Abstract
Objective
The insulin-like growth factor (IGF) axis is essential for the body’s metabolism. The hepatokine, insulin-like growth factor-binding protein 2 (IGFBP-2), acts as a major regulator of this metabolism. We aimed to evaluate the role of serum IGFBP-2 in the incidence of nonalcoholic fatty liver disease (NAFLD).
Methods
This hospital-based prospective cohort study recruited residents from a health program from January to November 2013, and re-invited them for follow-up in 2016. The occurrence of NAFLD was noted and IGFBP-2 levels were evaluated by enzyme-linked immunosorbent assay at both visits.
Results
Of 763 participants at baseline, 296 completed the re-evaluation. Baseline serum IGFBP-2 levels were significantly lower in subjects with NAFLD compared with those without NAFLD. Circulating IGFBP-2 levels were negatively correlated with body mass index, waist-to-hip ratio, alanine transaminase, triglycerides, fasting glucose, and insulin. IGFBP-2 levels at follow-up decreased in subjects who developed NAFLD compared with those who did not. Higher circulating levels of IGFBP-2 at baseline were negatively associated with the incidence of NAFLD.
Conclusion
These results indicate that IGFBP-2 levels are inversely associated with the risk of NAFLD. This offers new insights into the role of circulating IGFBP-2, as an IGF-axis hepatokine, in the pathogenesis of hepatic steatosis.
Keywords: Cohort study, hepatokine, insulin-like growth factor-binding protein 2, nonalcoholic fatty liver disease, insulin-like growth factor, metabolism
Introduction
Nonalcoholic fatty liver disease (NAFLD) is closely associated with obesity and diabetes, and is considered as a hepatic manifestation of metabolic syndrome (MetS).1 The liver plays a key role in the regulation of energy metabolism and insulin sensitivity, and consequently in the pathogenesis of metabolic disorders.2
The insulin-like growth factor (IGF) axis is an essential part of the body’s metabolism, and evidence supports a link between the IGF axis and metabolic diseases such as obesity and diabetes.3,4 Insulin-like growth factor-binding protein 2 (IGFBP-2) is a 34-kDa hepatokine secreted by the liver,5 originally identified through its binding to IGF-1 and IGF-2, and is one of six IGFBPs circulating in the plasma.6
Recent studies have revealed a beneficial effect of IGFBP-2 on metabolism. IGFBP-2 has been shown to improve insulin sensitivity and down-regulate adipogenesis,7 depending on IGF-1 signaling. IGFBP-2, as a liver-secreted protein,8 has also been shown to activate the phosphatidylinositol 3-kinase (PI3K) and protein kinase B signaling pathway, via binding to α5β1-integrin receptors on the cell surface, leading to intracellular translocation of glucose transporter type 4 and promotion of glucose uptake.3,9,10 Allen et al.11 found that older women had higher IGFBP-2 levels than younger women, and obese women had lower levels than lean women. Heald et al.12 found lower IGFBP-2 levels in subjects with MetS, based on a cohort study of 163 patients with type 2 diabetes, and low circulating levels of IGFBP-2 correlated with increased glucose, triglycerides, and low-density lipoprotein cholesterol, and were positively correlated with insulin sensitivity, suggesting that IGFBP-2 was negatively associated with MetS risk. Rajpathak et al.3 reported that subjects with type 2 diabetes had lower circulating IGFBP-2 levels, and IGFBP-2 levels were negatively correlated with body mass index (BMI), waist circumference, C-reactive protein, and insulin, and were inversely associated with type 2 diabetes risk. A cross-sectional study of 379 Caucasian men indicated that subjects with low IGFBP-2 levels had increased fat mass, high triglycerides, and impaired insulin sensitivity,5 while van den Beld and colleagues13 found that IGFBP-2 concentrations were negatively correlated with BMI and positively with insulin sensitivity and age. Wittenbecher et al.14 reported that higher circulating IGFBP-2 levels were associated with lower BMI, waist circumference, fatty liver index, triglycerides, fetuin A, alanine transaminase (ALT), and γ-glutamyltransferase, and a lower incidence of type 2 diabetes, while IGFBP-2 gene methylation levels were also associated with the incidence of type 2 diabetes.
A better understanding of the link between hepatokines and metabolism may promote the effective management of metabolic diseases. We therefore conducted a hospital-based prospective cohort study to evaluate the role of circulating IGFBP-2, as a novel hepatokine, in the incidence of NAFLD.
Methods
Study design and patients
This hospital-based cohort study recruited participants from a health program at the First Affiliated Hospital of Zhejiang University from January to November 2013. The participants were then re-invited for follow-up in 2016. Patients were excluded if they met any of the following criteria: malignant tumor, severe cardiopulmonary disorder, renal dysfunction, liver disease (other than NAFLD), pregnancy, endocrine disease, therapy history of estrogens or steroids, and excessive alcoholic consumption (>140 g/week men, >70 g/week women).
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University, in accordance with the Helsinki Declaration. Written informed consent was obtained from all subjects before participation.
Examinations
Fasting blood samples were obtained from all participants and stored for further evaluation, as reported previously.15,16 Circulating IGFBP-2 levels were measured by enzyme-linked immunosorbent assay (ELISA) (catalog no. CSB-E04588h; CUSABIO Corp., Wuhan, China). The intra- and interassay variations in circulating IGFBP-2 measurements were 4.9% and 10.2%, respectively. The IGFBP-2 ELISA results were validated in eight randomly selected serum samples by western blotting. Proteins were extracted using RIPA buffer with added protein and phosphatase inhibitor (Sigma, Darmstadt, Germany), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to polyvinylidine difluoride membranes. Membranes were immunoblotted with anti-IGFBP-2 (catalog no. 3922; Cell Signaling Technology, Danvers, MA, USA) (1:1000) and rabbit monoclonal glyceraldehyde 3-phosphate dehydrogenase (catalog no. ab128915, Abcam Inc., Cambridge, MA, USA) (1:2000) for 12 hours at 4°C. NAFLD was diagnosed according to the guidelines for the diagnosis and treatment of NAFLD issued by the Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association.17–19
Statistical methods
Variables were compared between groups using Student’s t-test or Mann–Whitney U test for continuous variables, and χ2 test for categorical variables. Differences in NAFLD according to quartiles of serum IGFBP-2 were analyzed by χ2 test. Baseline and follow-up values were compared by Wilcoxon’s matched-pairs signed rank test. The relationship between baseline IGFBP-2 levels and the incidence of NAFLD at follow-up was analyzed by adjusted odds ratios (ORs) and 95% confidence intervals (CIs) obtained by multivariate binary logistic regression. Correlations between serum IGFBP-2 levels and anthropometric/biomedical variables were determined by partial correlation coefficients. The performance of the ELISA kits was validated by Cronbach’s Alpha test. All analyses were performed using SPSS Statistics for Windows, Version 21.0 (SPSS Inc., Chicago, IL, USA). The sample size power (post hoc) was calculated using G*Power (version 3.1, Heinrich-Heine-Universität Düsseldorf, Germany).20 A two-sided value of P < 0.05 was considered significant.
Results
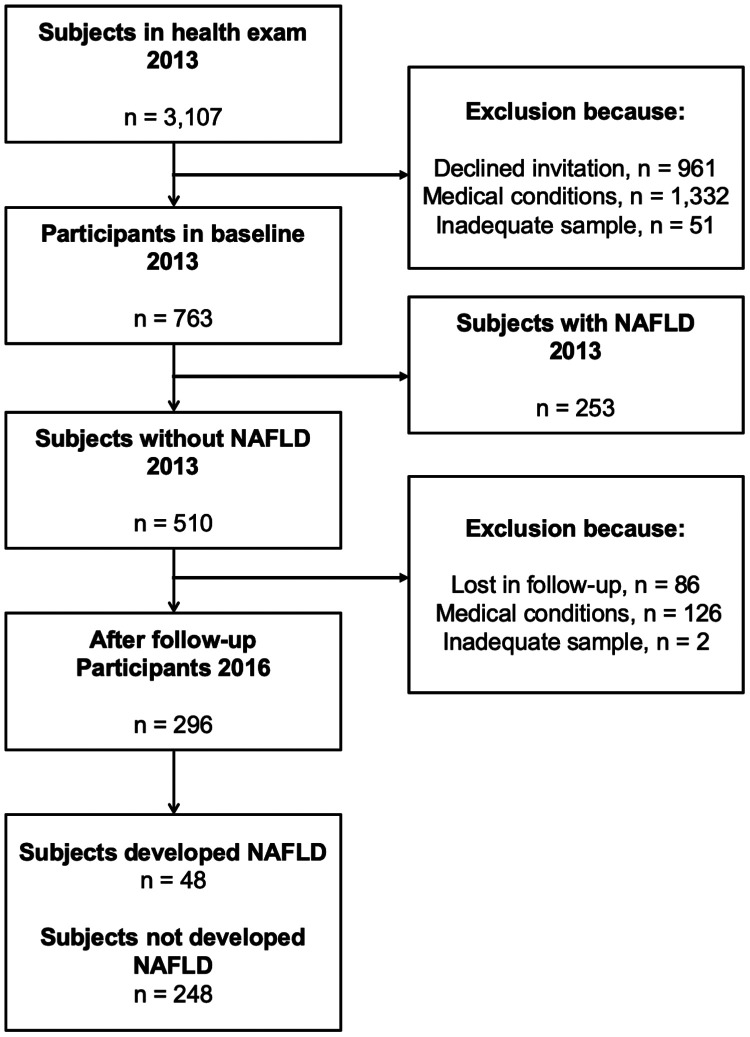
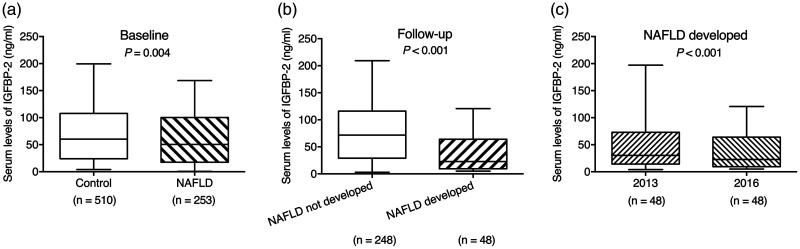
A total of 763 participants were included at baseline, of whom 296 completed re-evaluation (Figure 1). The subjects’ baseline characteristics according to the presence/absence of NAFLD are shown in Table 1. The ELISA results for IGFBP-1 showed excellent consistency with the quantified densitometry of the IGFBP-2/GAPDH ratio in western blotting (Cronbach’s Alpha based on standardized items = 0.850) (Figure S1). Serum IGFBP-2 levels (median [interquartile range]) were significantly lower in subjects with NAFLD (50.6 [17.6–100.4] ng/mL) compared with controls without NAFLD (60.2 [24.0–107.8] ng/mL) (P = 0.004) (Figure 2a). Given the circulating IGFBP-2 levels and numbers of subjects, the power of the sample size at baseline was 0.784 (effect size d = 0.211).
Figure 1.
Flow chart of the study population.
Table 1.
Baseline characteristics of subjects with and without nonalcoholic fatty liver disease.
| Parameter | Non-NAFLD | NAFLD | P value |
|---|---|---|---|
| Number of subjects | 510 | 253 | |
| Age (years) | 53.6 ± 12.7 | 54.0 ± 13.3 | 0.749 |
| Number of men | 332 | 162 | 0.809 |
| SBP (mmHg) | 112.1 ± 22.5 | 121.0 ± 23.1 | <0.001 |
| DBP (mmHg) | 73.8 ± 16.3 | 79.0 ± 16.4 | <0.001 |
| BMI (kg/m2) | 22.7 ± 3.3 | 23.4 ± 3.6 | 0.006 |
| WHR | 0.82 ± 0.09 | 0.87 ± 0.09 | <0.001 |
| ALT (U/L) | 20.0 [13.0–31.0] | 23.0 [15.0–33.0] | 0.003 |
| GGT (U/L) | 33.0 [23.0–49.0] | 32.0 [23.0–53.0] | 0.854 |
| TG (mmol/L) | 1.12 [0.82–1.85] | 1.40 [1.02–2.10] | <0.001 |
| HDL-C (mmol/L) | 1.11 [0.89–1.39] | 1.10 [0.92–1.38] | 0.628 |
| LDL-C (mmol/L) | 2.27 [1.73–2.83] | 2.45 [1.87–3.13] | 0.001 |
| FPG (mmol/L) | 3.90 [3.05–5.70] | 4.43 [3.74–5.92] | <0.001 |
| Insulin (mU/L) | 9.89 [6.66–14.1] | 10.0 [0.76–14.8] | 0.288 |
| HbA1c (%) | 6.28 [5.20–9.00] | 6.20 [5.20–8.85] | 0.958 |
| C-peptide (ng/ml) | 0.99 [0.65–1.44] | 0.99 [0.65–1.46] | 0.976 |
| UA (μmol/L) | 320.0 [260.0–378.8] | 313.0 [260.0–360.5] | 0.152 |
Data are mean ± standard deviation or median (interquartile range) for continuous variables. ALT, alanine transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GGT, γ-glutamyltransferase; HbA1c, hemoglobin A1C; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; SBP, systolic blood pressure; TG, triglycerides; UA, serum uric acid; WHR, waist-to-hip ratio.
Figure 2.
Comparison of serum insulin-like growth factor-binding protein 2 levels. (a) Baseline in 2013: control vs. nonalcoholic fatty liver disease (NAFLD) (P = 0.004); (b) follow-up in 2016: developed NAFLD vs. not developed (P < 0.001); (c) NAFLD developed after 3 years: baseline vs. follow-up (P < 0.001). Data presented as median (interquartile range). IGFBP-2, insulin-like growth factor-binding protein 2; NAFLD, nonalcoholic fatty liver disease.
Baseline IGFBP-2 levels were significantly negatively correlated with BMI, waist-to-hip ratio (WHR), ALT, triglycerides, fasting plasma glucose (FPG), and insulin (Table 2).
Table 2.
Partial correlations between serum insulin-like growth factor-binding protein 2 and various parameters at baseline.
| Parameter | r value | P value |
|---|---|---|
| SBP | −0.045 | 0.212 |
| DBP | −0.012 | 0.739 |
| BMI | −0.159 | <0.001 |
| WHR | −0.120 | 0.001 |
| ALT | −0.145 | <0.001 |
| GGT | −0.070 | 0.052 |
| TG | −0.164 | <0.001 |
| HDL-C | 0.009 | 0.814 |
| LDL-C | −0.034 | 0.348 |
| FPG | −0.170 | <0.001 |
| Insulin | −0.124 | 0.001 |
| HbA1c | −0.020 | 0.580 |
| C-peptide | −0.048 | 0.185 |
| UA | 0.022 | 0.548 |
Correlation coefficients calculated after adjusting for age and sex. Variables with a skewed distribution underwent log(x) transformation to achieve a normal distribution before analysis. ALT, alanine transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GGT, γ-glutamyltransferase; HbA1c, hemoglobin A1C; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglycerides; UA, serum uric acid; WHR, waist-to-hip ratio.
IGFBP-2 levels were significantly lower in subjects who developed NAFLD (22.9 [9.5–64.0] ng/mL) compared with those without NAFLD at follow-up (71.7 [29.2–116.1] ng/mL (P < 0.001) (Figure 2b). IGFBP-2 levels fell significantly between baseline and follow-up in subjects who developed NAFLD (P < 0.001) (Figure 2c).
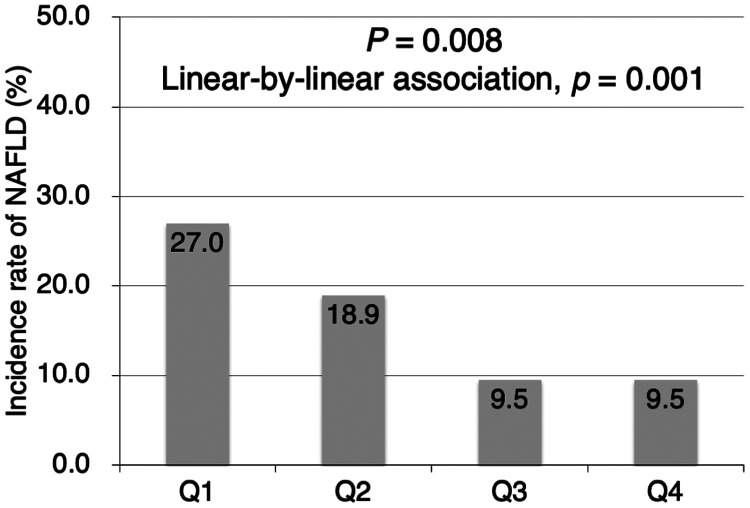
The incidence of NAFLD at the 3-year follow-up was 27.0% in participants stratified in the first quartile according to baseline IGFBP-2 levels, decreasing to 18.9% in the second and 9.5% in the third and fourth quartiles (P = 0.008, linear-by-linear association P = 0.001) (Figure 3).
Figure 3.
Incidence of nonalcoholic fatty liver disease according to insulin-like growth factor-binding protein 2 quartile. (P = 0.008, linear-by-linear association, P = 0.001). NAFLD, nonalcoholic fatty liver disease; Q, quartile.
Higher circulating levels of IGFBP-2 at baseline were negatively associated with the incidence of NAFLD (third quartile: adjusted OR = 0.492, 95% CI [0.282–0.861], P = 0.0013; fourth quartile: adjusted OR = 0.620, 95% CI [0.426–0.902], p = 0.012), compared with the first quartile (Table 3).
Table 3.
Baseline serum insulin-like growth factor-binding protein 2 prediction of development of nonalcoholic fatty liver disease at 3 years.
| Reference | Adjustment | Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|---|---|
| Quartile 1 (4.0–6.7 ng/mL) | Quartile 2 (26.7–62.9 ng/mL) | Model 1 | 0.630 | 0.290–1.368 | 0.243 |
| Model 2 | 0.543 | 0.218–1.350 | 0.189 | ||
| Quartile 3 (63.3–110.9 ng/mL) | Model 1 | 0.531 | 0.333–0.847 | 0.008 | |
| Model 2 | 0.492 | 0.282–0.861 | 0.013 | ||
| Quartile 4 (110.29–199.44 ng/mL) | Model 1 | 0.656 | 0.481–0.895 | <0.001 | |
| Model 2 | 0.620 | 0.426–0.902 | 0.012 |
Model 1: unadjusted; Model 2: adjusted for age, sex, systolic blood pressure, diastolic blood pressure, body mass index, waist-to-hip ratio, alanine transaminase, γ-glutamyltransferase, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, hemoglobin A1C, fasting plasma glucose, insulin, C-peptide, and serum uric acid.
Discussion
This study demonstrated that subjects with NAFLD had lower levels of circulating IGFBP-2 than those without NAFLD at baseline. IGFBP-2 was also correlated negatively with BMI, WHR, ALT, triglycerides, FPG, and fasting insulin. Furthermore, IGFBP-2 levels were lower in subjects who developed NAFLD at follow-up. Interestingly, even among the subjects who developed NAFLD, their IGFBP-2 levels decreased after 3-years of follow-up, compared with their baseline levels. Notably, circulating IGFBP-2 levels at baseline were negatively associated with the incidence of NAFLD at follow-up.
The bioavailability of IGFs is modulated by a series of binding proteins.21,22 IGFBP-2 has been reported to be a major regulator of metabolism,23 with both stimulatory and depressive effects on the cellular actions of IGF-1 and IGF-2.24 Hedbacker et al.25 found that IGFBP-2 markedly reduced hepatic glucose production and down-regulated hepatic fatty acid synthesis, which improved hepatic insulin sensitivity. Wheatcroft et al.9 found that IGFBP-2 overexpression protected mice from high-fat diet-induced obesity, insulin resistance, and other metabolic consequences in vivo, while recombinant IGFBP-2 ameliorated adipogenesis in adipocytes in vitro. Ahrens and colleagues26 reported that the IGFBP2 locus was hypermethylated and its transcript levels were significantly downregulated in patients with nonalcoholic steatohepatitis. Kammel et al.27 found that IGFBP-2 expression was inhibited in mice with diet-induced obesity, associated with methylation of hepatic IGFBP2, while genetic depression of IGFBP2 aggravated high-fat diet-induced hepatic steatosis. The mechanism of IGFBP-2 in the development of NAFLD might be attributed to its involvement in insulin pathways.25 Previous studies revealed that IGFBP-2 influenced insulin-dependent pathways in a compensatory way in diabetic models.25 Assefa et al.28 found that IGFBP-2 promoted glucose uptake via activation of PI3K, Akt, and 5′ AMP-activated protein kinase signaling, rather than via insulin or the IGF-1 receptor.
Previous reports showed lower circulating IGFBP-2 levels in subjects with obesity, diabetes, and MetS.5,11,13,14 However, limitations of study design meant that most of these studies failed to explore the cause-and-effect relationship. The current cohort study thus provides the first evidence for an inverse association between circulating IGFBP-2 and the incidence of NAFLD.
This study had some limitations. First, this was a single-center study that enrolled participants from one urban area, leading to potential selection bias, and the results thus need to be interpreted with caution. Second, the gold standard for the diagnosis of NAFLD is liver pathological examination rather than ultrasonography,18,29 though the latter has several advantages including being more economical, safer, and repeatable.30 Further investigations using other non-invasive radiological tools, such as magnetic resonance imaging, should therefore be performed to confirm these results. Finally, this study did not consider the impacts of variables such as physical activity, diet, alcohol consumption, and economic status because of a lack of data.
In conclusion, the results of this prospective cohort study support the existence of an inverse relationship between circulating IGFBP-2 levels and the risk of NAFLD. These findings offer new insights into the role of IGFBP-2, as an IGF-axis hepatokine, in the pathogenesis of hepatic steatosis, and highlight its potential as a target for the development of new diagnostic or therapeutic strategies.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520935219 for Circulating IGFBP-2 levels are inversely associated with the incidence of nonalcoholic fatty liver disease: A cohort study by Ji Yang, Wenjing Zhou, Yue Wu, Liqian Xu, Yuming Wang, Zherong Xu and Yunmei Yang in Journal of International Medical Research
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by the National Natural Science Foundation of China (Recipient: Yunmei Yang; No. 81771498), the Science and Technology Program of Medicine and Health of Zhejiang Province (2018KY372), The Construction Project of National Key Clinical Geriatrics Department, and Demonstration Base for Clinical Nutrition Implementation in Geriatrics.
ORCID iD
Yunmei Yang https://orcid.org/0000-0002-6646-4954
Supplemental material
Supplemental material for this article is available online.
References
- 1.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014; 59: 713–723. DOI: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of fatty liver. Endocr Rev 2008; 29: 939–960. DOI: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 3.Rajpathak SN, He M, Sun Q, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes 2012; 61: 2248–2254. DOI: 10.2337/db11-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandhu MS, Heald AH, Gibson JM, et al. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet 2002; 359: 1740–1745. DOI: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 5.Carter S, Li Z, Lemieux I, et al. Circulating IGFBP-2 levels are incrementally linked to correlates of the metabolic syndrome and independently associated with VLDL triglycerides. Atherosclerosis 2014; 237: 645–651. DOI: 10.1016/j.atherosclerosis.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Kelley KM, Schmidt KE, Berg L, et al. Comparative endocrinology of the insulin-like growth factor-binding protein. J Endocrinol 2002; 175: 3–18. [DOI] [PubMed] [Google Scholar]
- 7.Russo VC, Azar WJ, Yau SW, et al. IGFBP-2: the dark horse in metabolism and cancer. Cytokine Growth Factor Rev 2015; 26: 329–346. DOI: 10.1016/j.cytogfr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Wu Z, Ren G, et al. Expression patterns of insulin-like growth factor system members and their correlations with growth and carcass traits in Landrace and Lantang pigs during postnatal development. Mol Biol Rep 2013; 40: 3569–3576. DOI: 10.1007/s11033-012-2430-1. [DOI] [PubMed] [Google Scholar]
- 9.Wheatcroft SB, Kearney MT, Shah AM, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes 2007; 56: 285–294. DOI: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frommer KW, Reichenmiller K, Schutt BS, et al. IGF-independent effects of IGFBP-2 on the human breast cancer cell line Hs578T. J Mol Endocrinol 2006; 37: 13–23. DOI: 10.1677/jme.1.01955. [DOI] [PubMed] [Google Scholar]
- 11.Allen NE, Appleby PN, Kaaks R, et al. Lifestyle determinants of serum insulin-like growth-factor-I (IGF-I), C-peptide and hormone binding protein levels in British women. Cancer Causes Control 2003; 14: 65–74. [DOI] [PubMed] [Google Scholar]
- 12.Heald AH, Kaushal K, Siddals KW, et al. Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp Clin Endocrinol Diabetes 2006; 114: 371–376. DOI: 10.1055/s-2006-924320. [DOI] [PubMed] [Google Scholar]
- 13.Van Den Beld AW, Carlson OD, Doyle ME, et al. IGFBP-2 and aging: a 20-year longitudinal study on IGFBP-2, IGF-I, BMI, insulin sensitivity and mortality in an aging population. Eur J Endocrinol 2019; 180: 109–116. DOI: 10.1530/EJE-18-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittenbecher C, Ouni M, Kuxhaus O, et al. Insulin-like growth factor binding protein 2 (IGFBP-2) and the risk of developing type 2 diabetes. Diabetes 2019; 68: 188–197. DOI: 10.2337/db18-0620. [DOI] [PubMed] [Google Scholar]
- 15.Dai YN, Zhu JZ, Fang ZY, et al. A case-control study: association between serum neuregulin 4 level and non-alcoholic fatty liver disease. Metabolism 2015; 64: 1667–1673. DOI: 10.1016/j.metabol.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Zhou W, Wang Y, Wu Y, et al. Serum CTRP3 level is inversely associated with nonalcoholic fatty liver disease: a 3-y longitudinal study. Clin Chim Acta 2018; 479: 79–83. DOI: 10.1016/j.cca.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 2007; 22: 775–777. DOI: 10.1111/j.1440-1746.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 18.Zeng MD, Fan JG, Lu LG, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis 2008; 9: 108–112. DOI: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 19.Graif M, Yanuka M, Baraz M, et al. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Invest Radiol 2000; 35: 319–324. [DOI] [PubMed] [Google Scholar]
- 20.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41: 1149–1160. DOI: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 21.Bach LA, Headey SJ, Norton RS. IGF-binding proteins–the pieces are falling into place. Trends Endocrinol Metab 2005; 16: 228–234. DOI: 10.1016/j.tem.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol Metab 2016; 27: 375–391. DOI: 10.1016/j.tem.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Boney CM, Moats-Staats BM, Stiles AD, et al. Expression of insulin-like growth factor-I (IGF-I) and IGF-binding proteins during adipogenesis. Endocrinology 1994; 135: 1863–1868. DOI: 10.1210/endo.135.5.7525256. [DOI] [PubMed] [Google Scholar]
- 24.Hoeflich A, Reisinger R, Lahm H, et al. Insulin-like growth factor-binding protein 2 in tumorigenesis: protector or promoter? Cancer Res 2001; 61: 8601–8610. [PubMed] [Google Scholar]
- 25.Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab 2010; 11: 11–22. DOI: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Ahrens M, Ammerpohl O, Von Schonfels W, et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab 2013; 18: 296–302. DOI: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Kammel A, Saussenthaler S, Jahnert M, et al. Early hypermethylation of hepatic IGFBP2 results in its reduced expression preceding fatty liver in mice. Hum Mol Genet 2016; 25: 2588–2599. DOI: 10.1093/hmg/ddw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assefa B, Mahmoud AM, Pfeiffer AFH, et al. Insulin-like growth factor (IGF) binding protein-2, independently of IGF-1, induces GLUT-4 translocation and glucose uptake in 3T3-L1 adipocytes. Oxid Med Cell Longev 2017; 2017: 3035184. DOI: 10.1155/2017/3035184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc 2015; 90: 1233–1246. DOI: 10.1016/j.mayocp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123: 745–750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520935219 for Circulating IGFBP-2 levels are inversely associated with the incidence of nonalcoholic fatty liver disease: A cohort study by Ji Yang, Wenjing Zhou, Yue Wu, Liqian Xu, Yuming Wang, Zherong Xu and Yunmei Yang in Journal of International Medical Research