Abstract
Background
Individuals with a very high lifetime risk of developing pancreatic ductal adenocarcinoma; for example, hereditary pancreatitis and main-duct or mixed-type intraductal papillary mucinous neoplasm, may wish to discuss prophylactic total pancreatectomy but strategies to do so are lacking.
Objective
To develop a shared decision-making programme for prophylactic total pancreatectomy using decision tables.
Methods
Focus group meetings with patients were used to identify relevant questions. Systematic reviews were performed to answer these questions.
Results
The first tables included hereditary pancreatitis and main-duct or mixed-type intraductal papillary mucinous neoplasm. No studies focused on prophylactic total pancreatectomy in these groups. In 52 studies (3570 patients), major morbidity after total pancreatectomy was 25% and 30-day mortality was 6%. After minimally invasive total pancreatectomy (seven studies, 35 patients) this was, respectively, 13% and 0%. Exocrine insufficiency-related symptoms occurred in 33%. Quality of life after total pancreatectomy was slightly lower compared with the general population.
Conclusion
The decision tables can be helpful for discussing prophylactic total pancreatectomy with individuals at high risk of pancreatic ductal adenocarcinoma.
Keywords: Pancreatic ductal adenocarcinoma, mutation, cancer risk, hereditary pancreatitis, intraductal papillary mucinous neoplasm, prophylactic total pancreatectomy, diabetes mellitus
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a major cause of cancer-related deaths worldwide. Globally, PDAC was responsible for 432,242 new deaths in 2018.1 Overall survival is poor, even in the 17% of patients who can undergo resection 5-year survival is only 15–20%.2 Therefore, prevention of PDAC may be preferred over early detection and treatment.
There are some individuals with a high (15–62%) lifetime risk of developing PDAC.3–5 This high-risk population can be divided into three main groups: known hereditary conditions (e.g. hereditary pancreatitis), familial clustering and patients with specific clinical predisposition factors (e.g. main-duct or mixed-type intraductal papillary mucinous neoplasm (IPMN), involving the entire pancreatic duct).6–8 Once diagnosed, these patients have to live with the knowledge that PDAC may develop. This knowledge is a considerable burden for most, especially when considering the very poor survival of PDAC.2
In some countries, individuals with a high risk of developing PDAC may enter a dedicated screening programme in a research setting.9,10 Such a programme has also been introduced in The Netherlands. The goal of surveillance is to detect asymptomatic high-risk lesions such as high-grade dysplastic IPMN or early cancer in order to improve survival. The 3-year psychological burden of such an approach appears acceptable.11 International guidelines currently do not consider prophylactic total pancreatectomy as a treatment option in these patients, due to the relatively high mortality and morbidity associated with this procedure and reduced quality of life due to ‘brittle’ diabetes.6,12–14 However, surgical outcomes and diabetes treatment have improved in recent years.15,16
For such a preference-sensitive treatment dilemma as total pancreatectomy both the individual and the physician need to be well informed. A shared decision-making approach with decision tables may facilitate this process as it provides a structured and easily accessible evidence-based data overview.17 Decision tables, also called ‘option grids’, have been developed for multiple conditions. Currently more than 40 are available on the option grid website (https://health.ebsco.com/products/option-grid). A decision table consists of a one-page matrix of evidence-based answers to the most frequently asked questions by individuals who are facing the choice between multiple treatment options.
Based on a request by the Dutch patient organisation, we developed a shared decision-making programme for prophylactic total pancreatectomy (PROPAN) using decision tables for high-risk individuals with either hereditary pancreatitis or main-duct or mixed-type IPMN (based on the European evidence-based guidelines) who wish to discuss prophylactic total pancreatectomy.6 This study describes the development, including four systematic reviews and the design of this programme.
Methods
The PROPAN shared decision-making programme for individuals who wish to discuss the option of prophylactic total pancreatectomy in the case of a very high risk of developing PDAC was developed by the Dutch Pancreatic Cancer Group in collaboration with the pancreatic cancer patient organisation, Living with Hope (www.livingwithhope.nl).
Developing the PROPAN programme
A meeting was organised involving relevant stakeholders, specifically the patient organisation, a shared decision-making expert, clinical geneticist, gastroenterologists, surgeons and a diabetologist. During the initial meeting the possibility of shared decision-making was discussed regarding two possible (treatment) options for high-risk individuals (i.e. observation or prophylactic surgery). It was decided to take further steps to facilitate shared decision-making within specific groups (starting with main-duct or mixed-type IPNM or hereditary pancreatitis). Several steps were identified to develop the programme:
Step 1
Round table focus group meeting, composed of patients after total pancreatectomy and individuals at high risk of developing PDAC to ask for their input and opinions regarding which information they would seek in the decision-making process (e.g. the frequently asked questions).
Step 2
Identify other stakeholders, such as the familial pancreatic cancer risk screening programme (chair: Professor Marco Bruno, ErasmusMC Rotterdam), and professional organisations such as the Dutch Pancreatic Cancer Group.
Step 3
Start a nationwide, retrospective study to collect quality of life and clinical outcome data on patients after a total pancreatectomy.
Step 4
Perform a systematic literature review about the outcomes after total pancreatectomy to acquire the most up-to-date information.
Step 5
Create a concept ‘decision table’ for prophylactic total pancreatectomy, following the rules of the option grid collaborative association, based on steps 1 to 4.
Step 6
Organise a second, larger multidisciplinary meeting with all stakeholders to discuss the content of the PROPAN programme, (e.g. the points that need to be discussed when an individual with a high risk of developing PDAC comes to an outpatient department).
Step 7
Finalise and launch the PROPAN programme.
Systematic reviews
Four systematic reviews consistent with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were performed.18 We searched PubMed, Embase and the Cochrane Library for studies published in the past 25 years (1992–2017), restricted to the English language. The following exclusion criteria were applied: paediatric patients, familial pancreatic cancer, total pancreatectomy with islet auto transplantation, no total pancreatectomy-specific outcomes, case reports (only in the minimally invasive review), conference abstracts, or reviews. The complete search strategies are presented in Supplementary Appendices 1–4. The following four systematic reviews were performed:
Systematic review 1
Studies on prophylactic total pancreatectomy programmes: all clinical and comparative studies reporting on or mentioning prophylactic total pancreatectomy were included.5,19
Systematic review 2
Studies on patient groups with a greater than 10% lifetime risk of PDAC. All studies reporting on high-risk populations for PDAC were included.3–5,19–29
Systematic review 3
Studies addressing the outcomes of total pancreatectomy. All studies reporting on total pancreatectomy outcome were included.16,30–80
Systematic review 4
Results
Decision tables
After two meetings with all stakeholders, it was decided to start by developing decision tables for high-risk patients in whom the pancreas is already affected by disease. This included individuals with main-duct or mixed-type IPMN and hereditary pancreatitis. The European evidence-based guideline on pancreatic cystic neoplasms was used for the definition of main-duct and mixed-type IPMN and the indication for total pancreatectomy in these patients.6 For hereditary pancreatitis a PRSS-1 mutation was considered mandatory.7 For high-risk individuals without pancreatic abnormalities on imaging (e.g. Peutz–Jeghers and p16-Leiden), it was decided to include them in a second round of the PROPAN programme. Individuals with familial clustering were not included in the PROPAN programme because of the unclear risk of PDAC (wide confidence intervals).87,88
After receiving and implementing input from both stakeholders and individuals in the focus group, two decision tables were created (Table 1 and Table 2). The decision tables can be used when an individual visits the outpatient clinic rather than making the decision table available online for all individuals. This creates the opportunity to discuss whether a prophylactic total pancreatectomy could be an option for that patient’s individual circumstances and preferences.
Table 1.
Decision table for patients to discuss prophylactic total pancreatectomy in main-duct/mixed-type IPMN.
| Frequently asked questions | Repeated check-ups | Total pancreatectomy |
|---|---|---|
| What does my possible treatment entail? | After you are diagnosed with IPMN, every 6 to 12 months you will get an MRI or EUS, which detects whether there is cancer in the pancreas.1 | Your entire pancreas will be removed using a minimally invasive approach (if considered possible by the surgeon). Conversion to open surgery occurs in approximately 6 out of 100 patients. In addition, one in 10 people also need to have their spleen removed. Patients in whom IPMN is diagnosed before 55 years will undergo a total pancreatectomy around their 55th birthday.2,3 Surgery at a younger age is possible in the case of a relative or absolute indication for partial pancreatectomy. |
| What is my risk of getting pancreatic cancer? | If a benign main-duct IPMN becomes malignant, this usually occurs within 5 years.2 After the diagnosis of main-duct IPMN, 60 out of 100 people (60%) will get pancreatic cancer.4 This may also occur after 5 years. | There is no more pancreatic tissue present in which you can get cancer. |
| What is my risk of dying? | It has not yet been proved that repeated check-ups reduces this risk. Cancer could be found at an early stage or in a precancerous stage. When cancer is present and you are being operated, the cancer will return in 70–80% of the patients within 5 years.5 | A total of 2–5 out of 100 people will die from complications due to the operation in very high-volume pancreatic surgery centres. Death rates are higher in other centres and for this reason the programme will only be conducted in very high volume centres. |
| What are the consequences/ complications? | You will be visiting the hospital two to four times a year for check-ups. If on the MRI (or EUS) imaging a lesion in the pancreas is detected, you will undergo surgery. Afterwards, this lesion may turn out to be a non-life-threatening lesion. There will be ongoing uncertainty. | After surgery, you will have diabetes in a serious form. In addition, you will get a shortage of digestive juices, for which you need to take two to four tablets of pancreatic enzymes at each meal. |
| What is my risk of getting diabetes? | 18 out of 100 people will get diabetes.6 | All, 100 out of 100 people will get insulin-dependent and unstable diabetes. This is a serious type of diabetes, for which insulin injections are necessary. |
| What more should I know about diabetes? | Due to your illness, your pancreas is affected and diabetes can develop. When this happens and at what age is unpredictable. | Treating and dealing with diabetes will be an important part of your life. You need to calculate the amount of insulin you need four to six times a day based on your diet and self-measured sugar levels. You must inject the insulin and measure your sugar levels by means of finger pricks, an insulin pump and/or glucose monitoring devices. |
| After surgery, how much time will it take for me to recover fully? | Not applicable. | You will stay in the hospital for about 1 to 2 weeks if there are no complications (in about half of the patients). If complications occur: 2 to 3 weeks. Complete recovery takes about 3 months. |
1Del Chiaro et al. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018; 67: 789–804.
2Sohn et al. Intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2004; 239: 788–799.
3Winter et al. Recurrence and survival after resection of small intraductal papillary mucinous neoplasm-associated carcinomas (<=20 mm invasive component): a multi-institutional analysis. Ann Surg 2016; 263: 793–801.
4Salvia et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004; 239: 678–687.
5Marchegiani et al. Patterns of recurrence after resection of IPMN who, when, and how? Ann Surg 2015; 262: 1108–1114.
6Julie et al. Intraductal papillary mucinous neoplasms and the risk of diabetes mellitus in patients undergoing resection versus observation. J Gastrointest Surg 2015; 19: 1974–1981.
IPMN: intraductal papillary mucinous neoplasm; EUS: endoscopic ultrasound; MRI: magnetic resonance imaging.
Table 2.
Decision table for patients to discuss prophylactic total pancreatectomy in hereditary pancreatitis.
| Frequently asked questions | Waiting | Repeated check-ups | Total pancreatectomy |
|---|---|---|---|
| What does my possible treatment include? | You will only have to visit the hospital when experiencing new complaints. | From your 40th until your 75th year of age, you will receive a MRI (or CT) scan once a year to see if a tumour is visible. | Around the age of 50 years, your entire pancreas will be removed using a minimally invasive approach (if considered possible by the surgeon). Conversion to open surgery occurs in approximately 6 out of 100 patients. In addition, one in 10 people also need to have their spleen removed.1 |
| What is my risk of getting pancreatic cancer? | 40 out of 100 people who do not smoke will get pancreatic cancer at a certain age (average around the age of 57 years). In smokers, 70 out of 100 people will get pancreatic cancer.2–4 | It is not yet known whether check-ups help to detect the disease in time. Pancreatic cancer can occur in between two check-up moments. | There is no more pancreatic tissue present in which you can get cancer. |
| What is my risk of dying? | If pancreatic cancer develops, it will no longer be operable in 4 out of 5 people. Often these people die within 4 to 6 months.4 If the tumour is operable, it will return within 5 years in four out of five patients. | It has not yet been proved scientifically if the risk of dying with repeated check-ups is reduced. The hope is that the cancer is found at an early stage or possibly even in a precancerous stage whereby the chances of survival are higher than when you already have complaints. | In total, 2–5 out of 100 people will die from complications due to the operation in very high volume pancreatic surgery centres. Death rates are higher in other centres and for this reason the programme will only be conducted in very high volume centres. |
| What are the consequences/ complications? | If complaints develop from pancreatic cancer, the disease is often already in an advanced stage. The survival chances are then very small. After 5 years, 5 out of 100 patients will be still alive. | You will visit the hospital every year for an investigation. There may be lesions in the pancreas seen on the MRI (or CT) for which you will undergo surgery. Afterwards, this lesion may turn out to be a non-life-threatening lesion. There will be ongoing uncertainty. | After surgery, you will have diabetes in a serious form. In addition, you will get a shortage of digestive juices, for which you need to take two to four capsules of pancreatic enzymes with each meal. |
| What is my risk of getting diabetes? | 70 out of 100 people will get diabetes.6 | 70 out of 100 people will get diabetes.6 | All, 100 out of 100 people will get insulin-dependent and unstable diabetes. This is a serious type of diabetes, for which insulin injections are necessary. |
| What should I know more about diabetes (treatment)? | Due to your illness, your pancreas is affected and this may cause diabetes. When this happens and at what age is unpredictable. | Due to your illness, your pancreas is affected and diabetes could develop. When this happens and at what age is unpredictable. | Treatment of and dealing with diabetes will be an important part of your life. You need to calculate the amount of insulin you need 4 to 6 times a day based on your diet and self-measured sugar levels. The insulin you will inject yourself and you measure your sugar values by means of finger pricks, an insulin pump and/or glucose monitoring devices. |
| After surgery, how much time will it take for me to recover fully? | Not applicable. | Not applicable. | You will stay in the hospital for about 1 to 2 weeks if there are no complications (in about half of the patients). If complications occur: 2 to 3 weeks. Complete recovery takes about 3 months. |
1Rebours et al. The natural history of hereditary pancreatitis: a national series. Gut 2009; 58: 97.
2Lowenfels et al. Hereditary pancreatitis and the risk of pancreatic cancer. J Natl Cancer Inst 1997; 89: 442–446.
3Rebours et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol 2008; 103: 111–119.
4Lowenfels et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA 2001; 286: 169–170.
5Neoptolemos et al. Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol 2003; 14: 675–692.
6Howes et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004; 2: 252–261.
CT: computed tomography; MRI: magnetic resonance imaging.
Systematic reviews
To answer the identified questions in the decision tables, four systematic reviews were performed. The first systematic review revealed two studies on prophylactic total pancreatectomy, but none on existing formal programmes for prophylactic total pancreatectomy.5,19 The second systematic review identified four patient groups with a greater than 10% lifetime risk of PDAC: hereditary pancreatitis, main-duct or mixed-type IPMN, Peutz–Jeghers syndrome and p16-Leiden (CDKN2A mutation), see Table 3.3–5,19–29 The peak age of onset of cancer is 40 years in Peutz–Jeghers disease and 60 years in the other groups.3,4,20,23,26,28,29,89,90 Although it is strongly recommended to perform surgery for main-duct or mixed-type IPMN, this mostly involves partial pancreatectomy with life-long follow-up and not total pancreatectomy.6 In main-duct IPMN, a main pancreatic duct diameter of more than 10 mm is considered an absolute indication for surgery, whereas in a main pancreatic duct of 5–9 mm surgery is considered a relative indication for surgery. It is recommended to perform standardised surveillance.6,91 The third systematic review identified 52 studies with 3570 patients who underwent total pancreatectomy with a pooled 30-day mortality of 6% (95% confidence interval (CI) 4–8%).16,30–80 Of the four studies reporting on quality of life the mean global health status was 75% (on a scale of 0–100%) based on the EORTC QLQ-C30 questionnaire (102 patients) and moderately lower compared with the general population.32,35,48,61 During a median follow-up of 38 months, 49/273 patients (18%) were re-admitted for endocrine-related morbidity, with hypoglycemia-related mortality in 10/568 patients (1.8%). Exocrine insufficiency-related symptoms were reported by 178/547 patients (33%) during a median follow-up of 33 months.
Table 3.
Estimated life-time risk of PDAC in high-risk patients and individuals.
| Condition | Gene | Estimated lifetime risk of pancreatic cancer | Other cancers |
|---|---|---|---|
| High-risk patients in whom the pancreas is already affected by disease | |||
| Hereditary pancreatitis3,5,24,25,27 | PRSS1, CFTR, SPINK1, CTRC | 25–40% | |
| MD/MT-IPMN20–22,26 | – | 60% | |
| High-risk individuals in whom the pancreas is not yet affected by disease | |||
| Peutz–Jeghers syndrome3,4,23 | STK11/LKB1 | 11–36% | Colorectal 39% Stomach 29% Small bowel 13% |
| P16-Leiden mutation3,19,27–29 | CDKN2A | 17% | |
PDAC: pancreatic ductal adenocarcinoma; IPMN: intraductal papillary mucinous neoplasm.
The fourth systematic review showed that minimally invasive total pancreatectomy is poorly described in the literature, with seven studies, including a total of 35 patients.34,81–86 Morbidity and mortality were, respectively, 13% and 0% after minimally invasive total pancreatectomy (Table 4). One report compared minimally invasive total pancreatectomy to open total pancreatectomy and found similar complication rates, although the mean operation time was longer in the robot-assisted group than in the open surgery group: mean 600 minutes (range 400–800) versus 469 minutes (range 300–660), P = 0.014.34
Table 4.
Minimally invasive total pancreatectomy.
| First author | n | Type of operation | Indications (n) | Time, minutes (range) | Conversion | Hospital stay, days (range) | Mortality (%) | Morbidity (Clavien-Dindo ≥3) |
|---|---|---|---|---|---|---|---|---|
| Boggi et al.34 2015 |
11 | Laparoscopic robot-assisted | (Malignant) IPMN, PDAC, CP | 600 (400–800) |
0 | 27 (12–88) | 0* | 2 of 11 (18%) |
| Choi et al.81 2012 |
3 | Laparoscopic-assisted | IPMN | 423 | 1 | 20 | 0 | 0 |
| Dallemagne et al.82 2013 |
2 | Laparoscopic | IPMN, pNET | 390 | 0 | 8 | 0 | 0* |
| Giulianotti et al.83 2011 |
5 | Robotic TP | IPMN, PDAC, CP, pNET | 456 | - | 7.2 (5–11) | 0 | 2 of 5 (40%) |
| Kim et al.84 2011 |
1 | Laparoscopic-assisted | Malignant IPMN | 300 | - | 20 | 0 | 0 of 1 (0) |
| Wang et al.85 2017 |
3 | Laparoscopic/robotic | IPMN, pNET | 490 (450–540) |
0 | 18 (8–24) | 0 | 0 of 3 (0) |
| Zureikat et al.86 2015 |
10 | Robotic | IPMN, PDAC, CP | 528 | 1 | 10 ± 3 | 0* | 0 of 10 (0) |
| Total | 35 | 0 | 4 of 32 (13%) |
*90 day mortality/morbidity.
IPMN: intraductal papillary mucinous neoplasm; PDAC: pancreatic ductal adenocarcinoma; CP: chronic pancreatitis; pNET: pancreatic neuroendocrine tumor.
PROPAN programme
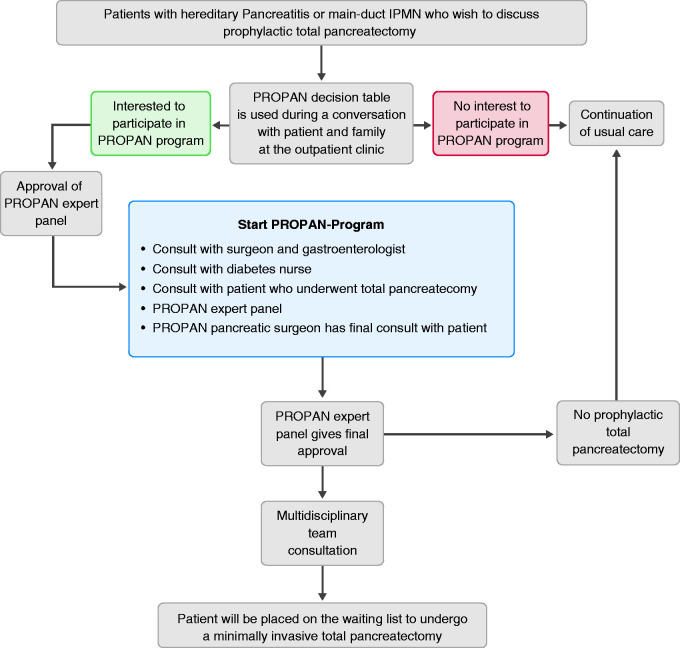
The design of the PROPAN programme is shown in Figure 1. Participants have several consultations during the programme. First, a combined consult with a gastroenterologist and pancreatic surgeon will take place in order to answer questions and fully inform the participant and relatives. Subsequently, a consultation with a diabetes nurse and a patient after total pancreatectomy will be organised. The surgeon will inform the participant about the procedure, expected postoperative course and the risk of complications. If the patient still wants to proceed, the specific case will be discussed in the PROPAN expert panel, consisting of a clinical geneticist, surgeon, gastroenterologist, internist/diabetologist and psychologist. The expert panel will check if the individual fulfils the inclusion criteria and give final approval. Next, the local pancreatic multidisciplinary team will be informed and asked for agreement on the decision to perform prophylactic (minimally invasive) total pancreatectomy based on the available imaging and reports of all completed consultations. Individuals will be advised to undergo this procedure at the age of 50 years, 10 years before the peak incidence of PDAC development.
Figure 1.
Flow chart of the PROPAN programme.
Implementation
It is expected that the number of Dutch patients eligible for participation in this programme is limited and that initially only a few will opt for minimally invasive total pancreatectomy. Therefore, the programme will only start in very high volume pancreatic centres which perform at least 80 pancreatoduodenectomies annually and have extensive experience with minimally invasive pancreatoduodenectomy and who can organise the programme as stated.92 Experience with minimally invasive pancreatectomy was guaranteed by participation in the LEALAPS, LAELAPS-2, and LAELAPS-3 structured nationwide training programmes in, respectively, laparoscopic distal pancreatectomy, laparoscopic pancreatoduodenectomy and robotic pancreatoduodenectomy, based on the IDEAL framework.93–95 An evaluation meeting with all involved stakeholders will be planned after five individuals have completed the full protocol as well as surgery.
Discussion
Together, the Dutch Pancreatic Cancer Group and the Dutch patient organisation for pancreatic cancer developed a shared decision-making programme for prophylactic total pancreatectomy using decision tables, to assist individuals at high risk of developing PDAC who wish to discuss the pros and cons of (minimally invasive) prophylactic total pancreatectomy.
The PROPAN programme addresses an important dilemma. Prophylactic total pancreatectomy for high-risk patients is not mentioned in current guidelines and no results are available in the literature. Traditionally, surgeons have been reluctant to perform total pancreatectomy because of postoperative exocrine and endocrine insufficiency, which both worsen quality of life.32 However, taking into account the improved surgical outcome of total pancreatectomy and the improved treatment of exocrine and endocrine insufficiency, the option to remove the pancreas prophylactically has been brought up by high-risk individuals and their family members.6,12–14 The PROPAN programme offers a conceptual and informative framework for shared decision-making in this treatment dilemma. It should be noted that this programme does not aim to lower the threshold for prophylactic total pancreatectomy. This programme does offer a framework for discussion between patients and caregivers in the situation that the European evidence-based guideline on pancreatic cystic neoplasms advises to perform total pancreatectomy because of main-duct or mixed-type IPMN. Clearly, there is room for discussion based on this advice. For instance, an earlier series showed that in elderly patients with main-duct IPMN with worrisome features or high-risk stigmata, disease-specific 5-year survival was 81%, which was comparable with patients after resection.96,97 In particular, a main pancreatic duct of 5–9 mm did not affect disease-specific survival.96 Furthermore, in an international expert survey and case vignette study, 97 experts disagreed on the indication for pancreatectomy in patients with main-duct or mixed-type IPMN in the entire pancreas with a nodule or tumour: 41% advised surveillance whereas 59% advised operative intervention.98 Of those who advised operative intervention, 46% would perform a total pancreatectomy and 31% pancreatoduodenectomy with follow-up. This again shows that there is room for shared decision-making.
Prophylactic surgery with removal of an entire target organ is already used in several genetic syndromes, such as hereditary breast and ovarian cancers, gynaecological cancers in Lynch syndrome, and has proved to improve survival.8 In these patients the risks of cancer are 40–70% and similar to the 60% risk in main-duct IPMN and 40% in hereditary pancreatitis.99,100 For hereditary pancreatitis, only patients with a PRSS1 mutation are included, because these patients have the highest risk of developing pancreatic adenocarcinoma. The relative roles of CFTR or SPINK1 mutations in carcinogenesis are not as well established compared with PRSS1 mutations and are therefore excluded in this first decision-making programme.101 In individuals with familial pancreatic cancer the exact pancreatic cancer risk is not known and, as concluded by Kekis et al. in 2001, prophylactic total pancreatectomy should only be used in the presence of high-grade dysplasia.102 The problem is how to diagnose or exclude high-grade dysplasia in these patients.
The diagnosis of hereditary pancreatitis is based on genetic testing and is therefore expected to be highly accurate. However, for patients with IPMN it has been demonstrated that there could be a considerable risk of misdiagnosis (approximately 20%).37,103,104 Naturally, a total pancreatectomy is not advised in patients without main-duct IPMN or with only main-duct IPMN in the pancreatic head. This emphasises the importance of the PROPAN expert panel, which is clearly aware of this risk of misdiagnosis. Diagnosis should be confirmed in a multidisciplinary team meeting, after careful discussion of imaging potentially in combination with endoscopic ultrasound according to current guidelines.6,91 In case of doubt about the diagnosis, patients are not eligible for the PROPAN programme.
It is yet unclear whether screening programmes improve the survival of individuals at high risk of developing PDAC. No randomised controlled trials are available and the only data available are from prospective cohort studies. Verna et al. showed, in a high-risk population of 51 patients enrolled in a screening programme, that six patients (12%) had neoplastic lesions in the pancreas.105 They concluded that screening is effective and identifies curable neoplasms that can be resected. Vasen et al. prospectively collected screening outcomes in patients with familial pancreatic cancer or families with a gene defect that predisposes to PDAC.28 Among 178 p16-Leiden mutation carriers, PDAC was detected in 13 patients (7.3%), whereas the resection rate was 75% and the 5-year survival rate was 24%. Two individuals (0.9%) in the familial pancreatic cancer cohort had a pancreatic tumour, including one with advanced PDAC and one with an early grade 2 neuroendocrine tumour. Four patients (1.9%) had high-risk lesions, out of 13 individuals with familial pancreatic cancer (6.1%) that underwent surgical resection for a suspected precursor lesion. The authors concluded that surveillance in this study of p16-Leiden mutation carriers is relatively successful, detecting most PDACs at a resectable stage, while the benefit of surveillance in families with familial pancreatic cancer is less evident.
All patients undergoing total pancreatectomy will instantly develop insulin-dependent diabetes. Glucose control in these patients may be challenging due to the complete loss of pancreatic endocrine parenchyma, secreting insulin and glucagon, which predisposes for difficult to control postoperative hypo and hyperglycemia. This will affect a patients’ life substantially and will therefore be emphasised by the surgeon and gastroenterologists, but also extensively discussed during the consult with the diabetes nurse and patient who underwent a total pancreatectomy. A recent systematic review on diabetes mellitus-related outcomes revealed a need for further improvement of diabetes management after total pancreatectomy, especially regarding the prevention of hypoglycaemia-related morbidity and even mortality.13 This study also showed that quality of life seems moderately affected by total pancreatectomy. One of the largest studies on this topic to date reported that quality of life with new-onset diabetes mellitus after total pancreatectomy is similar to that of patients with type 1 diabetes.14,106
Evidence about minimally invasive total pancreatectomy from large prospective studies is lacking. In a systematic literature review, Kuesters et al. included five studies with a total of 21 patients. They found 0% mortality after a short follow-up period ranging from 3 to 38 months and concluded that in this small group of selected patients this procedure is feasible, if carried out in centres with large expertise in minimally invasive and pancreatic surgery (see Table 4).107 Our group has recently initiated a European study to assess outcomes after minimally invasive total pancreatectomy within the European Consortium on Minimally Invasive Pancreatic Surgery.
In conclusion, the PROPAN programme provides a conceptual and informative framework with decision tables for both high-risk individuals and physicians who wish to discuss prophylactic total pancreatectomy. The programme includes preoperative counselling, weighing the pros and cons between the reduction in PDAC risk and the risks and long-term consequences of total pancreatectomy, as well as the uncertainty regarding lifelong surveillance as an alternative management approach.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620945534 for Prophylactic total pancreatectomy in individuals at high risk of pancreatic ductal adenocarcinoma (PROPAN): systematic review and shared decision-making programme using decision tables by Lianne Scholten, Anouk EJ Latenstein, Cora M Aalfs, Marco J Bruno, Olivier R Busch, Bert A. Bonsing, Bas Groot Koerkamp, I Quintus Molenaar, Dirk T Ubbink, Jeanin E van Hooft, Paul Fockens, Jolanda Glas, J Hans DeVries, Marc G Besselink and for the Dutch Pancreatic Cancer Group in United European Gastroenterology Journal
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
Not applicable
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Lianne Scholten received a grant from Zealand Pharma A/S for studies on postpancreatectomy diabetes management. Marc G Besselink received a grant from the Dutch Cancer Society (grant number UVA2013-5842) for studies on pancreatic cancer.
Informed consent
Not applicable
ORCID iD
Anouk EJ Latenstein https://orcid.org/0000-0003-3492-4968
References
- 1.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10: 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer 2020; 125: 83–93. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience J Gastrointest Surg 2012; 16: 771–783. [DOI] [PubMed] [Google Scholar]
- 4.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012; 142: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004; 2: 252–261. [DOI] [PubMed] [Google Scholar]
- 6.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018; 67: 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulrich CD; Consensus Committees of the European Registry of Hereditary Pancreatic Diseases, Midwest Multi-Center Pancreatic Study Group, International Association of Pancreatology. Pancreatic cancer in hereditary pancreatitis: consensus guidelines for prevention, screening and treatment. Pancreatology 2001; 1: 416–422. [DOI] [PubMed] [Google Scholar]
- 8.Peacock O, Waters PS, Otero de Pablos J, et al. A systematic review of risk-reducing cancer surgery outcomes for hereditary cancer syndromes. Eur J Surg Oncol 2019; 45: 2241–2250. [DOI] [PubMed] [Google Scholar]
- 9.Harinck F, Konings IC, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016; 65: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 10.DaVee T, Coronel E, Papafragkakis C, et al. Pancreatic cancer screening in high-risk individuals with germline genetic mutations. Gastrointest Endosc 2018; 87: 1443–1450. [DOI] [PubMed] [Google Scholar]
- 11.Bruenderman EH, Martin RC., II. High-risk population in sporadic pancreatic adenocarcinoma: guidelines for screening. J Surg Res 2015; 194: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrucciani N, Nigri G, Giannini G, et al. Total pancreatectomy for pancreatic carcinoma: when, why, and what are the outcomes? Results of a systematic review. Pancreas 2020; 49: 175–180. [DOI] [PubMed] [Google Scholar]
- 13.Scholten L, Stoop TF, Del Chiaro M, et al. Systematic review of functional outcome and quality of life after total pancreatectomy. Br J Surg 2019; 106: 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholten L, Latenstein AEJ, van Eijck CH, et al. Outcome including long-term quality of life after total pancreatectomy (Panorama): a nationwide cohort study. Surgery 2019; 166: 1017-1026. [DOI] [PubMed] [Google Scholar]
- 15.Pulvirenti A, Pea A, Rezaee N, et al. Perioperative outcomes and long-term quality of life after total pancreatectomy. Br J Surg 2019; 106: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 16.Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg 2009; 250: 282–287. [DOI] [PubMed] [Google Scholar]
- 17.Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ 2012; 344: e256. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Chiaro M, Verbeke CS, Kartalis N, et al. Short-term results of a magnetic resonance imaging-based Swedish screening program for individuals at risk for pancreatic cancer JAMA Surgery 2015; 150: 512–518. [DOI] [PubMed] [Google Scholar]
- 20.Akita H, Takeda Y, Hoshino H, et al. Mural nodule in branch duct–type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am J Surg 2011; 202: 214–219. [DOI] [PubMed] [Google Scholar]
- 21.Baiocchi GL, Portolani N, Missale G, et al. Intraductal papillary mucinous neoplasm of the pancreas (IPMN): clinico-pathological correlations and surgical indications. World J Surg Oncol 2010; 8: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bournet B, Vignolle-Vidoni A, Grand D, et al. Endoscopic ultrasound-guided fine-needle aspiration plus KRAS and GNAS mutation in malignant intraductal papillary mucinous neoplasm of the pancreas. Endosc Int Open 2016; 4: E1228–E1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canto MI, Coggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006; 4: 766–781. [DOI] [PubMed] [Google Scholar]
- 24.Ceppa EP, Pitt HA, Hunter JL, et al. Hereditary pancreatitis: endoscopic and surgical management. J Gastrointest Surg 2013; 17: 847–856. [DOI] [PubMed] [Google Scholar]
- 25.Joergensen MT, Gerdes AM, Sorensen J, et al. Is screening for pancreatic cancer in high-risk groups cost-effective? Experience from a Danish national screening program. Pancreatology 2016; 16: 584–592. [DOI] [PubMed] [Google Scholar]
- 26.Passot G, Lebeau R, Hervieu V, et al. Recurrences after surgical resection of intraductal papillary mucinous neoplasm of the pancreas: a single-center study of recurrence predictive factors. Pancreas 2012; 41: 137–141. [DOI] [PubMed] [Google Scholar]
- 27.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009; 104: 2175–2181. [DOI] [PubMed] [Google Scholar]
- 28.Vasen HF, Ibrahim I, Ponce CG, et al. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long-term prospective follow-up studies from three European expert centers. J Clin Oncol 2016; 34: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 29.Vasen HF, Wasser M, van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 2011; 140: 850–856. [DOI] [PubMed] [Google Scholar]
- 30.Amano H, Miura F, Toyota N, et al. Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? Hepatobiliary Pancreat Surg 2009; 16: 850–857. [DOI] [PubMed] [Google Scholar]
- 31.Bakkevold KE, Kambestad B. Morbidity and mortality after radical and palliative pancreatic cancer surgery. Risk factors influencing the short-term results. Ann Surg 1993; 217: 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbier L, Jamal W, Dokmak S, et al. Impact of total pancreatectomy: short- and long-term assessment. HPB (Oxford) 2013; 15: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumel H, Huguier M, Manderscheid JC, et al. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. Br J Surg 1994; 81: 102–107. [DOI] [PubMed] [Google Scholar]
- 34.Boggi U, Palladino S, Massimetti G, et al. Laparoscopic robot-assisted versus open total pancreatectomy: a case-matched study. Surg Endosc 2015; 29: 1425–1432. [DOI] [PubMed] [Google Scholar]
- 35.Casadei R, Monari F, Buscemi S, et al. Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg 2010; 62: 41–46. [DOI] [PubMed] [Google Scholar]
- 36.Casadei R, Ricci C, Taffurelli G, et al. Is total pancreatectomy as feasible, safe, efficacious, and cost-effective as pancreaticoduodenectomy? A single center, prospective, observational study. J Gastrointest Surg 2016; 20: 1595–1607. [DOI] [PubMed] [Google Scholar]
- 37.Crippa S, Pergolini I, Rubini C, et al. Risk of misdiagnosis and overtreatment in patients with main pancreatic duct dilatation and suspected combined/main-duct intraductal papillary mucinous neoplasms. Surgery 2016; 159: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 38.Crippa S, Tamburrino D, Partelli S, et al. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery 2011; 149: 79–86. [DOI] [PubMed] [Google Scholar]
- 39.Cuillerier E, Cellier C, Palazzo L, et al. Outcome after surgical resection of intraductal papillary and mucinous tumors of the pancreas. Am J Gastroenterol 2000; 95: 441–445. [DOI] [PubMed] [Google Scholar]
- 40.Datta J, Lewis RS, Jr, Strasberg SM, et al. Quantifying the burden of complications following total pancreatectomy using the postoperative morbidity index: a multi-institutional perspective. J Gastrointest Surg 2015; 19: 506–515. [DOI] [PubMed] [Google Scholar]
- 41.Edge SB, Schmieg RE, Jr, Rosenlof LK, et al. Pancreas cancer resection outcome in American University centers in 1989–1990. Cancer 1993; 71: 3502–3508. [DOI] [PubMed] [Google Scholar]
- 42.Epelboym I, Winner M, DiNorcia J, et al. Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. J Surg Res 2014; 187: 189–196. [DOI] [PubMed] [Google Scholar]
- 43.Fortner JG, Klimstra DS, Senie RT, et al. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann Surg 1996; 223: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujino Y, Matsumoto I, Ajiki T, et al. Clinical reappraisal of total pancreatectomy for pancreatic disease. Hepatogastroenterology 2009; 56: 1525–1528. [PubMed] [Google Scholar]
- 45.Fujino Y, Suzuki Y, Yoshikawa T, et al. Outcomes of surgery for intraductal papillary mucinous neoplasms of the pancreas. World J Surg 2006; 30: 1909–1914; discussion 1915. [DOI] [PubMed] [Google Scholar]
- 46.Golse N, Lebeau R, Lombard-Bohas C, et al. Lymph node involvement beyond peripancreatic region in pancreatic head cancers: when results belie expectations. Pancreas 2013; 42: 239–248. [DOI] [PubMed] [Google Scholar]
- 47.Gratian L, Pura J, Dinan M, et al. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol 2014; 21: 3515–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartwig W, Gluth A, Hinz U, et al. Total pancreatectomy for primary pancreatic neoplasms: renaissance of an unpopular operation. Ann Surg 2015; 261: 537–546. [DOI] [PubMed] [Google Scholar]
- 49.Hartwig W, Gluth A, Hinz U, et al. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br J Surg 2016; 103: 1683–1694. [DOI] [PubMed] [Google Scholar]
- 50.Hata T, Ishida M, Motoi F, et al. Clinical characteristics and risk factors for the development of postoperative hepatic steatosis after total pancreatectomy. Pancreas 2016; 45: 362–369. [DOI] [PubMed] [Google Scholar]
- 51.Ihse I, Anderson H, Andren S. Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg 1996; 20: 288–293; discussion 294. [DOI] [PubMed] [Google Scholar]
- 52.Jahromi HD, Jafarimehr E, Dabbous HN, et al. Curative resection of pancreatic adenocarcinoma with major venous resection/repair is safe procedure but will not improve survival. J Pancreas 2014; 15: 433–441. [DOI] [PubMed] [Google Scholar]
- 53.Jamil LH, Chindris AM, Gill KR, et al. Glycemic control after total pancreatectomy for intraductal papillary mucinous neoplasm: an exploratory study. HPB Surg 2012; 2012: 381328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janot MS, Belyaev O, Kersting S, et al. Indications and early outcomes for total pancreatectomy at a high-volume pancreas center. HPB Surg 2010; 2010: 686702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jethwa P, Sodergren M, Lala A, et al. Diabetic control after total pancreatectomy. Dig Liver Dis 2006; 38: 415–419. [DOI] [PubMed] [Google Scholar]
- 56.Karpoff HM, Klimstra DS, Brennan MF, et al. Results of total pancreatectomy for adenocarcinoma of the pancreas. Arch Surg 2001; 136: 44–47; discussion 48. [DOI] [PubMed] [Google Scholar]
- 57.Kitagawa M, Ikoma H, Ochiai T, et al. Total pancreatectomy for pancreatic carcinoma: evaluation of safety and efficacy. Hepatogastroenterology 2012; 59: 907–910. [DOI] [PubMed] [Google Scholar]
- 58.Kwak BJ, Kim SC, Song KB, et al. Prognostic factors associated with early mortality after surgical resection for pancreatic adenocarcinoma. Korean J Hepatobiliary Pancreat Surg 2014; 18: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Launois B, Franci J, Bardaxoglou E, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas with special reference to resection of the portal vein and multicentric cancer. World J Surg 1993; 17: 122–126; discussion 126–127. [DOI] [PubMed] [Google Scholar]
- 60.Lewis R, Drebin JA, Callery MP, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2013; 15: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müller MW, Friess H, Kleeff J, et al. Is there still a role for total pancreatectomy? Ann Surg 2007; 246: 966–974; discussion 974–965. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura M, Ohtsuka T, Nakashima H, et al. Extensive distal pancreatectomy for pancreatic tumor. Anticancer Res 2013; 33: 267–270. [PubMed] [Google Scholar]
- 63.Nathan H, Wolfgang CL, Edil BH, et al. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol 2009; 99: 87–92. [DOI] [PubMed] [Google Scholar]
- 64.Nikfarjam M, Low N, Weinberg L, et al. Total pancreatectomy for the treatment of pancreatic neoplasms. ANZ J Surg 2014; 84: 823–826. [DOI] [PubMed] [Google Scholar]
- 65.Onoue S, Katoh T, Chigira H, et al. Carcinoma of the head of the pancreas. Hepatogastroenterology 2002; 49: 549–552. [PubMed] [Google Scholar]
- 66.Parsaik AK, Murad MH, Sathananthan A, et al. Metabolic and target organ outcomes after total pancreatectomy: Mayo Clinic experience and meta-analysis of the literature. Clin Endocrinol (Oxf) 2010; 73: 723–731. [DOI] [PubMed] [Google Scholar]
- 67.Satoi S, Murakami Y, Motoi F, et al. Reappraisal of total pancreatectomy in 45 patients with pancreatic ductal adenocarcinoma in the modern era using matched-pairs analysis: multicenter study group of pancreatobiliary surgery in Japan. Pancreas 2016; 45: 1003–1009. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt CM, Glant J, Winter JM, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery 2007; 142: 572–578; discussion 578–580. [DOI] [PubMed] [Google Scholar]
- 69.Shi HJ, Jin C, Fu DL. Impact of postoperative glycemic control and nutritional status on clinical outcomes after total pancreatectomy. World J Gastroenterol 2017; 23: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000; 4: 567–579. [DOI] [PubMed] [Google Scholar]
- 71.Stauffer JA, Nguyen JH, Heckman MG, et al. Patient outcomes after total pancreatectomy: a single centre contemporary experience. HPB (Oxford) 2009; 11: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugiyama M, Atomi Y. Pylorus-preserving total pancreatectomy for pancreatic cancer. World J Surg 2000; 24: 66–70; discussion 70–61. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki S, Miura J, Shimizu K, et al. Clinicophysiological outcomes after total pancreatectomy. Scand J Gastroenterol 2016; 51: 1526–1531. [DOI] [PubMed] [Google Scholar]
- 74.Swope TJ, Wade TP, Neuberger TJ, et al. A reappraisal of total pancreatectomy for pancreatic cancer: results from US Veterans Affairs hospitals, 1987–1991. Am J Surg 1994; 168: 582–585; discussion 585–586. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi S, OY, Miyazaki H, et al. Aggressive surgery for pancreatic duct cell cancer: feasibility, validity, limitations. World J Surg 1995; 19: 653–659; discussion 660. [DOI] [PubMed] [Google Scholar]
- 76.Takami H, Fujii T, Kanda M, et al. Preservation of the pyloric ring confers little benefit in patients undergoing total pancreatectomy. World J Surg 2014; 38: 1807–1813. [DOI] [PubMed] [Google Scholar]
- 77.Venkat R, Puhan MA, Schulick RD, et al. Predicting the risk of perioperative mortality in patients undergoing pancreaticoduodenectomy: a novel scoring system. Arch Surg 2011; 146: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 78.Wagner M, Z’Graggen K, Vagianos CE, et al. Pylorus-preserving total pancreatectomy. Early and late results. Dig Surg 2001; 18: 188–195. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe Y, Ohtsuka T, Matsunaga T, et al. Long-term outcomes after total pancreatectomy: special reference to survivors' living conditions and quality of life. World J Surg 2015; 39: 1231–1239. [DOI] [PubMed] [Google Scholar]
- 80.Zakaria HM, Stauffer JA, Raimondo M, et al. Total pancreatectomy: short- and long-term outcomes at a high-volume pancreas center. World J Gastrointest Surg 2016; 8: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi SH, Hwang HK, Kang CM, et al. Pylorus- and spleen-preserving total pancreatoduodenectomy with resection of both whole splenic vessels: feasibility and laparoscopic application to intraductal papillary mucin-producing tumors of the pancreas. Surg Endosc 2012; 26: 2072–2077. [DOI] [PubMed] [Google Scholar]
- 82.Dallemagne B, de Oliveira de AT, Lacerda CF, et al. Full laparoscopic total pancreatectomy with and without spleen and pylorus preservation: a feasibility report. J Hepatobiliary Pancreat Sci 2013; 20: 647–653. [DOI] [PubMed] [Google Scholar]
- 83.Giulianotti PC, Addeo P, Buchs NC, et al. Early experience with robotic total pancreatectomy. Pancreas 2011; 40: 311–313. [DOI] [PubMed] [Google Scholar]
- 84.Kim DH, Kang CM, Lee WJ. Laparoscopic-assisted spleen-preserving and pylorus-preserving total pancreatectomy for main duct type intraductal papillary mucinous tumors of the pancreas: a case report Surg Laparosc Endosc Percutan Tech 2011; 21: 179–182. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Li Y, Cai Y, et al. Laparoscopic total pancreatectomy: case report and literature review. Medicine (Baltimore) 2017; 96: e5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zureikat AH, Nguyen T, Boone BA, et al. Robotic total pancreatectomy with or without autologous islet cell transplantation: replication of an open technique through a minimal access approach. Surg Endosc 2015; 29: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 2004; 64: 2634–2638. [DOI] [PubMed] [Google Scholar]
- 88.Matsubayashi H, Takaori K, Morizane C, et al. Familial pancreatic cancer and surveillance of high-risk individuals Gut Liver 2019; 13: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartsch DK, Sina-Frey M, Lang S, et al. CDKN2A Germline mutations in familial pancreatic cancer. Ann Surg 2002; 236: 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 1997; 89: 442–446. [DOI] [PubMed] [Google Scholar]
- 91.Tanaka M, Fernandes-del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017; 17: 738–753. [DOI] [PubMed] [Google Scholar]
- 92.Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 2020; 271: 1–14. [DOI] [PubMed] [Google Scholar]
- 93.Nota CL, Zwart MJ, Fong Y, et al. Developing a robotic pancreas program: the Dutch experience. J Vis Surg 2017; 3: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Rooij T, van Hilst J, Boerma D, et al. Impact of a nationwide training program in minimally invasive distal pancreatectomy (LAELAPS). Ann Surg 2016; 264: 754–762. [DOI] [PubMed] [Google Scholar]
- 95.de Rooij T, van Hilst J, Topal B, et al. Outcomes of a multicenter training program in laparoscopic pancreatoduodenectomy (LAELAPS-2). Ann Surg 2019; 269: 344–350. [DOI] [PubMed] [Google Scholar]
- 96.Crippa S, Bassi C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut 2017; 66: 495–506. [DOI] [PubMed] [Google Scholar]
- 97.Marchegiani G, Mino-Kenudson M, Sahora K, et al. IPMN involving the main pancreatic duct: biology, epidemiology, and long-term outcomes following resection. Ann Surg 2015; 261: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scholten L, van Huijgevoort NCM, Bruno MJ, et al. Surgical management of intraductal papillary mucinous neoplasm with main duct involvement: an international expert survey and case-vignette study. Surgery 2018; 164: 17–23. S0039-6060(18)30082-5. [DOI] [PubMed] [Google Scholar]
- 99.Schmeler KM, Lynch HT, Chen L-M, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006; 354: 261–269. [DOI] [PubMed] [Google Scholar]
- 100.Eisen A, Rebbeck TR, Wood WC, et al. Prophylactic surgery in women with a hereditary predisposition to breast and ovarian cancer. J Clin Oncol 2000; 18: 1980–1995. [DOI] [PubMed] [Google Scholar]
- 101.Ceppa EP, Pitt HA, Hunter JL, et al. Hereditary pancreatitis: endoscopic and surgical management. J Gastrointest Surg 2013; 17: 847–857. [DOI] [PubMed] [Google Scholar]
- 102.Kekis PB, Friess H, Kleeff J, et al. Timing and extent of surgical intervention in patients from hereditary pancreatic cancer kindreds. Pancreatology 2001; 1: 525–530. [DOI] [PubMed] [Google Scholar]
- 103.Salvia R, Malleo G, Marchegiani G, et al. Pancreatic resections for cystic neoplasms: from the surgeon’s presumption to the pathologist’s reality. Surgery 2012; 152: S135–S142. [DOI] [PubMed] [Google Scholar]
- 104.Del Chiaro M, Segersvärd R, Pozzi Mucelli R, et al. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol 2014; 21: 1539–1544. [DOI] [PubMed] [Google Scholar]
- 105.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010; 16: 5028–5037. [DOI] [PubMed] [Google Scholar]
- 106.Billings BJ, Christein JD, Harmsen WS, et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointestinal Surg 2005; 9: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 107.Kuesters S, Karcz WK, Hopt UT, et al. Anastomose laparoscopically assisted total pancreatectomy. Feasible minimally invasive alternative to open resection. Chirurg 2015; 86: 276–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620945534 for Prophylactic total pancreatectomy in individuals at high risk of pancreatic ductal adenocarcinoma (PROPAN): systematic review and shared decision-making programme using decision tables by Lianne Scholten, Anouk EJ Latenstein, Cora M Aalfs, Marco J Bruno, Olivier R Busch, Bert A. Bonsing, Bas Groot Koerkamp, I Quintus Molenaar, Dirk T Ubbink, Jeanin E van Hooft, Paul Fockens, Jolanda Glas, J Hans DeVries, Marc G Besselink and for the Dutch Pancreatic Cancer Group in United European Gastroenterology Journal