Abstract
In older patients (70±7 years) with chronic well-compensated heart failure with preserved ejection and controlled blood pressure, 6 months treatment with aliskiren (direct renin inhibitor) showed non-significant trends for modest improvements in peak exercise oxygen consumption (14.9±0.2 ml/kg/min versus 14.4±0.2 ml/kg/min; p=0.10, trend) and ventilatory anaerobic threshold (888±19 m//min versus 841±18ml/min; p=0.08).
Targeting the renin–angiotensin–aldosterone system (RAAS) pathways has long been considered a logical intervention for heart failure (HF) with preserved ejection fraction (HFpEF), due to its hypothesized link to left ventricular (LV) hypertrophy and fibrosis, and observations that HFpEF patients have abnormal activation of the RAAS. Aliskiren, as a direct renin inhibitor, functions through inhibition of angiotensin II effects as well as angiotensin II-independent effects mediated via the prorenin receptor.1 Direct renin inhibition has the theoretical benefit of upstream RAAS inhibition at the point of pathway activation. Furthermore, renin is a key variable in hypertension which is the most common risk factor for HFpEF, preceding the diagnosis −80% of HFpEF patients. We hypothesized that the severe exercise intolerance experienced by older patients with HFpEF can be improved with direct renin inhibition. To begin testing of this hypothesis, we performed a 6-month prospective, randomized, double-blind, placebo-controlled pilot trial with detailed measurements of exercise performance, health-related quality- of-life (QOL) scores, and cardiac structure and function.
As previously described, and in accord with the 2013 American College of Cardiology HF Guideline, HFpEF was defined as symptoms and signs of HF, a preserved LVEF(≥50%), and no other medical condition that could mimic HF symptoms, including significant ischemic, infiltrative, valvular, pericardial, or pulmonary disease, anemia or thyroid dysfunction.2;3 HF diagnosis was based on clinical criteria as previously described, including HF clinical score ≥3 from the National Health and Nutrition Examination Survey(NHANES)-I of,4 and those used by Rich et al,5 and verified by a board-certified cardiologist.2;3 The NHANES-1 criteria have been shown to have 94% specificity for HF, similar to the Framingham criteria.6 Patients were excluded if they had: peripheral artery revascularization or acute cerebrovascular syndrome within past 3 months; known significant bilateral renal artery stenosis; seated blood pressure (BP)≥160/90 mmHg at baseline screening; prior treatment with, intolerance of or contraindication to aliskiren; current treatment with antidepressant medication in the monoamine oxidase or selective serotine reuptake inhibitor class; baseline serum potassium >5.0 meq/L or serum creatinine ≥2.5 mg/dL.
Exercise testing was performed on a treadmill using the modified Naughton protocol and conducted by the same master's exercise physiologist as previously described.3;7 Metabolic gas exchange was measured continuously during exercise and averaged over 15-second intervals (Medgraphics Ultima, Medical Graphics Corp., St. Paul, Minnesota). Peak VO2 was defined as the average of the 2 highest VO2 values for a given 15-second interval within the last 90 seconds of exercise.7 We have shown peak VO2 measurements to be highly reproducibly specifically in HFpEF patients using these methods.7 Exercise time, VO2 at the ventilatory threshold (VAT), a key measure of submaximal exercise performance,7 and 6-minute walk distance (6MWD) were also assessed. Echo-Doppler examinations, including mitral annulus tissue and blood flow Doppler, were performed during supine rest at baseline and follow-up and analyzed as previously described in detail.3
Participants were randomly assigned to receive either aliskiren or placebo in a 1:1 ratio using permuted blocks and stratification by current angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB). Aliskiren vs placebo was initiated at 300mg or placebo, with no dose titrations. Outcomes were assessed using an intention-to-treat analysis. Comparisons of outcome measures between intervention groups were made by repeated measures analysis of covariance procedures. Analyses were adjusted for pre-randomization values of the outcome measure and other factors significantly associated with the outcome variable after adjusting for the other terms in the model. Data were presented as raw, unadjusted mean +/− standard deviation at each visit for each group, along with the P-value corresponding to the adjusted least squares outcomes means from the analysis of covariance procedures accounting for all data at all follow-up visits. Significance was set at p<0.05.
Fifty-two patients were enrolled. Patient characteristics were typical of HFpEF and had severely reduced exercise capacity (Table 1 and Table 2) and typical echo-Doppler findings, including LV hypertrophy, concentric LV remodeling, abnormal indices of diastolic function including reduced mitral annulus velocity and increased early filling to annulus velocity, and mildly increased left atrial size (supplemental Table 1). Twenty-four patients in the aliskiren group and 27 in the placebo group completed the 6-month follow-up. Compliance by pill count was excellent (94%). All patients tolerated full dose and there were no reductions in dose needed. Retention was excellent. There were 22 adverse events in18 individual patients, 6 in aliskiren and 16 in the placebo group: 3 acute exacerbations of HF in 3 separate patients, all in the placebo group; 5 hospitalizations, all in the placebo group (1 for viral gastroenteritis, 1 for small bowel obstruction, 1 for dehydration, and 2 for dyspnea; the remaining adverse events were minor.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Aliskiren (n=25) | Placebo (n=27) | p-value |
|---|---|---|---|
| Age(years) | 69.3±6.3 | 70.6±7.7 | 0.52 |
| Sex, Women | 19(76) | 23(85) | 0.49 |
| Race, Caucasian | 17(68) | 14(52) | 0.27 |
| Body weight (kg) | 87.0±12.9 | 90.4±19.6 | 0.46 |
| BSA(m2) | 1.91±0.15 | 1.95±0.23 | 0.43 |
| BMI(kg/m2) | 33.3±4.9 | 33.7±6.6 | 0.79 |
| LVEF(%) | 59±8 | 61±8 | 0.30 |
| Sinus rhythm | 24(96) | 25(93) | 1.0 |
| NYHA class | |||
| II | 20(80) | 17(63) | 0.23 |
| III | 5(21) | 10(39) | |
| Diabetes mellitus | 11(44) | 10(37) | 0.78 |
| History of hypertension | 24(96) | 26(96) | 1.0 |
| Systolic BP(mmHg) | 130±15 | 130±21 | 0.97 |
| Diastolic BP(mmHg) | 73±7 | 73±9 | 0.99 |
| Diastolic Filling Pattern | |||
| Normal | 0(0) | 0(0) | 0.74 |
| Delayed | 18(75) | 20(80) | |
| Pseudonormal | 6(25) | 5(20) | |
| Current Medication | |||
| ACE-Inhibitors | 9(36) | 11(41) | 0.78 |
| Beta-blockers | 11(44) | 13(48) | 0.79 |
| CA channel blockers | 9(36) | 10(37) | 1.0 |
| Digoxin | 0(0) | 1(4) | 1.0 |
| Diuretics | 17(68) | 20(74) | 0.76 |
| ARBs | 7(28) | 6(22) | 0.75 |
| Nitrates | 3(12) | 3(11) | 1.0 |
Data represented are mean ± SD or count (%)
Abbreviations: BSA: body surface area; BMI: body mass index; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; BP: blood pressure; Ea: early mitral annulus velocity; E: early mitral flow velocity; ACE: angiotensin converting enzyme; CA: calcium; ARB: angiotensin receptor blocker
Table 2.
Exercise Performance
| Aliskiren | Placebo | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Week | Final | LS mean* | Baseline | 12 week | Final | LS mean* | ||
| Peak Exercise | |||||||||
| Indexed VO2(ml/kg/min) | 14.4±2.9 | 15.0±3.2 | 15.0±3.2 | 14.9±0.2 | 14.2±1.8 | 14.2±2.0 | 14.4±2.1 | 14.4±0.2 | 0.10 |
| VO2(ml/min) | 1228±230 | 1279±255 | 1281±256 | 1304±16 | 1278±336 | 1303±332 | 1293±369 | 1275±15 | 0.19 |
| Time(sec) | 621±120 | 628±135 | 624±151 | 607±13 | 580±126 | 602±137 | 579±145 | 605±12 | 0.90 |
| Heart rate(bpm) | 131±25 | 135±23 | 132±25 | 130±2 | 125±24 | 126±22 | 122±23 | 127±2 | 0.17 |
| Respiratory rate(bpm) | 34±5 | 34±6 | 35±6 | 35±1 | 35±7 | 35± 6 | 34±7 | 34±1 | 0.67 |
| Oxygen pulse(ml/beat) | 9.8±3.4 | 10.0±3.6 | 10.1±3.3 | 10.4±0.1 | 10.5±2.9 | 10.5±2.6 | 10.7±2.8 | 10.3±0.1 | 0.72 |
| VCO2(ml/min) | 1433±288 | 1492±318 | 1477±323 | 1474±24 | 1422±399 | 1476±418 | 1445 ±451 | 1465±23 | 0.79 |
| VE(l/min) | 47±9 | 49±10 | 49±10 | 47±1 | 45±11 | 46±11 | 45±12 | 46±1 | 0.53 |
| RER | 1.17±0.05 | 1.17±0.07 | 1.15±0.06 | 1.14±0.01 | 1.11±0.07 | 1.13±0.09 | 1.11±0.08 | 1.14±0.01 | 0.91 |
| VE/VCO2 slope | 32.2±4.3 | 31.5±4.8 | 31.8±4.9 | 30.9±0.5 | 30.2±4.7 | 30.5±3.8 | 29.4±4.4 | 30.5±0.5 | 0.61 |
| VAT(ml/min) | 794±151 | 872±180 | 830±187 | 888±19 | 880±248 | 883±267 | 872±258 | 841±18 | 0.08 |
| 6 Minute walk(feet) | 1364±169 | 1378±172 | 1372±188 | 1360±15 | 1321±198 | 1366±180 | 1302±198 | 1348±16 | 0.58 |
Data represented are mean ± SD.
LS means ± SE represents combined follow-up visits following adjustment for the baseline value, age and gender. P-value represents comparison of LS means.
Abbreviations: VO2: oxygen consumption; VCO2: carbon dioxide production; VE: minute ventilation; RER: respiratory exchange ratio; VAT: ventilatory anaerobic threshold
At 6-month follow-up, peak VO2 was 0.5 ml/kg/min higher in aliskiren compared to placebo (14.9±0.2 ml/kg/min versus 14.4±0.2 ml/kg/min; p=0.10, trend, Figure 1). VAT was 5% higher in aliskiren compared to placebo (888±19 m//min versus 841±18ml/min; p=0.08, Figure 1). There was a small but significant reduction in resting systolic BP (−8mmHg; p=0.01) and pulse pressure (−7mmHg; p=0.01, supplemental Table 2). However, there was no relationship between change in systolic BP and change in peak VO2 (r: −0.12, p= 0.41). There were no significant differences in exercise time, 6-MWD, QOL, or LV structure and function. There were no significant changes in safety blood labs (supplemental Table 3).
Figure 1.
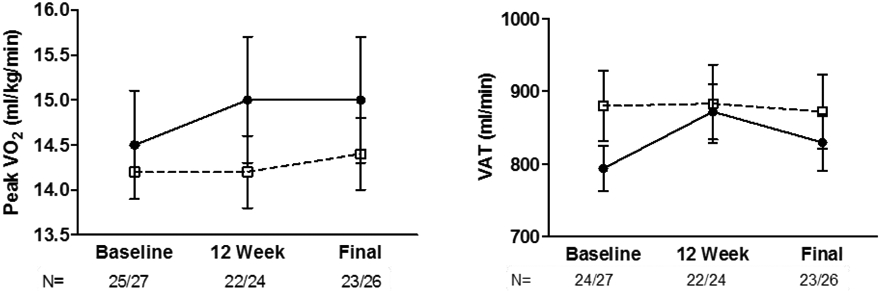
Raw, unadjusted means ±1 SE at baseline and 3-month and 6-month follow-up visits for aliskiren (circles) and placebo (squares) for peak exercise VO2 by expired gas analysis. The number of evaluable patients is shown for the specific outcome at each visit (aliskiren versus placebo).
The effect of aliskiren on exercise intolerance, which is an independent, clinically meaningful outcome, has not to our knowledge been examined in any type of HF. There are some data regarding the effect of aliskiren on other outcomes in patients with HFrEF. In the ALOFT study,8 plasma N-terminal-pro brain natriuretic peptide level was significantly reduced by aliskiren compared with placebo and there was reduction in echocardiographic parameters of LV remodeling. However, the ASTRONAUT trial showed no reduction in cardiovascular death or HF rehospitalization with the addition of aliskiren to standard therapy in hospitalized HFrEF patients.9 In the ATMOSPHERE study, the addition of aliskiren to enalapril did not result in a lower risk of death or HF hospitalization compared to aliskiren or enalapril monotherapy.10 Thus, although direct renin inhibition did not reduce clinical events in HFrEF patients, it reduced RAAS escape, natriuretic peptides and adverse LV remodeling.8;9;11
In this trial, we observed a trend (p=0.10) for improvement in peak VO2 with an effect size of 0.5 ml/kg/min. Although relatively modest, this is similar in magnitude to that achieved with exercise training (0.6ml/kg/min) in HF-ACTION, the largest trial of exercise outcomes in HF patients and is similar to that often achieved in pharmacological trials HFrEF patients.12 In addition, since older HFpEF patients have such markedly impaired exercise capacity, even modest effects sizes (in absolute units) can be proportionally substantial and clinically meaningful. Furthermore, since trials of drug therapy for improving exercise capacity in HFpEF have been negative to date, this finding is worth pursuing further.
The present study also provides other valuable information to facilitate the design of a subsequent, definitive trial. The inclusion/exclusion criteria yielded a patient group that was typical of HFpEF seen in population-based studies, and who had severe exercise intolerance, impaired diastolic function, and reduced QOL. The enrollment rate was reasonably efficient (4.7 patients per month), there was a good safety profile, and retention and compliance were excellent. A future trial should also utilize the Kansas City Cardiomyopathy Questionnaire, which recent reports suggest is more sensitive for assessing QOL in HFpEF patients than the instrument we used.3
Limitations:
The p-values for peak VO2 (0.10) and for VAT (0.08) suggest the study may have been underpowered, particularly for the effect size we observed which was less than our sample size estimate. We performed additional analyses which indicated that if the number of completed patients in the trial had been 119, and other results remained the same (such as effect size and variability), then the study would have been positive on peak VO2 at the 0.05 level of significance. Of course, that includes some key assumptions that might not be met in a subsequent study, so this additional analysis results should be interpreted with caution. However, these results do provide valuable data for sample size calculations for a subsequent, larger, definitive trial. Similarly we cannot exclude the possibility that a longer duration of treatment may have shown a statistically significant improvement in exercise capacity. Because this was a single-center study, results require confirmation in a larger, multicenter trial.
Another limitation of our study is lack of more detailed hemodynamic measures. Even though patients had controlled BP at baseline, and aliskiren treatment was accompanied by only a small (but significant) reduction in systolic BP that did not appear related statistically to the improved exercise performance, we cannot definitively exclude a role for improved arterial function and / ventricular-vascular coupling, since we did not perform detailed assessments of central arterial hemodynamics (aortic stiffness, aortic distensibility, stroke volume/pulse pressure) particularly during exercise. These should be examined in future studies.
Supplementary Material
Acknowledgements / potential conflicts of interest:
In addition to partial funding for the present study from Novartis (which had no role in study conduct, data analysis, interpretation, or manuscript generation), Dr. KItzman reports the following potential financial conflicts of interest: Consultant for Abbvie, Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer-Ingelheim, GSK, and Actavis, current research grant funding from Novartis, Bayer, and GSK, and stock ownership in Gilead Sciences. Dr. Upadhya has received research funding from Novarits and Corvia.
Funding Sources: This study was funded, in part, by an investigator-initiated grant from Novartis Pharmaceuticals. However, Novartis had no role in study conduct, data analysis, interpretation, or manuscript generation. Also funded in part by: NIH R01AG18915 and R01AG045551; The Claude D. Pepper Older Americans Independence Center of Wake Forest University NIH P30AG21332; Clinical and Translational Science Institute of Wake Forest School of Medicine NIH UL1TR001420; and the Kermit G. Phillips II Chair in Cardiovascular Medicine of Wake Forest School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT00982033
Reference List
- (1).Gross O, Girgert R, Rubel D, Temme J, Theissen S, Muller GA. Renal protective effects of aliskiren beyond its antihypertensive property in a mouse model of progressive fibrosis. Am J Hypertens 2011;24:355–361. [DOI] [PubMed] [Google Scholar]
- (2).Kitzman DW, Hundley WG, Brubaker P, Stewart K, Little WC. A randomized, controlled, double-blinded trial of enalapril in older patients with heart failure and preserved ejection fraction; effects on exercise tolerance, and arterial distensibility. Circ Heart Fail 2010;3:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kitzman DW, Brubaker P, Morgan T et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomised clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992;20:301–306. [DOI] [PubMed] [Google Scholar]
- (5).Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190–1195. [DOI] [PubMed] [Google Scholar]
- (6).Fonseca C, Oliveira AG, Mota T et al. Evaluation of the performance and concordance of clinical questionnaires for the diagnosis of heart failure in primary care. Eur J Heart Fail 2004;6:813–822. [DOI] [PubMed] [Google Scholar]
- (7).Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol 2012;110:1809–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).McMurray JJ, Pitt B, Latini R et al. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail 2008;1:17–24. [DOI] [PubMed] [Google Scholar]
- (9).Gheorghiade M, Bohn M, Greene S et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: The ASTRONAUT randomized trial. JAMA 2013;309:1125–1135. [DOI] [PubMed] [Google Scholar]
- (10).McMurray JJ, Krum H, Abraham WT et al. Aliskiren, Enalapril, or Aliskiren and Enalapril in Heart Failure. N Engl J Med 2016;374:1521–1532. [DOI] [PubMed] [Google Scholar]
- (11).Solomon SD, Shin SH, Shah A et al. Effect of the direct renin inhibitor aliskiren on left ventricular remodelling following myocardial infarction with systolic dysfunction. Eur Heart J 2011;32:1227–1234. [DOI] [PubMed] [Google Scholar]
- (12).O'Connor CM, Whellan DJ, Lee KL et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


