Highlights
-
•
Transfusion of sera of convalescent patients is currently in clinical evaluations to treat COVID-19 patients.
-
•
Neutralizing antibody levels vary among convalescent patients and fast and simple methods to identify suitable plasma donations are needed.
-
•
We compared three methods to determine the SARS-CoV-2 neutralizing activity of human convalescent plasma.
-
•
An ELISA-based sVNT assay required the lowest biosafety level, was fast and sufficient to identify highly neutralizing plasma samples.
-
•
Weakly neutralizing samples were more reliable detected by the more challenging lentiviral vector based assays or virus neutralization assays.
Keywords: SARS-CoV-2, Convalescent plasma, Neutralization, Pseudotyping
Abstract
Convalescent plasma is plasma collected from individuals after resolution of an infection and the development of antibodies. Passive antibody administration by transfusion of convalescent plasma is currently in clinical evaluations to treat COVID-19 patients. The level of neutralizing antibodies vary among convalescent patients and fast and simple methods to identify suitable plasma donations are needed. We compared three methods to determine the SARS-CoV-2 neutralizing activity of human convalescent plasma: life virus neutralization by plaque reduction assay, a lentiviral vector based pseudotype neutralization assay and a competition ELISA-based surrogate virus neutralization assay (sVNT). Neutralization activity correlated among the different assays; however the sVNT assay was overvaluing the low neutralizing plasma. On the other hand, the sVNT assay required the lowest biosafety level, is fast and is sufficient to identify highly neutralizing plasma samples. Though weakly neutralizing samples were more reliable detected by the more challenging lentiviral vector based assays or virus neutralization assays. Spike receptor binding competition assays are suitable to identify highly neutralizing plasma samples under low biosafety requirements. Detailed analysis of in vitro neutralization activity requires more sophisticated methods that have to be performed under higher biosafety levels.
1. Introduction
The ongoing worldwide pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, is a great threat to global public health. Currently no specific treatment or vaccines are available. Passive immunization by plasma therapy however, has the potential to be used as an emergency treatment. Plasma was applied to humans already in 1917 for the treatment of patients with acute poliomyelitis (Amoss and Chesney, 1917). Thereby, plasma collected from patients who have recovered from the disease, is transfused into acutely infected patients. This treatment has been successfully used as a safe and efficient therapy for infections with the coronaviruses SARS and MERS (Middle East respiratory coronavirus) and during the Ebola outbreak (Cheng et al., 2005; Ko et al., 2018; Winkler and Koepsell, 2015). Currently studies are ongoing and first successful treatments of COVID-19 patients are being published (Shen et al., 2020; Duan et al., 2020; Ye et al., 2020; Ahn et al., 2020). Apart from finding the right clinical parameters, e.g. stage of disease or symptoms, for plasma transfusion, identifying plasma sample with therapeutic potential is the main challenge for this type of therapy. Patients who have recovered from COVID-19 and have high neutralizing antibody titer are valuable donors. However, the amount of antibodies and the virus neutralizing activity in convalescent serum varies rigorously between patients (Robbiani et al., 2020). The availability of high titer convalescent plasma is less abundant than expected. A recent study of 175 Chinese patients, who recovered from mild COVID-19, described that 6 % of the patients did not produce detectable levels of neutralizing antibodies and 30 % of them only very low neutralizing titers (Wu et al., 2020a).
Here, we compared three different assay systems to determine in vitro SARS-CoV-2 neutralizing activity in convalescent plasma to identify a simple and reliable way to define samples with therapeutic potential.
2. Materials and methods
2.1. Cell culture
HEK293T-hACE2 (Glowacka et al., 2010), HEK293 T (ATCC CRL-3216) and Vero E6 cells (ATCC CRL-1586) were cultured at 37 °C under 5 % CO2 and grown in Dulbecco’s modified Eagle medium (DMEM; Lonza, Verviers, Belgium) supplemented with 10 % fetal bovine serum (PAA, Pasching, Austria) and 5 % l-glutamine (200 mM; Lonza, Verviers, Belgium) and 1% penicillin/streptavidin (Fisher Scientific, Schwerte, Germany).
2.2. Plasma samples
Human naïve and SARS-CoV-2 positive plasma was obtained from the German Red Cross from volunteer blood donors. Plasma samples were heat-inactivated at 56 °C for 30 min.
2.3. Virus neutralization assay
Plaque reduction neutralization tests were done as described before (Wölfel et al., 2020). In short, VeroE6 cells (4 × 105 cell/mL) were seeded in 24-well plates the day before. Prior to PRNT patient plasma were heat-inactivated at 56 °C for 30 min and diluted 1:20 up to 1:640. For each dilution step PRNT testing was done in duplicates. For PRNT samples were diluted in OptiPro (Fisher scientific, Schwerte, Germany) and mixed 1:1 with virus solution containing 100 plaque forming units of SARS-CoV-2 (EPI ISL 406862) and incubated at 37 °C for 1 h. The solution was added onto two wells of a 24-well plate. After 1 h at 37 °C the supernatants were discarded, the cells were washed once with PBS and supplemented with 1.2 % Avicel solution in DMEM (Merck, Darmstadt, Germany). After 3 days at 37 °C, the supernatants were removed and the 24-well plates were fixed and inactivated using a 6 % formaldehyde/PBS solution and stained with crystal violet as described (Herzog et al., 2008).
2.4. Pseudotype-based neutralization assay
Lentiviral vectors were prepared in HEK293 T cells by co-transfection using Lipofectamine 2000 (Thermo Fisher, Darmstadt, Germany) as described previously (Henss et al., 2019). Plasmids encoding HIV-1 Gag/pol, rev, the firefly luciferase encoding lentiviral vector genome and the SARS-Cov-2 full-length spike gene (#MN908947) or VSV-G were transfected. Vectors were concentrated by ultracentrifugation and stored at −80 °C. Pseudotyped vectors and serially diluted human plasma/serum (1:60 to 1:14,580) were incubated in triplicates for 30 min. at 37 °C and used to transduce HEK293T-hACE2 cells. After 48 h, luciferase substrate was added to measure luciferase activity. The reciprocal area under the curve (AUC) value calculated for each sample corresponds to the neutralization activity. AUC values were determined using the GraphPad Prism 7.04 software (La Jolla, CA, USA). Mean values and standard deviations were calculated in Excel.
2.5. sVNT assay
The spike protein (S) receptor binding domain (RBD) is responsible for recognizing the cell surface receptor, angiotensin converting enzyme-2 (ACE2). The RBD of SARS-CoV-2 S protein strongly interacts with hACE2. The SARS-CoV-2 sVNT Kit (Genscript; Leiden, Netherlands) is a blocking ELISA, which mimics this virus receptor binding process. The protein-protein interaction between a horseradish peroxidase (HRP) conjugated recombinant SARS-CoV-2 RBD fragment (HRP-RBD) and hACE2 can be blocked by neutralizing antibodies against the SARS-CoV-2 RBD and residual HRP activity is measured as a surrogate for neutralization. The assay was performed with 1:50 diluted plasma following the manufacturer’s instructions.
3. Results
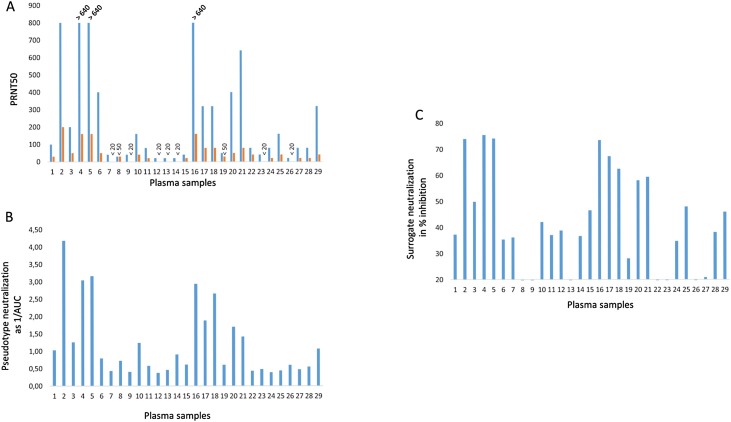
The SARS-Cov-2 neutralizing activity was determined by three different methods. First the gold standard method, virus neutralization assays was performed. Infectious SARS-Cov-2 was used to infect Vero E6 cells in the presence of decreasing amounts of convalescent plasma and the 50 % plaque reduction titer was determined (Fig. 1 A). The assay was performed a second time with higher plasma dilutions because for some samples the endpoint titer could not be determined (Fig. 1A in blue) and these values are depicted in orange in Fig. 1A. Several samples (# 2, 4, 5, 6, 16, 20 and 21) showed high neutralization activity above a PRNT50 of 1:350, which is recommended for plasma transfer by the US and European Comission authorities (https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/blood-derived-products/convalescent-plasma/ and (EU Ref. Ares(2020)3256185-23/06/2020; Tonn et al., 2020).
Fig. 1.
In vitro SARS-Cov-2 neutralization activity of human plasma samples.
Human plasma samples were analyzed by three assays. (A) Virus neutralization, which was done with two different dilution series of plasma. Lower dilutions are depicted in blue, higher dilution depicted in orange. SARS-CoV-2 neutralization is shown as plaque reduction neutralization titers 50 (PRNT50), which correspond to the plasma dilution that reduces the virus plaques by 50 %. Samples with titers >640 are indicated. (B) Pseudotyped vector neutralization is given as the reciprocal value of the area under the curve (AUC) obtained as relative light units with plasma dilutions. (C) The surrogate virus neutralization assay sVNT shows neutralization as inhibition of HRP-coupled RBD binding to the ACE2 receptor. Plasma samples were diluted 1:50 for the assay.
Next, the same samples were analyzed with lentiviral vectors pseudotyped with the full-length SARS-CoV-2 spike protein. These vectors acquire the host spectrum of the coronavirus and a rapid read out of the results is possible by the transfer of the luciferase gene, which serves as a measure of infection. Addition of neutralizing antibodies directed against SARS-Cov-2 reduces the luciferase activity. Neutralization activity of plasma was calculated by the area under the curve (AUC) generated by plasma dilutions and their reciprocal values are depicted (Fig. 1B). With this assay, samples 2, 4, 5, 16, 17, 18 and 20 showed the highest neutralization activity.
The third assay is a surrogate neutralization assay and is based on binding of the horseradish peroxidase-labeled SARS-CoV-2 receptor binding domain (RBD) to its receptor angiotensin converting enzyme 2 (ACE2) coated on ELISA plates. Neutralizing antibodies bind to RBD and compete with ACE2 binding. A 20 % inhibition of receptor binding was set as a negative cut-off of the assay. With this assay, samples number 2, 4, 5, 16, 17, 18, 20 and 21, showed high neutralizing activity (Fig. 1C).
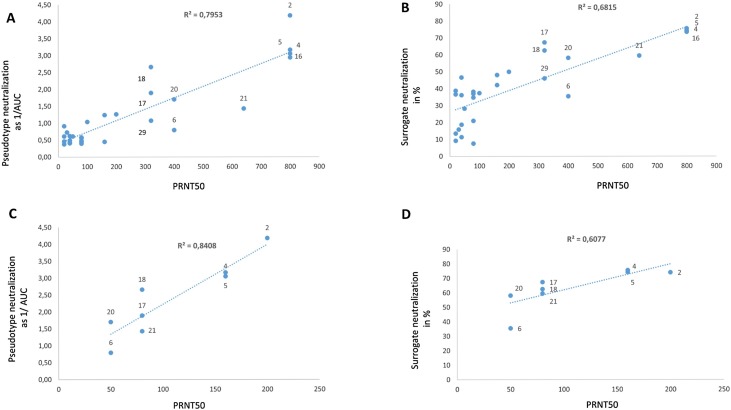
The different assay results were plotted against each other and showed linearity with a measure of certainty of r2 = 0.79 for pseudotype against the virus neutralization assay (Fig. 2 A). Slightly less linearity was observed for the sVNT and the virus neutralization with r2 = 0.68 (Fig. 2B). However, if the analysis was restricted to highly neutralizing plasma samples and the values of the virus neutralization assay with higher diluted plasma were used, a good correlation of pseudo-neutralization and virus neutralization corresponding to a measure of certainty of r2 = 0.84 was detectable (Fig. 2C). This was less significant for the comparison of the sVNT versus the virus neutralization assay with r2 = 0.60 (Fig. 2D).
Fig. 2.
Comparison of assays with the virus neutralization assay.
(A) Pseudotype neutralization was plotted against the virus neutralization activities. The r2 value indicates the certainty of the values to be at the trend line and shows with r2 = 0.79 comparability of the two assays. (B) sVNT assay values were plotted against the virus neutralization. The r2 = 0.68 shows less comparability of the two assays compared to A. (C) Pseudotype neutralization activities of highly neutralizing plasma samples were plotted against the virus neutralization activity. The r2 value indicates the certainty of the values to be at the trend line and shows with r2 = 0.84 high comparability of the two assays. (D) The sVNT activities of highly neutralizing plasma samples were plotted against the virus neutralization activity. The r2 value of 0.6 indicates less comparability of the two assays compared to C.
In general, the sVNT assay overestimated the samples with low neutralizing activities and was less reliable for these types of plasma samples (Fig. 2B, C and Fig. 1C samples 7, 15, 25, 28, 29). However, highly neutralizing samples could be identified, when using a cut-off of 50 % inhibition.
4. Discussion
Transfusion of convalescent plasma might be a therapeutic option for the treatment of COVID-19, but the identification of suitable plasma donors is hampered by the substantially variable levels of neutralizing antibodies in convalescent patients. Therefore, we evaluated here, which assay would be suitable to identify donors.
The three assays used are able to identify highly neutralizing plasma, but they require different biosafety levels to perform the assay. The virus neutralization has to be carried out under biosafety 3 level, the pseudotype neutralization assay requires biosafety level 2 and sVNT assay is a simple ELISA, which can be performed in any laboratory without special biosafety requirements. In addition, the pseudotype assay and the virus neutralization assay are time consuming and require 2 or 3 days respectively, until results are available. The sVNT assay can be performed in 2−3 h. However, the sVNT assay overestimated the samples with low neutralizing activities, but increasing the cut-off to 50 % inhibition enabled the identification of highly neutralizing samples. Therefore, the sVNT assay, although not yet approved for diagnostic application, is superior for a clinical setting and allows the fast screening of convalescent plasma samples in low biosafety level laboratories. Also other ELISA-based sVNT assay systems have been developed recently (Bošnjak et al., 2020; Abe et al., 2020; Ding et al., 2020) and different kits are commercially available. Assay comparisons were performed by multiple groups and overall, the sVNT assays correlated well with the other neutralization assays (Abe et al., 2020; Meyer et al., 2020; Bond et al., 2020; McGregor et al., 2020; Bošnjak et al., 2020; Ding et al., 2020). The sVNT assays are well suited for a rapid prescreening of patient plasma to identify donors with high neutralization activity. In addition, sVNT kits are useful to analyzed animal sera from preclinical vaccine studies, because no adaptation of the assay is required (Perera et al., 2020). A general limitation of these assays is that they are only able to detect neutralizing antibodies that function by blocking the interaction between the RBD and ACE2, although most neutralizing antibodies fulfill this requirement, single antibodies have been described that use other mechanisms for neutralization (Wang et al., 2020; Wu et al., 2020b).
Detailed analysis of recipients of the plasma donation might require the more sophisticated assays. For the clinical evaluation of this therapeutic option, also the recipients have to be tested because they might already have highly neutralizing antibodies and additional antibody treatment might not help (Arvind Gharbharan et al., 2020). These more detailed analyses of SARS-CoV-2 neutralization activity require either, virus neutralization or pseudotype assays. In addition, basic research on the induction of SARS-CoV-2 immune responses should be performed with these assays. Both assays are comparable and a direct calibration of two assays will be possible when an international standard is available.
Funding
Contributions by VMC were supported by the Berlin Institute of Health, the Berlin University Alliance, and the German Ministry of Health (Konsiliarlabor für Coronaviren). BSS was supported by a grant of the German Bundesministerium für Gesundheit (BMG).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Christine von Rhein: Data curation, Formal analysis, Investigation, Methodology. Tatjana Scholz: Data curation, Formal analysis, Investigation, Methodology. Lisa Henss: Data curation, Formal analysis, Investigation, Methodology. Romy Kronstein-Wiedemann: Data curation, Formal analysis, Investigation, Methodology. Tatjana Schwarz: Data curation, Formal analysis, Investigation, Methodology. Roman N. Rodionov: Data curation, Formal analysis, Investigation, Methodology. Victor M. Corman: Data curation, Formal analysis, Investigation, Methodology. Torsten Tonn: Data curation, Formal analysis, Investigation, Methodology. Barbara S. Schnierle: Data curation, Formal analysis, Investigation, Methodology.
Acknowledgments
We are thankful to Heike Baumann, Marie Schmidt, Anja Richter and Felix Walper for technical assistant.
References
- Abe K.T., Li Z., Samson R., Samavarchi-Tehrani P., Valcourt E.J., Wood H., Budylowski P., Dupuis A.P., Girardin R.C., Rathod B., Wang J.H., Barrios-Rodiles M., Colwill K., McGeer A.J., Mubareka S., Gommerman J.L., Durocher Y., Ostrowski M., McDonough K.A., Drebot M.A., Drews S.J., Rini J.M., Gingras A.-C. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight. 2020;5(19) doi: 10.1172/jci.insight.142362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., Roh J., Ahn M.Y., Chin B.S., Kim Y.S., Lee H., Yong D., Kim H.O., Kim S., Choi J.Y. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020;35(14):e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoss H.L., Chesney A.M. A report on the serum treatment of TWENTY-SIX cases of Epidemic Poliomyelitis. J. Exp. Med. 1917;25(4):581–608. doi: 10.1084/jem.25.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond K., Nicholson S., Lim S.M., Karapanagiotidis T., Williams E., Johnson D., Hoang T., Sia C., Purcell D., Mordant F., Lewin S.R., Catton M., Subbarao K., Howden B.P., Williamson D.A. Evaluation of serological tests for SARS-CoV-2: implications for serology testing in a low-prevalence setting. J. Infect. Dis. 2020;222(8):1280–1288. doi: 10.1093/infdis/jiaa467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bošnjak B., Stein S.C., Willenzon S., Cordes A.K., Puppe W., Bernhardt G., Ravens I., Ritter C., Schultze-Florey C.R., Gödecke N., Martens J., Kleine-Weber H., Hoffmann M., Cossmann A., Yilmaz M., Pink I., Hoeper M.M., Behrens G.M.N., Pöhlmann S., Blasczyk R., Schulz T.F., Förster R. 2020. Low Serum Neutralizing anti-SARS-CoV-2 S Antibody Levels in Mildly Affected COVID-19 Convalescent Patients Revealed by Two Different Detection Methods. Cellular & Molecular Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L., Chan P., Wong K.C., Leung C.B., Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Laumaea A., Benlarbi M., Beaudoin-Bussières G., Gasser R., Medjahed H., Pancera M., Stamatatos L., McGuire A.T., Bazin R., Finzi A. Antibody binding to SARS-CoV-2 S glycoprotein correlates with but does not predict neutralization. Viruses. 2020;12(11) doi: 10.3390/v12111214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbharan Arvind, Jordans Carlijn C.E., GeurtsvanKessel Corine, Hollander Jan Gden, Karim Faiz, Mollema Femke P.N., Stalenhoef Janneke E., Dofferhoff Anton, Ludwig Inge, Ad Koster, Hassing Robert-Jan, Bos Jeannet C., Pottelberge Geert Rvan, Vlasveld Imro N., Ammerlaan Heidi S.M., Segarceanu Elena, Miedema Jelle, Eerden Mennovander, Papageorgiou Grigorios, Broekhorst Peterte, Swaneveld Francis H., Katsikis Peter D., Mueller Yvonne, Okba Nisreen M.A., Koopmans Marion P.G., Haagmans Bart L., Rokx Casper, Rijnders Bart. Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. 2020:2020. 07.01.20139857. [Google Scholar]
- Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., Eichler J., Drosten C., Pöhlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henss L., Yue C., Kandler J., Faddy H.M., Simmons G., Panning M., Lewis-Ximenez L.L., Baylis S.A., Schnierle B.S. Establishment of an alphavirus-specific neutralization assay to distinguish infections with different members of the Semliki Forest complex. Viruses. 2019;11(1) doi: 10.3390/v11010082. pii:E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog P., Drosten C., Müller M.A. Plaque assay for human coronavirus NL63 using human colon carcinoma cells. Virol. J. 2008;5:138. doi: 10.1186/1743-422X-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.-H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H., Kim Y.-J., Park J.K., Chung C.R., Kang E.-S., Cho D., Müller M.A., Drosten C., Kang C.-I., Chung D.R., Song J.-H., Peck K.R. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir. Ther. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- McGregor R., Whitcombe A.L., Sheen C.R., Dickson J.M., Day C.L., Carlton L.H., Sharma P., Lott J.S., Koch B., Bennett J., Baker M.G., Ritchie S.R., Fox-Lewis S., Morpeth S.C., Taylor S.L., Roberts S.A., Webb R.H., Moreland N.J. Collaborative networks enable the rapid establishment of serological assays for SARS-CoV-2 during nationwide lockdown in New Zealand. PeerJ. 2020;8:e9863. doi: 10.7717/peerj.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Reimerink J., Torriani G., Brouwer F., Godeke G.-J., Yerly S., Hoogerwerf M., Vuilleumier N., Kaiser L., Eckerle I., Reusken C. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT) Emerg. Microbes Infect. 2020;9(1):2394–2403. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.A.P.M., Ko R., Tsang O.T.Y., Hui D.S.C., Kwan M.Y.M., Brackman C.J., To E.M.W., Yen H.-L., Leung K., Cheng S.M.S., Chan K.H., Chan K.C.K., Li K.-C., Saif L., Barrs V.R., Wu J.T., Sit T.H.C., Poon L.L.M., Peiris M. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat and hamster sera. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.02504-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., Hagglof T., Oliveira T.Y., Viant C., Hurley A., Hoffmann H.-H., Millard K.G., Kost R.G., Cipolla M., Gordon K., Bianchini F., Chen S.T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A.W., Waltari E., Pak J.E., Huey-Tubman K.E., Koranda N., Hoffman P.R., West A.P., Rice C.M., Hatziioannou T., Bjorkman P.J., Bieniasz P.D., Caskey M., Nussenzweig M.C. 2020. Convergent Antibody Responses to SARS-CoV-2 Infection in Convalescent Individuals. bioRxiv: The Preprint Server for Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn T., Corman V.M., Johnsen M., Richter A., Rodionov R.N., Drosten C., Bornstein S.R. Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma. Lancet Microbe. 2020;1(2):e63. doi: 10.1016/S2666-5247(20)30037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11(1):2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Koepsell S.A. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr. Opin. Hematol. 2015;22(6):521–526. doi: 10.1097/MOH.0000000000000191. [DOI] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., Ling Y., Zhang Y., Xun J., Lu L., Jiang S., Lu H., Wen Y., Huang J. 2020. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. [Google Scholar]
- Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y., Gong Y., Xiao H., Fan Z., Tan S., Wu G., Tan W., Lu X., Fan C., Wang Q., Liu Y., Zhang C., Qi J., Gao G.F., Gao F., Liu L. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science (New York, N.Y.) 2020;368(6496):1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., Xia X., Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]