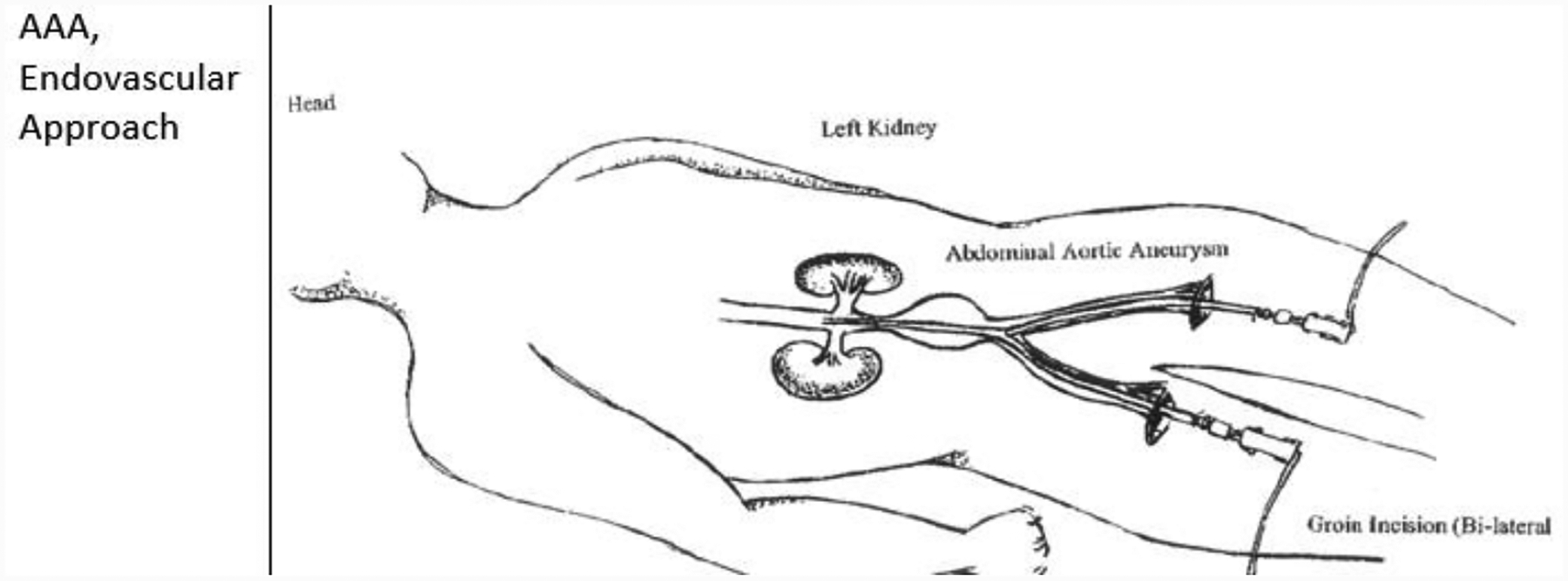
Approach to Endovascular AAA Repair
FIGURE H.
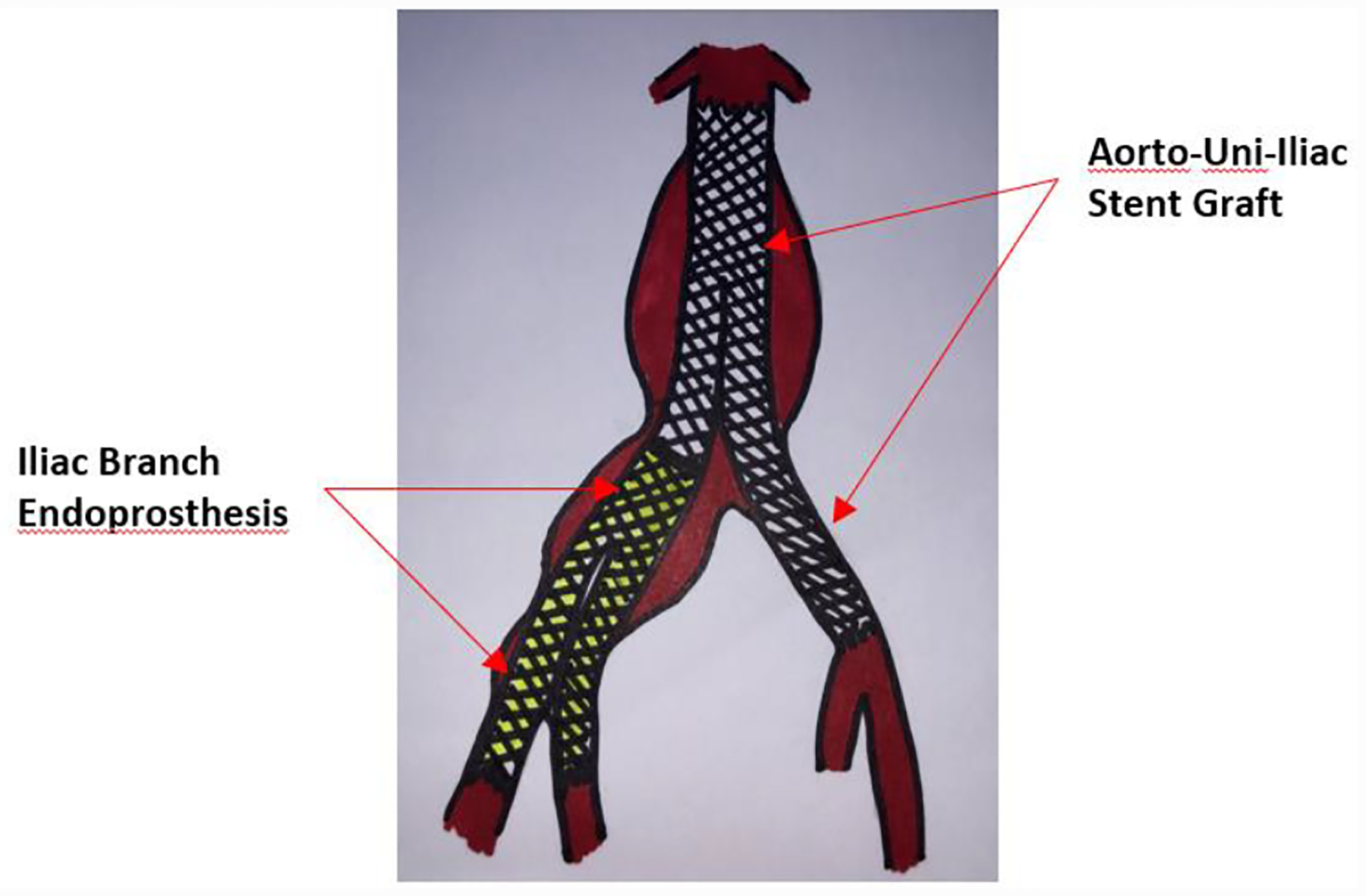
Diagram of Iliac Branch Endoprothesis
FIGURE I.
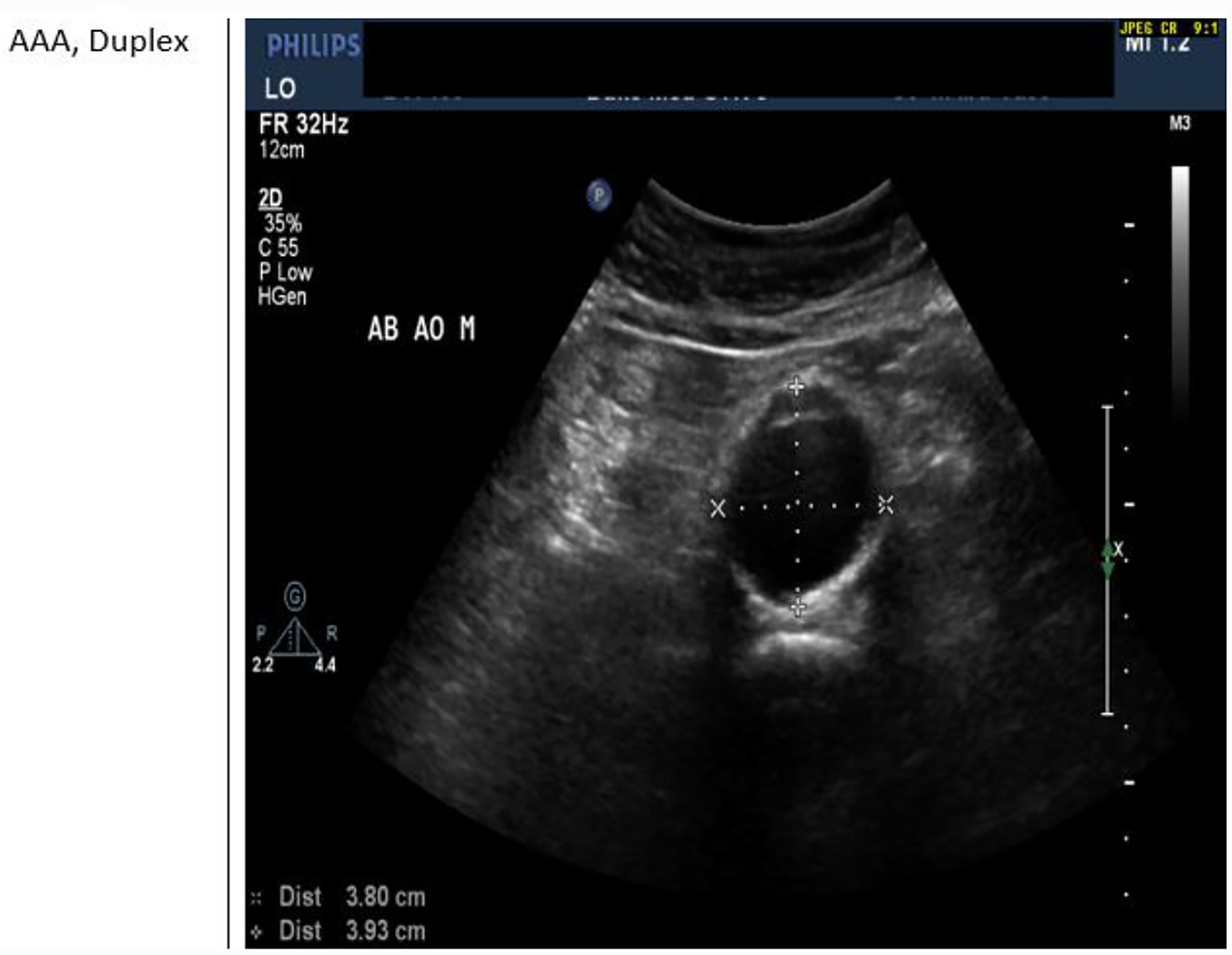
Abdominal Aortic Duplex
FIGURE J.
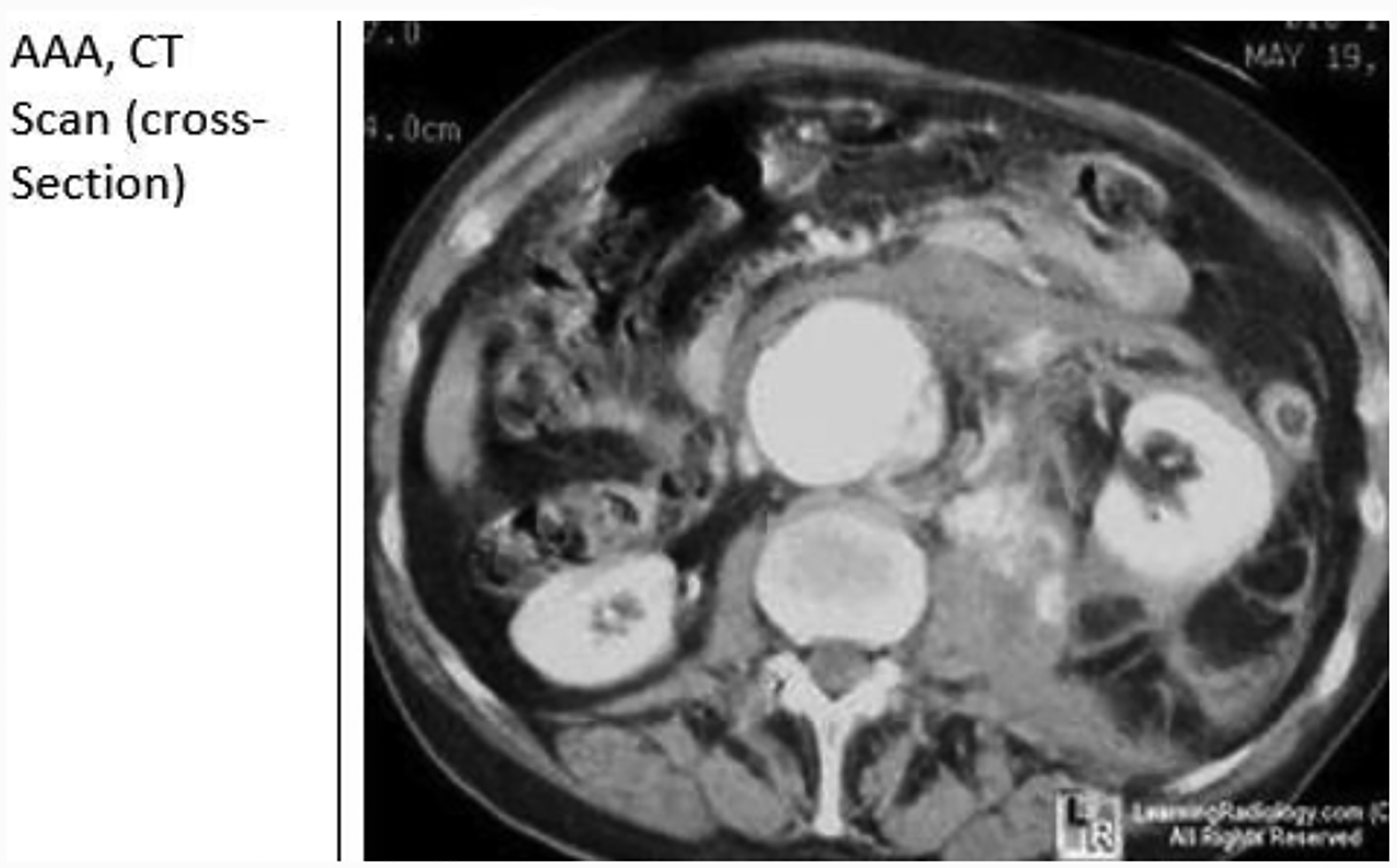
Cross Section of Abdominal Aneurysm CT Scan
FIGURE K.
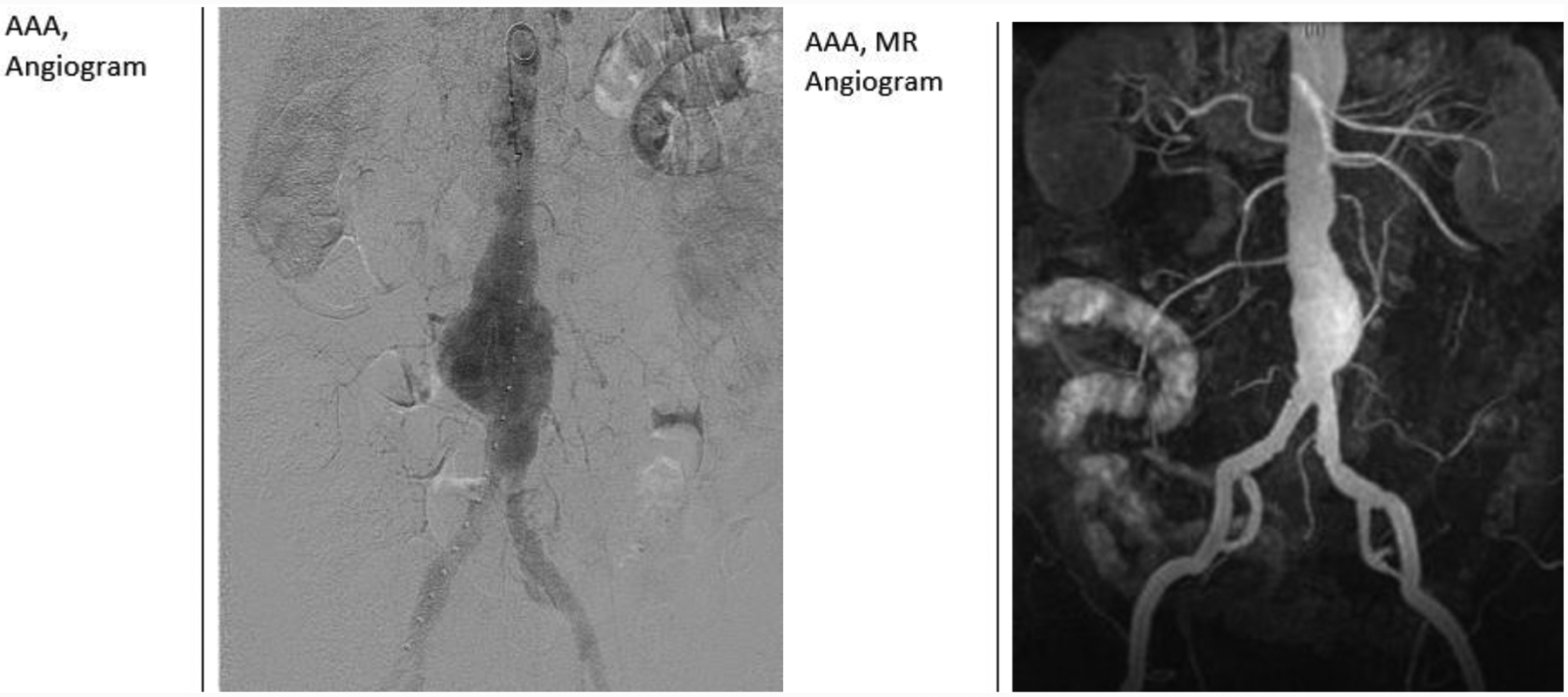
Images of Angiogram (left) and MR Angiogram (right)
Appendix A: Nursing Activities Checklist in Caring for EVAR Patient
| EVAR Nursing Activities** | |||
|---|---|---|---|
| Pre-Operative | Yes | No | NA |
| • Assess for co-morbid conditions, risk factors & previous abdominal/groin surgery. Notify MD if present | |||
| • Obtain baseline vital signs (BP both arms, apical/radial pulse, respirations, SpO2, temperature) | |||
| • Obtain list of current medications & assess for any allergies | |||
| • Perform a targeted physical assessment. Notify MD of abnormal results. | |||
| • Verify ordered diagnostic tests are done. Notify MD of any abnormal results. | |||
| • Start 1–2 peripheral IV sites according to institutional protocol. | |||
| • Cleanse skin using chlorhexidine shower or disposable wipes. | |||
| • Maintain NPO status for at least 4 hours prior to the procedure | |||
| • Provide patient education regarding medications, risk factors, peri-procedural/post-procedural care | |||
| • Record baseline pedal pulse exam | |||
| • Administer medications as ordered (antiplatelet, lipid lowering, antihypertensives excluding diuretics). Withhold oral hypoglycemic agents the morning of the procedure. Administer insulin based upon institutional protocol. Withhold antithrombotic medications as specified. | |||
| • Assist surgeon/interventionalist as indicated in surgical/procedural site marking. | |||
| • Perform pre-procedure verification process according to institutional protocol. | |||
| • Administer antibiotic as ordered within one (1) hour of incision. | |||
| Intra-Operative | Yes | No | NA |
| • Assemble surgical/procedural instruments per MD preference. | |||
| • Attach ECG monitor, non-invasive BP cuff, SpO2 probe to patient. Obtain baseline BP, heart rate, respirations, SpO2, ECG rhythm. | |||
| • Assist anesthesia as indicated with setup and insertion of arterial line. | |||
| • Assist with positioning of the patient. | |||
| • Monitor vital signs, ECG rhythm and complications during procedure. Notify MD if present. | |||
| • Administer ordered medications as indicated (unfractionated heparin, antihypertensives) | |||
| Post-Operative | Yes | No | NA |
| • Monitor vital signs, ECG rhythm, pain and sedation levels at specified intervals. Include assessment of groin/access site dressing(s) & incision for hematoma or drainage with assessments. Notify MD of vital sign changes or groin hematoma develops. | |||
| • Perform pulse assessment upon arrival and at scheduled intervals | |||
| • Titrate IV antihypertensive medication to targeted systolic or mean arterial blood pressure | |||
| • Titrate oxygen if ordered to maintain a SpO2 of 94% in acutely ill patients & 92% in patients at risk for hypercapneic respiratory failure | |||
| • Maintain patent IV site and continuous ECG monitoring until discharge or as specified by the MD | |||
| • Remove any groin/access incision dressings at time interval specified. | |||
| • Keep the head of the bed at 45 degrees & maintain bed-rest for specified time interval | |||
| • Resume clear liquids & progress diet when ordered. | |||
| • Assess for post-procedure complications – notify MD immediately if any occur | |||
| • Record post-procedural pedal pulse exam | |||
| • Provide patient education regarding risk factor modification, medications, activity restrictions, incision care, signs and symptoms to report to MD | |||
| • Administer post-procedure medications (antiplatelet agents, antihypertensives). Follow institutional protocol for oral antithrombotics. If diabetic: hypoglycemics may be restarted the morning after the procedure. If angiographic confirmation of revascularization performed during the procedure, withhold metformin/derivatives for 48-hours after the procedure. | |||
| • Schedule follow up completion imaging studies (such as CTA or Abdominal Duplex) as ordered | |||
| • Assist in data collection/outcome reporting as indicated based upon institutional requirements | |||
These are general recommendations – follow your healthcare facility protocol
References
- 1.Smith D, 2009 clinical practice guideline for patients undergoing endovascular repair of abdominal aortic aneurysms (AAA). Journal of Vascular Nursing, 2009. 27(2): p. 48–63. [DOI] [PubMed] [Google Scholar]
- 2.Melnyk BF-OE, Making the case for evidence-based practice and cultivating a spirit of inquiry, in Evidence-based nursing practice in nursing and healthcare: a guide to best practice. 2005, Lippincott, Williams & Wilkins: Philadelphia: p. 3–24. [Google Scholar]
- 3.Payne MM, Charles Theodore Dotter. The father of intervention. Texas Heart Institute journal, 2001. 28(1): p. 28–38. [PMC free article] [PubMed] [Google Scholar]
- 4.Palmaz J Julio Palmaz papers, University of Texas Health Sciences Center 2012; Available from: https//legacy.lib.utexas.edu/taro/uthscsa/00022/hscsa-00022.html.
- 5.Parodi JC, Palmaz JC, and Barone HD, Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg, 1991. 5(6): p. 491–9. [DOI] [PubMed] [Google Scholar]
- 6.Jackson BM and Carpenter JP, Devices used for endovascular aneurysm repair: past, present, and future. Seminars in interventional radiology, 2009. 26(1): p. 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White GH, y.W., May J, Stephen MS, Waugh RC, A new nonstented balloon-expandable graft doe straight or bifurcated endoluminal bypass. Journal of Endovascular Surgery, 1994. 1: p. 16–24. [DOI] [PubMed] [Google Scholar]
- 8.Ivancev K, et al. , Abdominal aortic aneurysms: experience with the Ivancev-Malmo endovascular system for aortomonoiliac stent-grafts. J Endovasc Surg, 1997. 4(3): p. 242–51. [DOI] [PubMed] [Google Scholar]
- 9.Ohki T, et al. , Varying strategies and devices for endovascular repair of abdominal aortic aneurysms. Semin Vasc Surg, 1997. 10(4): p. 242–56. [PubMed] [Google Scholar]
- 10.Gerhard-Herman MD, et al. , 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Chaikof EL, et al. , The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of vascular surgery, 2018. 67(1): p. 2–77.e2. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf SW, et al. , Emergency endovascular repair of leaking aortic aneurysm. Lancet (London, England), 1994. 344(8937): p. 1645. [DOI] [PubMed] [Google Scholar]
- 13.May J, et al. , Concurrent comparison of endoluminal versus open repair in the treatment of abdominal aortic aneurysms: analysis of 303 patients by life table method. Journal of vascular surgery, 1998. 27(2): p. 213–1. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi SA Abdominal aortic aneurysm. 2019; Available from: https://www.medscape.com/answers/1979501-30941/what-is-the-prevalence-of-abdominal-aortic-aneurysm-aaa-in-the-us
- 15.Jahangir E, et al. , Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18 782 persons aged above 65 years in the Southern Community Cohort Study. Journal of epidemiology and community health, 2015. 69(5): p. 481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar AOK, Handbook of endovascular interventions. 2012: Springer Science and Business Media. [Google Scholar]
- 17.Christensen C,LP, Core curriculum for vascular nursing 2014: Society for Vascular Nursing, Lippincott Williams and Wilkins. [Google Scholar]
- 18.Songür A, et al. , Abdominal Aorta and Its Branches: Morphometry - Variations In Autopsy Cases. European Journal of General Medicine, 2010. 7(3): p. 321–325. [Google Scholar]
- 19.Johnston KW, et al. , Suggested standards for reporting on arterial aneurysms. Journal of Vascular Surgery, 1991. 13(3): p. 452–458. [DOI] [PubMed] [Google Scholar]
- 20.Wanhainen A, et al. , European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. European Journal of Vascular and Endovascular Surgery, 2019. 57(1): p. 8–93. [DOI] [PubMed] [Google Scholar]
- 21.Cheung J Epidemiology, risk factors, pathogenesis, and natural history of abdominal aortic aneurysm. 2017. 01/02/2020 [cited 2020 01/10/2020]; Available from: https://www.uptodate.com/contents/epidemiology-risk-factors-pathogenesis-and-natural-history-of-abdominal-aortic-aneurysm.
- 22.Jim JT, R. Clinical features and diagnosis of abdominal aortic aneurysms. 2018. 10/15/2019 [cited 2020 01/10/2020]; Available from: https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-abdominal-aortic-aneurysm?sectionName=Ruptured%20AAA&topicRef=15191&anchor=H15830929&source=see_link#H15830929.
- 23.Norman PE, et al. , Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ (Clinical research ed.), 2004. 329(7477): p. 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboyans V, et al. , 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). European heart journal, 2018. 39(9): p. 763–816. [DOI] [PubMed] [Google Scholar]
- 25.USPSTF. USPSTF Releases 2014 guidelines to clinical prventive services. 2014. July 2014 [cited 2020 1/10/2020]; Available from: https://www.uspreventiveservicestaskforce.org/Announcements/News/Item/uspstf-releases-2014-guide-to-clinical-preventive-services.
- 26.Larsson E, et al. , Analysis of aortic wall stress and rupture risk in patients with abdominal aortic aneurysm with a gender perspective. Journal of vascular surgery, 2011. 54(2): p. 295–9. [DOI] [PubMed] [Google Scholar]
- 27.Dalman RL, Mell M Management of asymptomatic abdominal aortic aeurysm. 2019. [cited 2020 01/10/2020]; Available from: https://www.uptodate.com/contents/management-of-asymptomatic-abdominal-aortic-aneurysm?sectionName=INTRODUCTION&topicRef=15191&anchor=H611107773&source=see_link#H611107773.
- 28.Rooke TW, et al. , 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 2011. 58(19): p. 2020–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo RC, et al. , Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg, 2013. 57(5): p. 1261–8, 1268.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaer R Endovascular repair of abdominal aortic aneurysm. 2019. [cited 2020 01/10/2020]; Available from: https://www.uptodate.com/contents/endovascular-repair-of-abdominal-aortic-aneurysm.
- 31.Roche-Nagle G, Hazel M, and Rajan DK, Financial Impact of PEVAR Compared With Standard Endovascular Repair in Canadian Hospitals. Can Assoc Radiol J, 2018. 69(2): p. 215–219. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Liu J, and Li Y, Femoral Artery Closure Versus Surgical Cutdown for Endovascular Aortic Repair: A Single-Center Experience. Med Sci Monit, 2018. 24: p. 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaer R Complications of endovascular abdominal aortic repair. 2019. [cited 2020 1/10/2020]; Available from: https://www.uptodate.com/contents/complications-of-endovascular-abdominal-aortic-repair.
- 34.Bertges DJ, et al. , The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg, 2016. 64(5): p. 1411–1421.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballard DJ, et al. , Clinical practice change requires more than comparative effectiveness evidence: abdominal aortic aneurysm management in the USA. J Comp Eff Res, 2012. 1(1): p. 31–44. [DOI] [PubMed] [Google Scholar]
- 36.Karthikesalingam A, et al. , Locoregional anesthesia for endovascular aneurysm repair. J Vasc Surg, 2012. 56(2): p. 510–9. [DOI] [PubMed] [Google Scholar]
- 37.Sternbergh III W, Technique: endovascular aneurysm repair, in Rutheford’s Vascular Surgery, Cronenwett KJJ, Editor. 2014, Elsevier: Philadelphia: p. 1338–1356. [Google Scholar]
- 38.Society of Cardiovascular Angiography and, I. Educational guidelines for invasive cardiovascular technology personnel in the cardiovascular catheterization laboratory. 2015. April 30 2015 [cited 2020 1/10/2020]; Available from: https://www.cathlabdigest.com/article/Society-Invasive-Cardiovascular-Professionals-New-2015-Educational-Guidelines-Invasive.
- 39.Duffy JM, Rolph R, and Waltham M, Stent graft types for endovascular repair of abdominal aortic aneurysms. Cochrane Database Syst Rev, 2015(9): p. Cd008447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tefera GM, J., Aortic stents and stent grafts, in Rutherfords Vascular Surgery, Cronenwett KJJ, Editor. 2014, Elsevier: Philadelphoa: p. 1431–1442. [Google Scholar]
- 41.Matsumura J, J. C, Endovascular repair of abdominal aortic aneurysm, in Endovascular Surgery, Moore W, Editor. 2011, Elsevier: Philadelphia: p. 467–476. [Google Scholar]
- 42.Norris E, Anesthesia for vascular surgery, in Miller’s Anesthesia, Miller R, Editor. 2014, Elsevier Saunders: Philadelphia: p. 2096–2105. [Google Scholar]
- 43.Nelson PR, et al. , A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg, 2014. 59(5): p. 1181–93. [DOI] [PubMed] [Google Scholar]
- 44.Vatakencherry G, et al. , Percutaneous access planning, techniques and considerations for endovascular aortic repair (EVAR). Cardiovasc Diagn Ther, 2018. 8(Suppl 1): p. S184–s190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eagleton M, Upchurch G, Endovascular therapy for abdominal aortic aneurysms, in Vascular Medicine: A Companion to Braunwald’s Heart Disease, Creager JBM, Loscalzo J, Editor. 2012, Elsevier Saunders: Philadelphia: p. 493–505. [Google Scholar]
- 46.Krajcer Z and Matos JM, Totally percutaneous endovascular abdominal aortic aneurysm repair: 30-day results from the independent access-site closure study of the PEVAR trial. Tex Heart Inst J, 2013. 40(5): p. 560–1. [PMC free article] [PubMed] [Google Scholar]
- 47.Ullery BW, Snorkel/chimney versus fenestrated endovascular aneurysm repair: What works and when? Endovascular Today, 2016. 5(3): p. 76–81. [Google Scholar]
- 48.Dossabhoy SS, et al. , Fenestrated endovascular aortic aneurysm repair using physician-modified endovascular grafts versus company-manufactured devices. J Vasc Surg, 2018. 67(6): p. 1673–1683. [DOI] [PubMed] [Google Scholar]
- 49.Eagleton MJ, 10 key lessions learned in complex EVAR. Endovascular Today, 2016(3): p. 56–61. [Google Scholar]
- 50.Glebova NO, et al. , Fenestrated endovascular repair of abdominal aortic aneurysms is associated with increased morbidity but comparable mortality with infrarenal endovascular aneurysm repair. J Vasc Surg, 2015. 61(3): p. 604–10. [DOI] [PubMed] [Google Scholar]
- 51.Oderich G, Fenestrated stent graft repair for complex aneurysms. Endivascular Today, 2013. 5: p. 16–26. [Google Scholar]
- 52.Kansagra K, et al. , Advanced endografting techniques: snorkels, chimneys, periscopes, fenestrations, and branched endografts. Cardiovasc Diagn Ther, 2018. 8(Suppl 1): p. S175–s183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oderich G, de Almeida Sandri G, Tenorio E Techniques of endovascular aortoiliac repair using an iliac branch endoprosthesis. Endovascular Today, 2017. 16(8): p. 17–21. [Google Scholar]
- 54.AORN AORN -Guideline Quick View:Medication Safety. AORN Journal, 2018. 107(1): p. 155–158. [DOI] [PubMed] [Google Scholar]
- 55.Accetta D and Kelly KJ, Recognition and management of the latex-allergic patient in the ambulatory plastic surgical suite. Aesthet Surg J, 2011. 31(5): p. 560–5. [DOI] [PubMed] [Google Scholar]
- 56.Williams JS, Brown SM, and Conlin PR, Videos in clinical medicine. Blood-pressure measurement. N Engl J Med, 2009. 360(5): p. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coogan N, Marra A, and Lomonaco EA, Assessing accurate BP measurement: size and technique matter! Nursing, 2015. 45(4): p. 16–8. [DOI] [PubMed] [Google Scholar]
- 58.Kohlman-Trigoboff D, The missing vital sign: The significance of bilateral arm blood pressures. J Vasc Nurs, 2015. 33(3): p. 127–30. [DOI] [PubMed] [Google Scholar]
- 59.Eagleton MJ and Kang J, Preoperative management. Rutherford’s Vascular Surgery; 8th Edition, 2014: p. 466–479. [Google Scholar]
- 60.Seidel H, Ball J, Dains J, et al. ,, Objective data - physical findings, in Physical Examination Handbook, Seidel HBJDJFJSB and Strward R, Editors. 2010, Mosby-Elsevier: St. Louis: p. 306–314. [Google Scholar]
- 61.Kohlman-Trigoboff D, Utility of the hand-held continuous wave Doppler in the vascular examination: A review. Journal of vascular nursing : official publication of the Society for Peripheral Vascular Nursing, 2016. 34(4): p. 160–162. [DOI] [PubMed] [Google Scholar]
- 62.Wolf YG, et al. , Duplex ultrasound scanning versus computed tomographic angiography for postoperative evaluation of endovascular abdominal aortic aneurysm repair. Journal of vascular surgery, 2000. 32(6): p. 1142–1148. [DOI] [PubMed] [Google Scholar]
- 63.Craig M, Essentials of Sonography and Patient Care. 3rd ed. 2012: Ellsevier Saunders. [Google Scholar]
- 64.Lau C, et al. , Imaging for surveillance and operative management for endovascular aortic aneurysm repairs. Journal of thoracic disease, 2017. 9(Suppl 4): p. S309–S316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu PS and Platt JF, CT angiography in the abdomen: a pictorial review and update. Abdominal imaging, 2014. 39(1): p. 196–214. [DOI] [PubMed] [Google Scholar]
- 66.Shammas NW, et al. , Intravascular Ultrasound Assessment and Correlation With Angiographic Findings Demonstrating Femoropopliteal Arterial Dissections Post Atherectomy: Results From the iDissection Study. The Journal of invasive cardiology, 2018. 30(7): p. 240–244. [PubMed] [Google Scholar]
- 67.Nederkoorn PJ, van der Graaf Y, and Hunink MG, Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review. Stroke, 2003. 34(5): p. 1324–1332. [DOI] [PubMed] [Google Scholar]
- 68.Yuan C, et al. , In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation, 2001. 104(17): p. 2051–2056. [DOI] [PubMed] [Google Scholar]
- 69.Lowe G, Smith RP, and Costabile RA, A catalog of magnetic resonance imaging compatibility of penile prostheses. The journal of sexual medicine, 2012. 9(5): p. 1482–1487. [DOI] [PubMed] [Google Scholar]
- 70.Weidman EK, et al. , MRI safety: a report of current practice and advancements in patient preparation and screening. Clinical imaging, 2015. 39(6): p. 935–937. [DOI] [PubMed] [Google Scholar]
- 71.Grist TM, MRA of the abdominal aorta and lower extremities. Journal of magnetic resonance imaging : JMRI, 2000. 11(1): p. 32–43. [DOI] [PubMed] [Google Scholar]
- 72.Remonda L, et al. , Contrast-enhanced 3D MR angiography of the carotid artery: comparison with conventional digital subtraction angiography. AJNR. American journal of neuroradiology, 2002. 23(2): p. 213–219. [PMC free article] [PubMed] [Google Scholar]
- 73.Lee O-H, et al. , Peripheral artery disease is associated with poor clinical outcome in patients with abdominal aortic aneurysm after endovascular aneurysm repair. International journal of cardiology, 2018. 268: p. 208–213. [DOI] [PubMed] [Google Scholar]
- 74.Beckmann M, et al. , Risk Stratification of Patients with Peripheral Arterial Disease and Abdominal Aortic Aneurysm Using Aortic Augmentation Index. PloS one, 2015. 10(10): p. e0139887–e0139887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta DK, et al. , Heart failure risk across the spectrum of ankle-brachial index: the ARIC study (Atherosclerosis Risk In Communities). JACC. Heart failure, 2014. 2(5): p. 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bader AM, Advances in preoperative risk assessment and management. Current problems in surgery, 2012. 49(1): p. 11–40. [DOI] [PubMed] [Google Scholar]
- 77.Feely MA, et al. , Preoperative testing before noncardiac surgery: guidelines and recommendations. American family physician, 2013. 87(6): p. 414–418. [PubMed] [Google Scholar]
- 78.Fleischmann KE, et al. , 2009 ACCF/AHA focused update on perioperative beta blockade: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation, 2009. 120(21): p. 2123–2151. [DOI] [PubMed] [Google Scholar]
- 79.Fleisher LA, Fleischmann KE, Auerbach AD Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN, 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014. 130(24): p. e278–333. [DOI] [PubMed] [Google Scholar]
- 80.Aggarwal G, et al. , Advantages of type and screen policy: Perspective from a developing country! Asian journal of transfusion science, 2018. 12(1): p. 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poirer P, et al. , Cardiovascular evaluation and management of severely obese patients undergoing surgery. Circulation, 2009. 120: p. 86–95. [DOI] [PubMed] [Google Scholar]
- 82.Ronco M, et al. , Patient education outcomes in surgery: a systematic review from 2004 to 2010. International journal of evidence-based healthcare, 2012. 10(4): p. 309–323. [DOI] [PubMed] [Google Scholar]
- 83.American Society of Anesthesiologists Task, F., Practical advisory for preanesthesia evaluation. Anesthesiology, 2012. 116(3): p. 1–17. [DOI] [PubMed] [Google Scholar]
- 84.Delaisse J, Varaada S, Au S, Pope A, Manders E, Ramirez-Fort MK, Peri-operative management of the patient with body piercings. Journal of Dermatology and Clinical Research, 2014. 2(1). [Google Scholar]
- 85.Slade S, Evidence Summary. Analgesia: narcotic 2016: The Joanna Briggs Institute EBP Database, JBI@Ovid. [Google Scholar]
- 86.Tanner J, Woodings D, and Moncaster K, Preoperative hair removal to reduce surgical site infection. Cochrane Database of Systematic Reviews, 2006. 3: p. 004122. [DOI] [PubMed] [Google Scholar]
- 87.Edminston C, et al. , Evidence for using chlorhexidine gluconate preoperative cleansing to reduce the risk of surgical site infection. AORN, 2010. 92(5): p. 509–518. [DOI] [PubMed] [Google Scholar]
- 88.Ortiz J, et al. , Preoperative patient education: can we improve satisfaction and reduce anxiety? Revista brasileira de anestesiologia, 2015. 65(1): p. 7–13. [DOI] [PubMed] [Google Scholar]
- 89.Saratzis A, et al. , Pharmacotherapy before and after endovascular repair of abdominal aortic aneurysms. Curr Vasc Pharmacol, 2008. 6(4): p. 240–9. [DOI] [PubMed] [Google Scholar]
- 90.Whinney C, Perioperative medication management: general principles and practical applications. Clev Clinic J Med, 2009. 76(4): p. S126–S132. [DOI] [PubMed] [Google Scholar]
- 91.Kuwajerwala N and Schwer W, Perioperative medication management. 2013.
- 92.O’Donnell TFX, et al. , Statin therapy is associated with higher long-term but not perioperative survival after abdominal aortic aneurysm repair. Journal of vascular surgery, 2018. 68(2): p. 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Martino R, et al. , Preoperative management with antiplatelet and statin medication is associated with reduced mortality following vascular surgery. J Vasc Surg, 2014. 59(6): p. 1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez L, Helkin A, and Gahtan V, Dyslipidemia Part 2: Review of Dyslipidemia Treatment in Patients With Noncoronary Vascular Disease. Vascular and endovascular surgery, 2016. 50(2): p. 119–135. [DOI] [PubMed] [Google Scholar]
- 95.Ferrebee CB and Dawson PA, Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids. Acta pharmaceutica Sinica. B, 2015. 5(2): p. 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golden SH, et al. , The Case for Diabetes Population Health Improvement: Evidence-Based Programming for Population Outcomes in Diabetes. Current diabetes reports, 2017. 17(7): p. 51-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.American Diabetes, A., 14. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2018. Diabetes care, 2018. 41(Suppl 1): p. S144–S151. [DOI] [PubMed] [Google Scholar]
- 98.Sweitzer BJ, Holt NF, Crowley M Preanesthesia evaluation for noncardiac surgery. 2019. 4/23/2019 [cited 2019 1/16/2020]; Available from: https://www.uptodate.com/contents/preanesthesia-evaluation-for-noncardiac-surgery?search=preanesthesia%20evaluation&source=search_result&selectedTitle=1~62&usage_type=default&display_rank=1.
- 99.Saber W, Perioperative medication management: a case-based review of general principles. Clev Clin J Med, 2006. 73(Suppl1): p. S82–S87. [DOI] [PubMed] [Google Scholar]
- 100.Doherty JU, et al. , 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Report of the American College of Cardiology Clinical Expert Consensus Document Task Force. Journal of the American College of Cardiology, 2017. 69(7): p. 871–898. [DOI] [PubMed] [Google Scholar]
- 101.Mar PL, et al. , Periprocedural management of anticoagulation in patients taking novel oral anticoagulants: Review of the literature and recommendations for specific populations and procedures. International journal of cardiology, 2016. 202: p. 578–585. [DOI] [PubMed] [Google Scholar]
- 102.Douketis J, et al. , Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest, 2012. 141(2 Suppl): p. e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.monograph P.d.p. 2019, Boehringer Ingelheim Pharmaceuticals, Inc.
- 104.monograph X.r.p. 2019, Janssen Pharmaceuticals, Inc.
- 105.monograph E.a.p. 2019, Bristol-Meyers Squibb.
- 106.monograph S.e.p. 2019, Daiichi Sankyo., LTD
- 107.Wang C-Z, Moss J, and Yuan C-S, Commonly Used Dietary Supplements on Coagulation Function during Surgery. Medicines (Basel, Switzerland), 2015. 2(3): p. 157–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levy I, et al. , Perioperative Risks of Dietary and Herbal Supplements. World journal of surgery, 2017. 41(4): p. 927–934. [DOI] [PubMed] [Google Scholar]
- 109.Rosado Ingelmo A, et al. , Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Contrast Media. Journal of investigational allergology & clinical immunology, 2016. 26(3): p. 144–155. [DOI] [PubMed] [Google Scholar]
- 110.Weisbord SD, et al. , Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. The New England journal of medicine, 2018. 378(7): p. 603–614. [DOI] [PubMed] [Google Scholar]
- 111.Subramaniam RM, et al. , Effectiveness of Prevention Strategies for Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Annals of internal medicine, 2016. 164(6): p. 406–416. [DOI] [PubMed] [Google Scholar]
- 112.World Health, O., WHO guidelines for safe surgery.
- 113.Commission, J. 2020 National patient safety goals. 2020. [cited 2020 1/16/2020]; Available from: https://www.jointcommission.org/standards/national-patient-safety-goals/hospital-2020-national-patient-safety-goals/.
- 114.Lyons V and Popejoy L, Meta-analysis of surgical safety checklist effects on teamwork, communication, morbidity, mortality and safety. Western J Nurs Res, 2014. 36: p. 245–261. [DOI] [PubMed] [Google Scholar]
- 115.Varon J and Marik P, Perioperative hypertension management. Vasc Health Risk Manag, 2008. 4(3): p. 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lonjaret L, et al. , Optimal perioperative management of arterial blood pressure. Integrated blood pressure control, 2014. 7: p. 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bratzler D, Dellinger E, and Olsen K, ASHP report: clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst Pharm, 2013. 70: p. 195–283. [DOI] [PubMed] [Google Scholar]
- 118.Malignant Hyperthermia Association of the United, S. Safe and unsafe anesthetics for the mh-susceptible patient. 2020. [cited 2020 1/16/2020]; Available from: https://www.mhaus.org/healthcare-professionals/be-prepared/safe-and-unsafe-anesthetics/.
- 119.Glahn KP, et al. , Recognizing and managing a malignant hyperthermia crisis: guidelines from the European Malignant Hyperthermia Group. British journal of anaesthesia, 2010. 105(4): p. 417–420. [DOI] [PubMed] [Google Scholar]
- 120.Malignant Hyperthermia Association of the United, S. Managing a crisis. 2020. [cited 2020 Web Page]; Available from: https://www.mhaus.org/healthcare-professionals/managing-a-crisis/.
- 121.Ultee KHJ, et al. , Conversion from endovascular to open abdominal aortic aneurysm repair. Journal of vascular surgery, 2016. 64(1): p. 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bashir MR, et al. , Endoleaks after endovascular abdominal aortic aneurysm repair: management strategies according to CT findings. AJR. American journal of roentgenology, 2009. 192(4): p. W178–W186. [DOI] [PubMed] [Google Scholar]
- 123.Dumville JC, et al. , Dressings for the prevention of surgical site infection. Cochrane Database of Systematic Reviews, 2014(9). [DOI] [PubMed] [Google Scholar]
- 124.Naidu SS, et al. , Clinical expert consensus statement on best practices in the cardiac catheterization laboratory: Society for Cardiovascular Angiography and Interventions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions, 2012. 80(3): p. 456–464. [DOI] [PubMed] [Google Scholar]
- 125.Mehaffey JH, et al. , Targets to prevent prolonged length of stay after endovascular aortic repair. Journal of vascular surgery, 2015. 62(6): p. 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.American Society of PeriAnesthesia, N., Perianesthesia Nursing Standards and Practice Recommendations 2010–2012. 2010, Chery Hill, NJ: ASPAN. [Google Scholar]
- 127.Fernandez R and Griffiths R, A comparison of an evidence based regime with the standard protocol for monitoring postoperative observation: a randomised controlled trial. Australian Journal of Advanced Nursing, 2005. 23(1): p. 15–21. [PubMed] [Google Scholar]
- 128.Krenzischek DA, Wilson L, and Aspan, An introduction to the ASPAN pain and comfort clinical guideline. Journal of PeriAnesthesia Nursing, 2003. 18(4): p. 228–236. [DOI] [PubMed] [Google Scholar]
- 129.Abbott/American Association of Critical-Care, N. and M. Saint Thomas Health System Sedation Expert Panel, Consensus conference on sedation assessment. A collaborative venture by Abbott Laboratories, American Association of Critical-Care Nurses, and Saint Thomas Health System. Critical care nurse, 2004. 24(2): p. 33–41. [PubMed] [Google Scholar]
- 130.O’Driscoll B, Howard L, and Davison A, BTS guideline for emergency oxygen use in adult patients. Thorax, 2008. 68(Suppl VI): p. vi1–vi68. [DOI] [PubMed] [Google Scholar]
- 131.Gordon PA and Toursarkissian B, Treatment of abdominal aortic aneurysms: the role of endovascular repair. AORN journal, 2014. 100(3): p. 241–259. [DOI] [PubMed] [Google Scholar]
- 132.Buckley SD and Buckley CJ, Endovascular aortoiliac aneurysm repair: surgical progress through new treatment paradigms and innovative endograft design. AORN journal, 2014. 100(3): p. 271–279. [DOI] [PubMed] [Google Scholar]
- 133.Shepherd A, Measuring and managing fluid balance. Nursing times, 2011. 107(28): p. 12–16. [PubMed] [Google Scholar]
- 134.Gould C, Umscheid c., Agarwal R, Kuntz G, Pegues DA, and the Healthcare Infection Clontrol Practice Committee (HICPAC). Guideline for prevention of catheter associated urinary tract infections 2009. 2019. June 6, 2019 [cited 2020 1/18/2020]; Available from: https://www.cdc.gov/infectioncontrol/guidelines/cauti/.
- 135.Toon CD, et al. , Early versus delayed dressing removal after primary closure of clean and clean-contaminated surgical wounds. Cochrane Database of Systematic Reviews, 2013(9). [DOI] [PubMed] [Google Scholar]
- 136.Orsted H, et al. , Best practice recommendations for the prevention and management of open surgical wounds. Wound Care Canada, 2010. 8(1): p. 6–34. [Google Scholar]
- 137.Inui T and Bandyk DF, Vascular surgical site infection: risk factors and preventive measures. Seminars in vascular surgery, 2015. 28(3–4): p. 201–207. [DOI] [PubMed] [Google Scholar]
- 138.Walker TG, et al. , Clinical practice guidelines for endovascular abdominal aortic aneurysm repair: written by the Standards of Practice Committee for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. Journal of vascular and interventional radiology : JVIR, 2010. 21(11): p. 1632–1655. [DOI] [PubMed] [Google Scholar]
- 139.Picel AC and Kansal N, Essentials of endovascular abdominal aortic aneurysm repair imaging: postprocedure surveillance and complications. AJR. American journal of roentgenology, 2014. 203(4): p. W358–W372. [DOI] [PubMed] [Google Scholar]
- 140.Schanzer A, et al. , Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation, 2011. 123(24): p. 2848–2855. [DOI] [PubMed] [Google Scholar]
- 141.Mignatti A, Friedmann P, and Slovut DP, Targeting the safe zone: A quality improvement project to reduce vascular access complications. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions, 2018. 91(1): p. 27–32. [DOI] [PubMed] [Google Scholar]
- 142.Barone C, Pablo c., and Baron G, Postanesthetic care in the critical care unit. Crit Care Nurse, 2004. 24(1): p. 38–45. [PubMed] [Google Scholar]
- 143.Nicholau D, The postanesthesia care unit, in Miller’s Anesthesia, Miller R, et al. , Editors. 2009, Churchill Livingstone: Orlando: p. 2707–2728. [Google Scholar]
- 144.Norred CL, Anesthetic-induced anaphylaxis. AANA journal, 2012. 80(2): p. 129–140. [PubMed] [Google Scholar]
- 145.Misiakos EP, et al. , Advents in the Diagnosis and Management of Ischemic Colitis. Frontiers in surgery, 2017. 4: p. 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kirkpatrick AW, et al. , Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive care medicine, 2013. 39(7): p. 1190–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang G, et al. , Limb graft occlusion following endovascular aortic repair: Incidence, causes, treatment and prevention in a study cohort. Experimental and therapeutic medicine, 2017. 14(2): p. 1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wikström J, et al. , Lower extremity artery stenosis distribution in an unselected elderly population and its relation to a reduced ankle-brachial index. Journal of vascular surgery, 2009. 50(2): p. 330–334. [DOI] [PubMed] [Google Scholar]
- 149.Saric M Embolism from atherosclerotic plaque: Atheroembolism (cholesterol crystal embolism). 2019. 5/23/2019 [cited 2020 1/16/2020]; Available from: https://www.uptodate.com/contents/embolism-from-atherosclerotic-plaque-atheroembolism-cholesterol-crystal-embolism?search=embolism%20from%20athero.
- 150.Arnaoutoglou E, et al. , Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: influence on patients’ 30 day outcome. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery, 2015. 49(2): p. 175–183. [DOI] [PubMed] [Google Scholar]
- 151.Awad H, et al. , Spinal cord injury after thoracic endovascular aortic aneurysm repair. Canadian journal of anaesthesia = Journal canadien d’anesthesie, 2017. 64(12): p. 1218–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zettervall SL, et al. , Predictors of renal dysfunction after endovascular and open repair of abdominal aortic aneurysms. Journal of vascular surgery, 2017. 65(4): p. 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aicher B, et al. , Infrainguinal wound infections in vascular surgery: An antiquated challenge without a modern solution. Journal of vascular nursing : official publication of the Society for Peripheral Vascular Nursing, 2017. 35(3): p. 146–156. [DOI] [PubMed] [Google Scholar]
- 154.American Diabetes A, 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes care, 2018. 41(Suppl 1): p. S73–S85. [DOI] [PubMed] [Google Scholar]
- 155.Kothandan H, et al. , Anesthetic considerations for endovascular abdominal aortic aneurysm repair. Annals of cardiac anaesthesia, 2016. 19(1): p. 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Riles TS, General principles of vascular surgery, in Atlas of Vascular Surgery abd Endovascular Therapy: Anatomy and Technique, Chaikof RPCEL, Editor. 2014, Saunders/Elsevier; p. 29–40. [Google Scholar]
- 157.Noori VJ and Eldrup-Jørgensen J, A systematic review of vascular closure devices for femoral artery puncture sites. Journal of vascular surgery, 2018. 68(3): p. 887–899. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz BG, et al. , Review of vascular closure devices. The Journal of invasive cardiology, 2010. 22(12): p. 599–607. [PubMed] [Google Scholar]
- 159.Hodgson CL, et al. , Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Critical care (London, England), 2014. 18(6): p. 658–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Castanheira L, Fresco P, and Macedo AF, Guidelines for the management of chronic medication in the perioperative period: systematic review and formal consensus. Journal of clinical pharmacy and therapeutics, 2011. 36(4): p. 446–467. [DOI] [PubMed] [Google Scholar]
- 161.Whelton PK, et al. , 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex. : 1979), 2018. 71(6): p. e13–e115. [DOI] [PubMed] [Google Scholar]
- 162.Miura S, et al. , Postoperative initial 2-day blood pressure management facilitates the shrinkage of abdominal aortic aneurysm after endovascular aneurysm repair by reducing the incidence of type II endoleak. Journal of vascular surgery, 2018. 67(1): p. 166–173. [DOI] [PubMed] [Google Scholar]
- 163.Giancarelli A, et al. , Hypocalcemia in trauma patients receiving massive transfusion. The Journal of surgical research, 2016. 202(1): p. 182–187. [DOI] [PubMed] [Google Scholar]
- 164.Polomano RC, et al. , Multimodal Analgesia for Acute Postoperative and Trauma-Related Pain. The American journal of nursing, 2017. 117(3 Suppl 1): p. S12–S26. [DOI] [PubMed] [Google Scholar]
- 165.Gan TJ, Poorly controlled postoperative pain: prevalence, consequences, and prevention. Journal of pain research, 2017. 10: p. 2287–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jungquist CR, et al. , Assessing and Managing Acute Pain: A Call to Action. The American journal of nursing, 2017. 117(3 Suppl 1): p. S4–S11. [DOI] [PubMed] [Google Scholar]
- 167.Naughton CA, Drug-induced nephrotoxicity. American family physician, 2008. 78(6): p. 743–750. [PubMed] [Google Scholar]
- 168.Perazella MA, Renal vulnerability to drug toxicity. Clinical journal of the American Society of Nephrology : CJASN, 2009. 4(7): p. 1275–1283. [DOI] [PubMed] [Google Scholar]
- 169.McCarberg B, Washington State Opioid Prescribing Guidelines. Pain medicine (Malden, Mass.), 2015. 16(8): p. 1455–1456. [DOI] [PubMed] [Google Scholar]
- 170.American Society of Anesthesiologists Task Force on Acute Pain, M., Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology, 2012. 116(2): p. 248–273. [DOI] [PubMed] [Google Scholar]
- 171.Soulez G, et al. , Pain and quality of life assessment after endovascular versus open repair of abdominal aortic aneurysms in patients at low risk. Journal of vascular and interventional radiology : JVIR, 2005. 16(8): p. 1093–1100. [DOI] [PubMed] [Google Scholar]
- 172.Harlin SA, et al. , A Novel Anesthetic Technique for PEVAR. Annals of vascular surgery, 2016. 34: p. 106–110. [DOI] [PubMed] [Google Scholar]
- 173.Anderson JL, et al. , Management of Patients With Peripheral Artery Disease (Compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation, 2013. 127(13): p. 1425–1443. [DOI] [PubMed] [Google Scholar]
- 174.Stone N, et al. , 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol, 2014. 63(25): p. 2889–2934. [DOI] [PubMed] [Google Scholar]
- 175.Adhyaru BB and Jacobson TA, New cholesterol guidelines for the management of atherosclerotic cardiovascular disease risk: a comparison of the 2013 American College of Cardiology/American Heart Association cholesterol guidelines with the 2014 National Lipid Association recommendations for patient-centered management of dyslipidemia. Cardiology clinics, 2015. 33(2): p. 181–196. [DOI] [PubMed] [Google Scholar]
- 176.Berger JS and Hiatt WR, Medical therapy in peripheral artery disease. Circulation, 2012. 126(4): p. 491–500. [DOI] [PubMed] [Google Scholar]
- 177.Veith F, Cayne N, Berland T, Mayer D, Lachat M, Open surgical and endovascular management of ruptured abdominal aortic aneurysm, in Vascular and Endovascular Surgery: A comprehensive Review, Moore W, Editor. 2013, Elsevier: Philadelphia: p. 685–690. [Google Scholar]
- 178.Le L, Evidence summary Assessment of constipation: Hospitalized patients 2017: The Joanna Briggs Institute EBP Database, JBI@Ovid. [Google Scholar]
- 179.Rao S Constipation in the older adult. 2018. 5/21/2018 [cited 2020 1/18/2020]; Available from: https://www.cdc.gov/infectioncontrol/guidelines/cauti/.
- 180.Zierler RE, et al. , The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. Journal of vascular surgery, 2018. 68(1): p. 256–284. [DOI] [PubMed] [Google Scholar]
- 181.American College of Sports, M., ACSM’s guidelines for exercise testing and prescription, ed. Thompson WR, Gordon NF, and Pescatello LS. 2010, Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- 182.Dolinger C and Strider DV, Endovascular interventions for descending thoracic aortic aneurysms: The pivotal role of the clinical nurse in postoperative care. Journal of vascular nursing : official publication of the Society for Peripheral Vascular Nursing, 2010. 28(4): p. 147–153. [DOI] [PubMed] [Google Scholar]
- 183.Hamel WJ, Femoral artery closure after cardiac catheterization. Critical Care Nurse, 2009. 29(1): p. 39–46. [DOI] [PubMed] [Google Scholar]
- 184.Kassaian SE, et al. , Glycosylated hemoglobin (HbA1c) levels and clinical outcomes in diabetic patients following coronary artery stenting. Cardiovascular diabetology, 2012. 11: p. 82–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arnett DK, et al. , 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019. 140(11): p. e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Weber M, et al. , Clinical practice guidelines for the management of hypertension int he community. J Clin Hypertens, 2014. 16(1): p. 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cronenwett JL, Kraiss LW, and Cambria RP, The Society for Vascular Surgery Vascular Quality Initiative. Journal of vascular surgery, 2012. 55(5): p. 1529–1537. [DOI] [PubMed] [Google Scholar]
- 188.Krajcer Z, et al. , Perioperative Outcomes From the Prospective Multicenter Least Invasive Fast-Track EVAR (LIFE) Registry. J Endovasc Ther, 2018. 25(1): p. 6–13. [DOI] [PubMed] [Google Scholar]


