Abstract
Objective
To investigate Acacia honey from different altitudes regarding total phenols and flavonoids, laser-induced fluorescence (LIF) spectra and anticancer activity against human cancer cell lines.
Methods
Anticancer activity was investigated using sulforhodamine B cytotoxicity assays in the following human cancer cell lines: HCT116 (colon); MCF7 (breast), and HepG2 (liver). Total phenols and flavonoids were measured using spectrophotometric methods and LIF was used to differentiate between low and high-altitude honey.
Results
The LIF spectra differed between low and high-altitude Acacia honey. High altitude Acacia honey was characterized by significantly lower total phenol content (81.47 ± 1.25 mg gallic acid equivalent [GAE]/100 g) and increased total flavonoids (10.63 ± 0.53 mg quercetin equivalent [QE]/100 g) versus low altitude Acacia honey (91.33 ± 0.96 mg GAE/100 g and 8.78 ± 0.23 mg QE/100 g, respectively). Low altitude Acacia honey displayed increased IC50 values against HCT116 and MCF7 cells (264.17 ± 10.5 and 482.65 ± 20.3 µg/ml, respectively) versus high altitude Acacia honey (117.99 ± 12.7 and 189.82 ± 15.8 µg/ml, respectively).
Conclusions
High altitude Acacia honey had significantly more effective anticancer activity against HCT116 and MCF7 cells compared with low altitude honey.
Keywords: Bee honey, high and low altitudes, HCT116, MCF7, IC50, arbitrary units
Introduction
Honey is a natural substance produced by honeybees from plant nectar and secretions, and from the excretions of plant sucking insects (raw materials). Honeybees collect these raw materials, add some chemicals of their own, such as carbohydrates, enzymes, and vitamins, and leave the mixture in honeycombs to mature.1 The colour of honey ranges from clear to dark amber and it is found in four states; liquid, viscous, crystalline, and a mixture of liquid and crystalline. Chemically, honey contains many compounds, such as carbohydrates (glucose, fructose, and sucrose), enzymes (catalase and glucose oxidase), acids (gluconic and phenolic acids), proteins, flavonoids, hydrogen peroxide, dicarbonyl compounds, vitamins, and minerals.2,3 The composition of honey facilitates its nutritional and medicinal uses. For example, honey is used as an antimicrobial agent because of its dicarbonyl compounds (methylglyoxal) and hydrogen peroxide content, and it acts as an antioxidant because it contains flavonoids, vitamins and minerals.4,5
Altitude has been reported to significantly and positively affect the concentration of many honey ingredients, such as antioxidants, including total phenols and flavonoids, minerals, and vitamin C.5–7 Many studies have reported that the content of bee honey is variable regarding phenols, total flavonoids, and total phenolic acids, the content of which may be used as indicators for the botanical origin of the honey.8–17 The phenolic and flavonoids concentration in honey is associated with its antimicrobial, antitumor and antioxidant activities.5,12,13
The laser-induced fluorescence (LIF) technique has proved to be useful, simple, and nondestructive for the confirmation of floral origin and authentication of bee honey.18–20
Regarding the anticancer properties of honey, some studies have shown that honey samples have anticancer activity, while others have reported that honey is ineffective as an anticancer agent.21–24
The aim of the present study was to analyse Acacia honey samples from high altitude (2 246 m above sea level) and low altitude (437 m above sea level) in terms of the concentration of total polyphenols and flavonoids, the LIF spectra, and the activity against human colon cancer (HCT116), breast cancer (MCF7) and liver cancer (HepG2) cell lines.
Materials and methods
Study area
This study was conducted in the Asir region in the southwest of Saudi Arabia. The Asir region comprises an area of 80 000 km2 with an estimated population of 2.19 million, and is located between the latitudes of 17.25 and 19.5 North and longitudes of 41.3 and 44.3 East.25
Asir land is divided into three regions: the Tehama region, which is located between the red sea and the mountains of Alsarah; the Alsarah, which comprises the Alsarah mountains that extend up to the Yemen; and a region of flat land at the eastern part. The altitude of Asir ranges from the red sea level up to more than 3 000 m above sea level at Alsouda.25,26 The Asir climate is moderate, with a 20–25 °C mean temperature and year-round rainfall. However, climate differences exist between the three regions of Asir according to their geographical location; near the red sea, high altitudes and the flat land in the eastern part.25
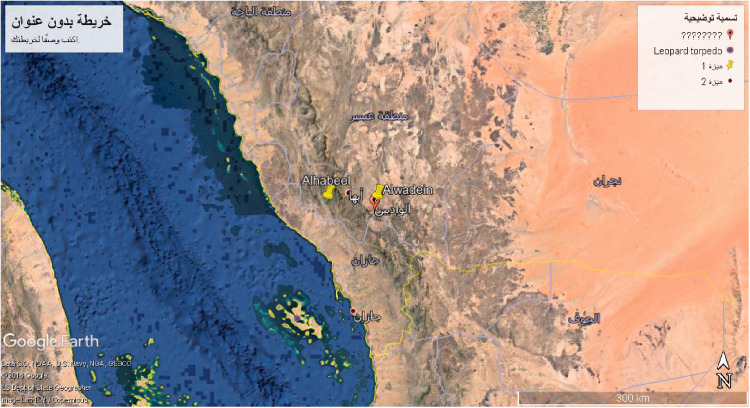
The bee farms from which the honey samples were collected are located at two elevations; 437 m 05° 18′ 56.51′′ N; 13° 42′ 52.72′′ E) and 2 246 m (05° 18′ 50.52′′ N; 48° 42′ 54.12′′ E; Figure 1).
Figure 1.
Satellite view of the two bee farm locations from which honey samples were collected for the present study: Alhabeel (437 m above sea level) and Alwadein (2 246 m above sea level).
Honey samples
Sample collection and treatment
Three Acacia honey samples from Alwadein (2 246 m above sea level) and three Acacia honey samples from Alhabeel (437 m above sea level) were collected directly from the hives of the bee farms (to avoid adulterated honey samples) during the summer season of 2018. Three Acacia species were flowering during the collection period; Acacia tortilis, Acacia ehrenbergiana, and Acacia origena. The Acacia honey was separated from the honeycomb by filtration using a nylon filter.
Confirmation of floral origin
The botanical origin was confirmed microscopically as follows: 10 ml of each honey sample was diluted with 10 ml distilled water and centrifuged for 5 min at 4 024.8 g. The supernatant was discarded, the pellet was mounted on a microscope slide, and the pollens were counted in 10 microscopic fields. Acacia pollens are characterized by circular shapes with four central monads (chambers) surrounded by eight monads. The percentage of Acacia pollens was determined according to the following equation:
Authentication of honey samples
Three quality parameters, namely pH, conductivity, and moisture percentage, were measured and compared to their value in the Codex Alimentaruis standards, USA National Honey Board reference guide, and the gulf countries standardization organization.27–29 The pH and conductivity were determined according to the methods of the International Honey Commission.30,31 Moisture percentage was obtained using the oven drying method according to the Association of Official Analytical Chemists (AOAC).32
Determination of total phenols and flavonoids
Honey sample treatment
A 5 g portion of each honey sample was placed in a 100 ml beaker, thoroughly mixed with 50 ml deionised water and magnetically stirred for 5–10 min at room temperature. The obtained honey solution was filtered through grade 1 Whatman® qualitative filter paper to obtain 0.1 g/ml honey solution, which was kept for the determination of phenolic compounds and flavonoids concentration.
Measurement of total phenols
First, 0.2N Folin–Ciocalteu reagent was prepared from 2N Folin–Ciocalteu reagent (code, 47641-100ML-F; Sigma-Aldrich; St Louis, MI, USA) as follows: 10 ml of 2N Folin–Ciocalteu reagent were diluted with 90 ml of distilled water in a 100 ml volumetric flask. A 1 ml aliquot of each 0.1 g/ml honey sample was then added to 5 ml of 0.2 N Folin–Ciocalteu reagent in a test tube. The mixture was shaken for 5 min then 4 ml of saturated sodium carbonate solution (7.5% w/v; 13418-1KG-R; Sigma-Aldrich) was added. The test tube was covered with silver foil and incubated at room temperature for 90 min with intermittent shaking. After incubation, the absorbance of the reaction mixture was measured in a spectrophotometer at 760 nm against the blank. The mean of three readings was calculated, and total phenolic content was presented as mg of gallic acid equivalent (GAE) per 100 g of honey.33
The gallic acid standard curve was produced as follows: A stock solution of 5 mg/ml gallic acid was prepared by weighing 0.500 g of dry gallic acid (G7384-250G; Sigma-Aldrich) in a 100 ml volumetric flask and dissolving in 10 ml water: methanol solution (1: 1). Deionized distilled water: methanol solution (1: 1) was added to bring the volume up to the mark. A range of gallic acid standards (0, 0.005, 0.01, 0.02, 0.025, 0.05, 0.1, 0.2 mg/ml) were prepared using water: methanol (1: 1) solution. These standards were used to produce the calibration curve which was analysed in the same way as the honey samples.
The concentration of total phenols was calculated according to the following equation: T = (G × V × 100)/M, where T = total phenolic compounds content (mg GAE/100 g); G = concentration of gallic acid in mg/ml; V = volume of honey sample solution in ml (50 ml); and M = weight of honey sample in g (5 g).
Measurement of flavonoids concentration
First, 2 ml of a 2% aluminium chloride methanolic reagent (237051-500G; Sigma-Aldrich) was added to the same volume of honey extract solution (2 mg/ml). After 30 min of incubation, the absorbance was read at 415 nm and compared with a blank sample consisting of a honey solution along with methanol without AlCl3. The total flavonoid content was calculated using a quercetin calibration curve. The mean of three readings was calculated and results were presented as mg of quercetin equivalent per 100 g of honey (mg QE/100 g).34
A stock solution of 0.5 mg/ml quercetin (Q4951-100G; Sigma-Aldrich) was prepared by dissolving 0.050 g in 10 ml methanol (95%) in a 100 ml volumetric flask. Methanol was then added to bring the volume up to the final volume. A range of quercetin standards (0.0005, 0.001, 0.002, 0.005, 0.01, 0.02, 0.025 and 0.05 mg/ml) were prepared. Quercetin standard solutions were used to produce the calibration curve, which was analysed in the same manner as the honey samples.
The concentration of total flavonoids in honey samples was determined according to the following equation: T = (G × V × 100)/M, where T = total flavonoids content (mg QE/100 g); G = concentration of quercetin in mg/ml; V = volume of honey sample solution in ml (50 ml); and M = weight of honey sample in g (5 g).
Laser induced fluorescence (LIF) experimental setup
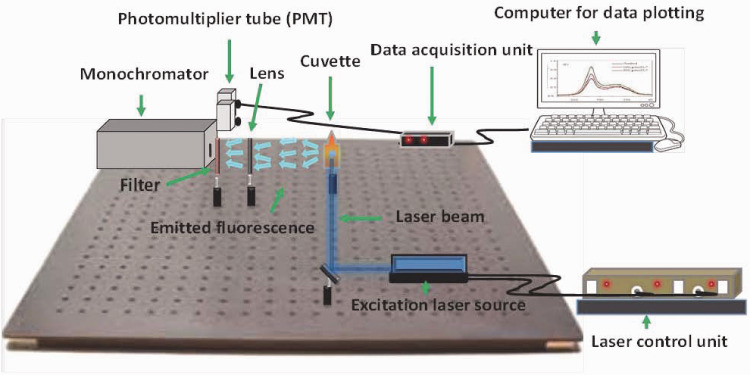
The LIF experimental set up is shown in Figure 2. A diode laser (Pro100; Toptica Photonics Inc., Munich, Germany) with a maximum average power output of 29 mW at wavelength 398 nm was used as an excitation source in the experiment.
Figure 2.
Schematic of laser induced fluorescence experimental setup.
The output power of the laser beam was controlled by means of a laser control unit that changed the current and temperature of the diode. For this experiment, an average output power of 2 mW was used. The laser beam was focused to 2 mm onto one side of a quartz cuvette containing the honey sample. The emitted fluorescence radiation was collected using a lens that focused the radiation and sent it to a monochromator (ScienceTech 9055; ScienceTech lnc., London, Canada). A long pass filter (FEL0450; Thorlabs Inc., Newton, NJ, USA) was used to block the excitation laser and at the same time to pass the fluorescence radiation only. The sample holder and the monochromator were set to be perpendicular to each other. The monochromator slit was opened at a width of 0.2 mm. This arrangement offered a spectral resolution of 0.2 nm. The fluorescence signal was analysed inside the monochrome and then exited through another slit to fall on a photomultiplier tube (PMT). The PMT converted the light signal to a voltage. The signal was then passed to the data acquisition unit connected to a computer, to read and draw the signal as a function of the wavelength change (Figure 2). LIF values are presented as arbitrary units (a.u.).
Sulforhodamine B cytotoxicity assay
The anticancer activity of honey samples from two different altitudes was assessed using the calorimetric Sulforhodamine B (SRB) cytotoxicity assay, with all cells and reagents purchased from Sigma-Aldrich, as follows: Human cancer cell lines (HCT116, MCF7 and HepG2) were maintained in the appropriate optimum media (Dulbecco's Modified Eagle's Medium [DMEM]; D6429-500ML) or (Roswell Park Memorial Institute [RPMI]-1640; R0883-500ML), containing 100 U/ml penicillin (13752-5G-F), 10% v/v heat inactivated fetal bovine serum (F4135-500ML), and 100 µg/ml streptomycin (S9137-100G). Cells were grown in a humidified incubator with 5% CO2 atmosphere at 37 °C. Exponentially growing cells were collected using 0.25% w/v trypsin-EDTA (T4049-500ML) and seeded at 1–2 × 103 cells/well in 96-well plates. Cells were then treated with serial concentrations of the Acacia honey samples for 72 h and compared to untreated control cells. Doxorubicin (44583-50MG) was tested against the same cancer cell lines for comparison purposes. Cells were fixed by incubation with 10% trichloroacetic acid (TCA; T8657-250G) for 1 h at 4 °C; washed three times with distilled water; subjected to 0.4% w/v SRB solution (230162-5G) for 15 min in the dark; and then washed with 1% v/v glacial acetic acid (27225-2.5L-R). SRB-stained cells were dissolved in Tris-HCl (pH 7.4; 93313-1L) and the colour intensity was assessed at 570 nm. Dose-response curves were fitted using Sigma Plot software, version 12.0 (Systat software, San Jose, CA, USA).35 The half maximum inhibitory concentrations (IC50 values) were calculated for high and low altitude honey samples and for the doxorubicin positive control.
Statistical analyses
Data are presented as mean ± SD, and differences between mean values of the studied parameters in the different honey samples were statistically analysed using Student’s t-test. All statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc. Chicago, IL, USA). A P value ≤0.05 was considered statistically significant.
Results
All of the Acacia honey samples were found to be monofloral (more than 50% of the pollens were Acacia pollens). The pH and moisture percentage values of all the Acacia honey samples were within their ranges in the Codex Alimentaruis standards and the US national honey board reference guide.27,28 The conductivity of the high altitude honey (1650.6 µS/cm) exceeded the Codex standards for honey (<800 µS/cm), but was within the range determined by the gulf countries standardization organization (<2 000 µS/cm).29 Altitude was shown to significantly affect the studied quality parameters (Table 1).
Table 1.
Concentration of total phenol compounds and total flavonoids, and authentication parameters, in Acacia honey samples obtained from low and high altitudes.
| Sample | Total phenols, mg GAE/100 g | Total flavonoids, mg QE/100 g | pH | Conductivity,μS/cM | Moisture, % |
|---|---|---|---|---|---|
| Acacia honey from 437 m | 91.33 ± 0.96 | 8.78 ± 0.23 | 4.36 ± 0.08 | 184.6 ± 4.51 | 13.5 ± 0.76 |
| Acacia honey from 2246 m | 81.47 ± 1.25 | 10.63 ± 0.53 | 5.4 ± 0.05 | 1650.6 ± 7.15 | 15.7 ± 0.84 |
| Statistical significance | P = 0.0016 | P = 0.031 | P < 0.001 | P < 0.001 | P = 0.043 |
Data presented as mean ± SD.
GAE, gallic acid equivalent; QE, quercetin equivalent.
The high-altitude Acacia honey samples (from 2 246 m above sea level) were characterized by a significantly lower concentration of total phenolic compounds and higher concentration of flavonoids compared with low-altitude Acacia honey samples (from 437 m above sea level; P < 0.05; Table 1).
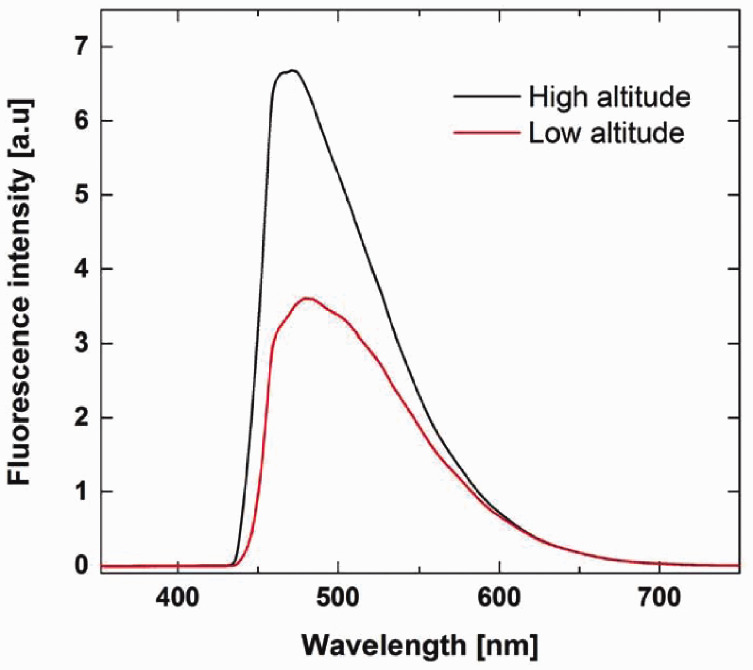
The LIF ʎ value of high altitude Acacia honey was 6.68513 a.u at 471.3 nm, while the ʎ value of low altitude Acacia honey was 3.60067 a.u at 479.45 and 479.5 nm (Figure 3). In terms of total phenols and flavonoids, there was a similar trend between flavonoids and fluorescence intensity, in that they were both higher in the high-altitude honey samples, suggesting that the fluorescence may be due to the flavonoid concentration (Table 1) and (Figure 3). This suggests that LIF may be effective in differentiating between low and high-altitude honey samples from the same floral origin.
Figure 3.
Laser induced fluorescence spectra of the low and high-altitude Acacia honey samples. The ʎ values of the high and low altitude Acacia honey were 6.68513 arbitrary units (a.u) and 3.60067 a.u, respectively.
The SRB cytotoxicity assay revealed that Acacia honey samples were toxic against all of the cancer cell lines, shown by the IC50 values (Table 2). The Acacia honey samples from the 2 246 m altitude had significantly higher anti-HCT116 and anti-MCF7 activity compared with the honey samples from the 437 m altitude, shown by statistically significant differences in IC50 values (P < 0.001; Table 2). There was no statistically significant difference in cytotoxicity against HepG2 cells between honey samples from the two different altitudes (Table 2). Doxorubicin displayed the lowest IC50 values against all of the cancer cell lines.
Table 2.
Cytotoxicity (IC50) of honey samples from high and low altitudes, and doxorubicin, against three human cancer cell lines.
| Human cell line |
Honey sample IC50 value, µg/ml |
Statisticalsignificance | Doxorubicin IC50 value, µg/ml | |
|---|---|---|---|---|
| High altitude | Low altitude | |||
| HCT116 | 117.99 ± 12.7 | 264.17 ± 10.5 | P = 0.0006 | 1.2 ± 0.036 |
| MCF7 | 189.82 ± 15.8 | 482.65 ± 20.3 | P = 0.0002 | 1.09 ± 0.044 |
| HepG2 | 150.93 ± 19.1 | 173.05 ± 25.6 | NS | 0.92 ± 0.046 |
Data presented as mean ± SD.
NS, no statistically significant between-group difference (P > 0.05, Student’s t-test).
Discussion
In the current study, the mean values for total phenolic compounds in Acacia honey samples from high altitude (2 246 m) were significantly lower than values from low altitude (437 m), being 81.47 ± 1.25 and 91.33 ± 0.96 mg GAE/100 g, respectively. Conversely, the high-altitude Acacia honey contained an increased concentration of total flavonoids (10.63 ± 0.53 mg QE/100 g) compared with low altitude Acacia honey (8.78 ± 0.23 mg QE/100 g). The significant differences between the low and high-altitude Acacia honey samples may be due to climate differences between low and high altitudes. In a previously published paper, the present authors showed that high altitude is characterized by disintegrated pollens, which may contribute to the significantly different physicochemical properties and chemical composition of honey samples.36 Furthermore, differences between the low and high altitude honey may be due to the presence of pollens other than Acacia pollens, and to the fact that bees living at high altitude depend partly on plant and insect secretions, which leads to the production of honeydew beside the blossom honey.37
Previous studies have reported variable results regarding the concentration of total phenols and flavonoids in honey samples. For example, three studies reported that the total flavonoids concentration in Yemeni honey of different botanical origins was 122–5482 µg/100 g and 261–1646 µg/100 g,13–15 and the concentration of total flavonoids in the Yemni honey was less than that of the honey samples of the present study, which may be due to the different floral origins. The Yemni honey research studied polyfloral and cactus honey samples, while the present study investigated total flavonoids in Acacia honey samples.13–15 Pontis et al.38 reported total flavonoids in honey samples from Brazil that ranged from 9 to 4.8 mg QE/kg, which was very low compared with the findings of the present study. Omani honey samples registered high concentrations of total phenols and total flavonoids compared with the findings of the present study, ranging from 162.4–289.8 mg GAE/100 g and 161.3–289.0 mg QE/100 g, respectively.39 Similar to the present findings, Cabrera et al.40 measured total phenols and flavonoids in Acacia honey samples from Burkina Faso, and reported concentrations of 93.4 ± 0.87 mg GAE/100 g and 6.14 ± 0.35 mg QE/100 g, respectively. Another Saudi study reported comparable total phenols in Acacia honey samples, ranging from 74–84 mg GAE/100 g).41 A comparison between the present study findings and those of previously published studies regarding the concentration of total phenols and flavonoids in honey samples is presented in Table 3.
Table 3.
Comparison between the concentration of total phenols and flavonoids in honey samples from the present research and from previously published studies.
| Study | Total phenols | Total flavonoids | Botanical origin | Geographical origin |
|---|---|---|---|---|
| Present study | ||||
| Low altitude honey (437 m) | 91.33 ± 0.96 mg GAE/100 g | 8.78 ± 0.23 mg QE/100 g | Acacia | Saudi Arabia |
| High altitude honey (2 246 m) | 81.47 ± 1.25 mg GAE/100 g | 10.63 ± 0.53 mg QE/100 g | Acacia | Saudi Arabia |
| Akbari et al. (2020)16 | 33.21 ± 5.64 mg/100 g | 2.71 ± 0.17 mg/100 g | Acacia | Iran |
| Al-Farsi et al. (2018)39 | 1624–2898 mg GAE/kg | 1613–2890 mg QE/kg | Acacia (Sumer), Ziziphus and multiflora | Sultanate of Oman |
| Cabrera et al. (2017)40 | 93.4 ± 0.87 mg GAE/100 g | 6.14 ± 0.35 mg QE/100 g | Acacia | Burkina Faso |
| Alqarni et al. (2016)41 | 0.74–0.84 mg GAE/g | – | Acacia | Saudi Arabia |
| Ahmed et al. (2016)14 | – | 122–5482 μg/100 g | Polyfloral and Cactus | Yemen |
| Ahmed et al. (2014)13 | – | 261–1646 μg/100 g | Ziziphus jujuba | Yemen |
| Pontis et al. (2014)38 | – | 9–4.8 mg QE/kg | Multifloral | Brazil |
Data presented as mean ± SD, or range.
GAE, gallic acid equivalent; QE, quercetin equivalent.
Differences in total phenols and flavonoids concentrations may be due to different botanical or geographical origin.
A search of the literature did not reveal any published articles regarding the use of LIF as a technique to differentiate between low and high-altitude honey samples. However, two previous studies showed the possibility of using spectra obtained with the LIF technique for detecting honey adulteration and in honey authentication.18,20 In addition, Ruoff et al.19 showed that the laser fluorescence spectra of bee honey may be used for determining bee honey’s floral origin. The results of the present study suggest that LIF may be used for differentiating between low and high-altitude honey samples because of their flavonoid concentration, and not the total phenols.
It is well known that dark honeys are characterized by their high content of polyphenols and flavonoids, which may be the cause of their antioxidant and anticancer capacities.38–40 Previous research has shown that bee honey has variable in vitro effects on cancer cell lines depending on the plant source. For example, some types of honey have anti-proliferative effects against human cancer cell lines, such as thyme honey, while others (e.g. Fir honey) have been shown to stimulate the viability of human cancer cell lines, such as MCF7.21–24 In the present study, Acacia honey from both high and low altitudes were shown to exhibit cytotoxic effects against three human cancer cell lines. High altitude honey displayed increased cytotoxicity against HCT116 and MCF7 cells, and also contained increased levels of flavonoids, versus the low altitude honey.
The results of the present study are limited due to the small number of honey samples obtained from only two locations. Thus, it is not possible to generalize the conclusions, and further research is required to validate the results.
The present study has provided a basis for further research on the effect of altitude on the physicochemical properties and biological activities of honey samples. However, the major findings of this study are that: (1) High-altitude Acacia honey may have higher activity against human cancer cell lines compared with low altitude Acacia honey; and (2) The laser induced fluorescence technique may be used as a simple, rapid, and nondestructive technique for differentiating between low and high-altitude honey samples, because of the flavonoid content of the honey rather than the total phenols content.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for the endless support and encouragement.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received funding from the Deanship of Scientific Research at King Khalid University (grant number GRP/124/41).
ORCID iD
Moammed Elimam Ahamed Mohammed https://orcid.org/0000-0003-3909-3788
References
- 1.COUNCIL DIRECTIVE 2001/110/EC . Relating to honey. Official Journal of the European Communities 2002; L10: 47–52. [Google Scholar]
- 2.Bogdanov S, Jurendic T, Sieber R, et al. Honey for nutrition and health: a review. J Am Coll Nutr 2008; 27: 677–689. [DOI] [PubMed] [Google Scholar]
- 3.El Sohaimy SA, Masry SHD, Shehata MG. Physicochemical characteristics of honey from different origins. Ann Agric Sci 2015; 60: 279–287. [Google Scholar]
- 4.Arena E, Ballistreri G, Tomaselli F, et al. Survey of 1,2-dicarbonyl compounds in commercial honey of different floral origin. J Food Sci 2011; 76: C1203–C1210. [DOI] [PubMed] [Google Scholar]
- 5.Mohammed MEA, Alaergani W, Suleiman MHA, et al. Hydrogen peroxide and dicarbonyl compounds concentration in honey samples from different botanical origins and altitudes in the south of Saudi Arabia. Curr Res Nutr Food Sci J 2019; 7: 150–160. [Google Scholar]
- 6.Nayik GA, Suhag Y, Majid I, et al. Discrimination of high altitude Indian honey by chemometric approach according to their antioxidant properties and macro minerals. Journal of the Saudi Society of Agricultural Sciences 2018; 17: 200–207. [Google Scholar]
- 7.Al-Mosa A, Brima EI, Fawy KF, et al. Antioxidant vitamins in honey samples from different floral origins and altitudes in Asir region at the south-western part of Saudi Arabia. Curr Nutr Food Sci 2019; 15: 296–304. [Google Scholar]
- 8.Liben T, Atlabachew M, Abebe A. Total phenolic, flavonoids and some selected metal content in honey and propolis samples from South Wolo zone, Amhara region, Ethiopia. Cogent Food Agric 2018; 4: 1475925. [Google Scholar]
- 9.Lachman J, Hejtmánková A, Sýkora J, et al. Contents of major phenolic and flavonoid antioxidants in selected Czech honey. Czech J Food Sci 2010; 28: 412–426. [Google Scholar]
- 10.Moniruzzaman M, An CY, Rao PV, et al. Identification of phenolic acids and flavonoids in monofloral honey from Bangladesh by high performance liquid chromatography: determination of antioxidant capacity. Biomed Res Int 2014; 2014: 737490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastos DHM, Dos Santos MCM, Mendonça S, et al. Antioxidant capacity and phenolic content of stingless bee honey from Amazon in comparison to Apis bee honey. Acta Hortic 2009; 841: 483–486. [Google Scholar]
- 12.Bueno-Costa FM, Zambiazi RC, Bohmer BW, et al. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT Food Sci Technol 2016; 65: 333–340. [Google Scholar]
- 13.Badjah Hadj Ahmed AY, Obbed MS, Wabaidur SM, et al. High-performance liquid chromatography analysis of phenolic acid, flavonoid, and phenol contents in various natural Yemeni honeys using multi-walled carbon nanotubes as a solid-phase extraction adsorbent. J Agric Food Chem 2014; 62: 5443–5450. [DOI] [PubMed] [Google Scholar]
- 14.Badjah Hadj Ahmed AY, Wabaidur SM, Siddiqui MR, et al. Simultaneous determination of twenty-five polyphenols in multifloral and cactus honeys using solid-phase extraction and high-performance liquid chromatography with photodiode array detection. Eur Food Res Technol 2016; 242: 943–952. [Google Scholar]
- 15.Wabaidur SM, Ahmed YBH, Alothman ZA, et al. Ultra high performance liquid chromatography with mass spectrometry method for the simultaneous determination of phenolic constituents in honey from various floral sources using multiwalled carbon nanotubes as extraction sorbents. J Sep Sci 2015; 38: 2597–2606. [DOI] [PubMed] [Google Scholar]
- 16.Akbari E, Baigbabaei A, Shahidi M. Determination of the floral origin of honey based on its phenolic profile and physicochemical properties coupled with chemometrics. Int J Food Prop 2020; 23: 506–519. [Google Scholar]
- 17.Jibril FI, Hilmi ABM, Manivannan L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull Natl Res Cent 2019; 43: 4. [Google Scholar]
- 18.El-Bialee NM, Rania KI, El-Bialee AM, et al. Discrimination of honey adulteration using laser technique. Aust J Basic & Appl Sci 2013; 7: 132–138. [Google Scholar]
- 19.Ruoff K, Karoui R, Dufour E, et al. Authentication of the botanical origin of honey by front-face fluorescence spectroscopy. A preliminary study. J Agric Food Chem 2005; 53: 1343–1347. [DOI] [PubMed] [Google Scholar]
- 20.Nikolova K, Eftimov T, and Aladjadjiyan A. Fluorescence spectroscopy as method for quality control of honey. Adv Res 2014; 2: 95–108. [Google Scholar]
- 21.Tsiapara AV, Jaakkola M, Chinou I, et al. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: profile analysis of extracts. Food Chem 2009; 116: 702–708. [Google Scholar]
- 22.Othman NH. Does honey have the characteristics of national cancer vaccine? J Tradit Complement Med 2012; 2: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porcza LM, Simms C, Chopra M. Honey and cancer: current status and future directions. Diseases 2016; 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed S, Othman NH. Honey as a potential natural anticancer agent: a review of its mechanisms. Evid Based Complement Alternat Med 2013; 2013: 829070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asir Municipality. Asir region in brief, https://ars.gov.sa/Ar/AsirRegion/AsirInLines/Pages/default.aspx (accessed 27 July 2019).
- 26.Youssef AM, Maerz NH. Overview of some geological hazards in the Saudi Arabia. Environ Earth Sci 2013; 70: 3115.– . [Google Scholar]
- 27.Codex Alimentarius: International food standards. CXS 12-1981 Standard for honey 1981. Revised 2019.
- 28.National Honey Board. A reference guide to nature’s sweetener. Firestone, USA: National Honey Board, 2005. [Google Scholar]
- 29.GCC Standardization Organization (GSO): Gulf Technical Regulation. GSO 147:2008. Honey, 2008.
- 30.Bogdanov S. Harmonized methods of the International Honey Commission. International Honey Commission, 2009; pp.21–23.
- 31.Bogdanov S. Harmonized methods of the International Honey Commission. International Honey Commission, 2009; pp.16–18.
- 32.AOAC. Official Methods of Analysis. 13th ed. Washington: Association of Official Analytical Chemists, 1980, pp.56–132. [Google Scholar]
- 33.Agbor GA, Vinson JA, Donnelly PE. Folin-Ciocalteau reagent for polyphenolic assay. Int J Food Sci Nutr Diet 2014; 3: 147–156. [Google Scholar]
- 34.Meda A, Lamien CE, Romito M, et al. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 2005; 91: 571–577. [Google Scholar]
- 35.Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990; 82: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 36.Mohammed EAM, Alfifi A, Aal-Mudawi A, et al. Some physiochemical properties of acacia honey from different altitudes of the Asir region in Southern Saudi Arabia. Czech J Food Sci 2017; 35: 321–327. [Google Scholar]
- 37.Robertson LM, Edlin JS, Edwards JD. Investigating the importance of altitude and weather conditions for the production of toxic honey in New Zealand. New Zeal J Crop Hort 2010; 38: 87–100. [Google Scholar]
- 38.Pontis JA, Da Costa LAMA, Da Silva SJR, et al. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci Technol, Campinas 2014; 34: 69–73. [Google Scholar]
- 39.Al-Farsi M, Al-Amri A, Al-Hadhrami A, et al. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018; 4: e00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabrera M, Perez M, Gallez L, et al. Colour, antioxidant capacity, phenolic and flavonoid content of honey from the humid Chaco region, Argentina. Phyton, Int J Exp Bot 2017; 86: 124–130. [Google Scholar]
- 41.Alqarni AS, Owayss AA, Mahmoud AA. Physicochemical characteristics, total phenols and pigments of national and international honeys in Saudi Arabia. Arab J Chem 2016; 9: 114–120. [Google Scholar]