Abstract
Introduction
Long-term outcomes of patients with ulcerative proctitis (UP) have been poorly investigated, since these patients are excluded from participation in randomized controlled clinical trials.
Objective
The aim of this study was to investigate the prognostic and therapeutic long-term outcomes of patients with UP.
Methods
A retrospective study of patients with UP followed at our referral centre between 1 January 1998 and 1 January 2019 was performed. Treatment success was defined as clinical response (significant improvement in UP-related symptoms) and endoscopic response (mayo endoscopic sub-score of 0 or 1) if available at last follow-up.
Results
From a total of 1561 patients with ulcerative colitis, 118 patients with UP were identified. A total of 36 (31%) patients were refractory to rectal and oral therapy with 5-ASA and corticosteroids, necessitating azathioprine as monotherapy in 19 (16%) patients and/or biological therapies in 33 (28%) patients. After a median follow-up of 71 months (interquartile range 29–149 months), treatment success was observed in 103/118 (87%) UP patients and in 25/36 (69%) patients with refractory UP. Clinical response rates were significantly higher for refractory UP patients treated with biologicals (23/33; 70%) compared to ones treated with azathioprine (2/19; 11%; p = 0.001).
Conclusion
Good clinical outcomes were recorded in UP, with treatment success in 87% of patients. Nevertheless, 28% needed escalation to biologicals. Long-term outcome in patients on biologicals was superior to azathioprine.
Keywords: Ulcerative colitis, refractory ulcerative proctitis, azathioprine, anti-TNF, vedolizumab
Introduction
Ulcerative proctitis (UP) is a form of ulcerative colitis (UC), where intestinal inflammation is limited to the rectal mucosa.1 While the incidence rate of UC varies from 0.5 to 24.5/100,000 person-years worldwide,2,3 25–55% of patients present with UP at the time of diagnosis.4 Although the disease is limited to the rectum, UP is often responsible for distressing symptoms such as tenesmus, urgency, incontinence and rectal bleeding, leading to a reduced quality of life.4 Proximal extension of UP occurs in up to 28% of patients after 5 years of follow-up.4–7 Effective and timely management of patients with UP is therefore important not only to control symptoms and improve quality of life, but also potentially to delay or prevent proximal extension of inflammation.7–11
First-line treatment for mild or moderately active UP is rectal therapy with 5-aminosalicylic acid (5-ASA).7,12–14 Topical 5-ASA is more effective than topical steroids and oral 5-ASA.12,13 When there is insufficient response, the next step is to combine topical 5-ASA with topical corticosteroids and/or with oral 5-ASA. In patients who fail to improve despite combination therapy, oral corticosteroids are often necessary.9,12 Refractory UP is defined as active UP which fails rectal and oral therapy with 5-ASA and corticosteroids.9 Treatment of refractory UP remains challenging because these patients are systematically excluded from randomized controlled trials with drugs with new modes of action.9,15–19 In the absence of controlled data, recommendations for the management of UP are therefore often extrapolated from data in more extended UC or from small real-world evidence.9 Refractory UP may require treatment with intravenous steroids, immunomodulators or biologicals.13 A small retrospective multicentre study assessed short- and long-term outcomes of refractory UP treated with azathioprine. Only 20% had treatment success at last follow-up, with a median follow-up of 46 months.20 Another retrospective multicentre study analysed the efficacy of anti-tumour necrosis factor (anti-TNF) therapy in patients with refractory UP.21 After a median follow-up of 24 months, 64% (67/104) of patients were in clinical remission. There are no data with other biologicals or small molecules in UP. A small randomized, placebo controlled, double-blinded trial showed that topical tacrolimus was more effective than placebo for achievement of clinical remission and mucosal healing in patients with UP.22 As outcome data in patients with UP remain scarce, the aim of our study was to assess the prognostic and therapeutic outcomes of patients with UP followed at a single tertiary referral centre over a period of 20 years.
Methods
Study population
All patients with diagnosis of UC included in the inflammatory bowel disease (IBD) care programme of our tertiary referral centre between 1 January 1998 and 1 January 2019 were selected. Diagnosis of UC was based on clinical, endoscopic and/or histological criteria. The electronic medical records (EMR) of these patients were searched automatically for the keywords ‘proctitis’ or ‘rectitis’, and all identified EMR were reviewed. UP was defined according to the Montreal classification as involvement limited to the rectum, with the proximal extent of inflammation distal to the rectosigmoid junction.23 Refractory UP was defined by the absence of response to topical and peroral administration of 5-ASA and steroids.9 The use of intravenous steroids was not required.
Only patients who had a limitation of UC to the rectum were included. If patients with UP developed colonic extension while under therapy for UP, this was treated as treatment failure. The following demographic and clinical data were collected: age, sex, smoking status at time of diagnosis of UC, date of diagnosis of UP, duration of disease until last follow-up and treatments received with the date they were started and stopped. This study was approved by the ethics committee of the Catholic University of Leuven (MP006745, 25 September 2018). All patients included in the analysis had given written consent to participate in the Institutional Review Board approved IBD Biobank Research (B322201213950/S53684). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Outcomes
Clinical response was defined as partial or complete disappearance of UP-related symptoms as judged by the treating physician. Treatment success was defined as clinical response at last follow-up, no disease extension to left-sided or extensive colitis during follow-up, no need for systemic steroids or treatment switch at last follow-up, absence of colectomy and endoscopic inactive disease (mayo endoscopic sub-score of 0 or 1 on sigmoidoscopy) if endoscopy was available. We assured the absence of disease progression by reviewing medical reports of all endoscopic procedures during follow-up. All other patients were considered as treatment failure, except patients who maintained clinical response after stopping therapy due to adverse events. We also collected data on adverse events (infections, malignancies, hospitalizations and colectomy rates) until last follow-up.
Statistical analysis
Descriptive statistics were used to analyse patient characteristics. Quantitative variables were described as medians with interquartile ranges (IQR 25–75%). Categorical variables were presented as counts and percentages of the cohort. Proportions of patients with clinical response over time were described using Kaplan–Meier survival analysis. The starting date corresponded to the date of diagnosis or the date of start of azathioprine or first biological. Time to event was defined as time between the start date and the event of interest (therapy failure under latest therapy). For patients who did not have an event of interest, the observation was censored at the date of maximal follow-up. A p-value of <0.05 was considered significant. Patient characteristics were compared using Fisher’s exact test for categorical variables and the Mann–Whitney test for continuous variables. Statistical analyses were performed using IBM SPSS Statistics for Windows v25.0 (IBM Corp., Armonk, NY).
Results
Patient characteristics
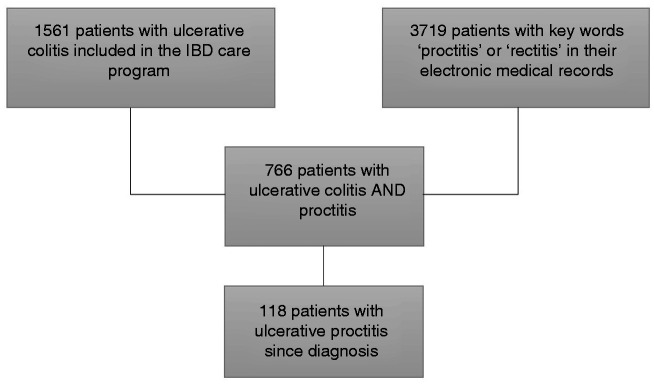
Starting from the total cohort of 1561 patients with UC, 766 patients had an episode of UP. From these, 118 patients fulfilled the inclusion criteria. Patient selection is shown in Figure 1. The baseline characteristics of the study population are summarized in Table 1. The median age at diagnosis of UC was 34 years (IQR 26–44 years), and the median duration of follow-up was 71 months (IQR 29–149 months). A total of 25 patients (21%) were active smokers at diagnosis of UC.
Figure 1.
Patient selection. IBD: inflammatory bowel disease.
Table 1.
Patient characteristics.
| Total group (N = 118) | |
|---|---|
| Sex, n (%) | |
| Female | 66 (56) |
| Median age (years) at UC diagnosis (IQR) | 34 (26–44) |
| Smoking status at UC diagnosis, n (%) | |
| Smoker | 25 (21) |
| Former smoker | 31 (26) |
| Never smoker | 46 (39) |
| Unknown | 16 (14) |
| Topical treatment, n (%) | |
| Topical 5-ASA | 50 (42) |
| Topical steroids | 1 (1) |
| Combination | 65 (55) |
| Unknown | 2 (2) |
| Oral treatment, n (%) | |
| Oral 5-ASA | 34 (29) |
| Oral steroids | 6 (5) |
| Combination | 42 (36) |
| Therapy-refractory patients, n (%) | 36 (31) |
| Azathioprine, n (%) | 22 (19) |
| Median duration (months) of UP prior to azathioprine (IQR) | 20 (8–72) |
| Azathioprine started as monotherapy, n (%) | 19 (16) |
| Azathioprine started in combination with infliximab, n (%) | 3 (3) |
| Biologicals, n (%) | 33 (28) |
| Median duration (months) of UP prior to biologicals (IQR) | 76 (17–144) |
| Anti-TNF as first biological, n (%) | 25 (21) |
| Vedolizumab as first biological, n (%) | 8 (7) |
| Azathioprine as concomitant therapy with first biological, n (%) | 6 (5) |
| Appendectomy, n (%) | 4 (3) |
| Median duration (months) of UP at last follow-up (IQR) | 71 (29–149) |
5-ASA: 5-aminosalicylic acid; anti-TNF: anti-tumour necrosis factor; IQR: interquartile range; UC: ulcerative colitis; UP: ulcerative proctitis.
Long-term outcomes
First-line therapy with 5-ASA and steroids
Topical 5-ASA was prescribed in 115 (97%) patients, and topical steroids were prescribed in 66 (56%) patients. The most commonly applied combination therapy was topical 5-ASA, together with topical steroids (65 patients; 55%). Oral therapy was started in 82/118 (69%) patients, of whom 76 (64%) received oral 5-ASA. A course of oral steroids (beclomethasone or prednisone) was necessary in 48 (41%) patients.
Azathioprine
Eventually, 22/118 (19%) patients were prescribed azathioprine for refractory UP. Median disease duration at azathioprine initiation was 20 months (IQR 8–72 months). Azathioprine was started as monotherapy in 19/118 (16%) patients, while it was initiated in association with anti-TNF in three (3%) patients. The patients in this last group were regarded as patients receiving biological therapy. Of the total 19 patients who initiated azathioprine as monotherapy, five (26%) achieved remission, with a median follow-up of 3 months (IQR 2–56 months). In all five patients, azathioprine was stopped because of lasting remission (n = 2; after treatment duration of 17 and 184 months), adverse events (n = 1; delayed wound healing, despite efficacy of the therapy during 23 months) and non-compliance with therapy (n = 2). Two of these five patients had treatment success at last follow-up. The other three patients maintained in remission at first but eventually needed step-up therapy to biologicals. The remaining 14 (74%) patients were non-responders to azathioprine. Out of those, 13 were switched to biologicals. One patient had colonic extension from UP while under therapy with azathioprine and was considered as treatment failure. Taken together, only 2/19 (11%) patients receiving azathioprine as monotherapy had long-term treatment success at the end of follow-up.
Biologicals
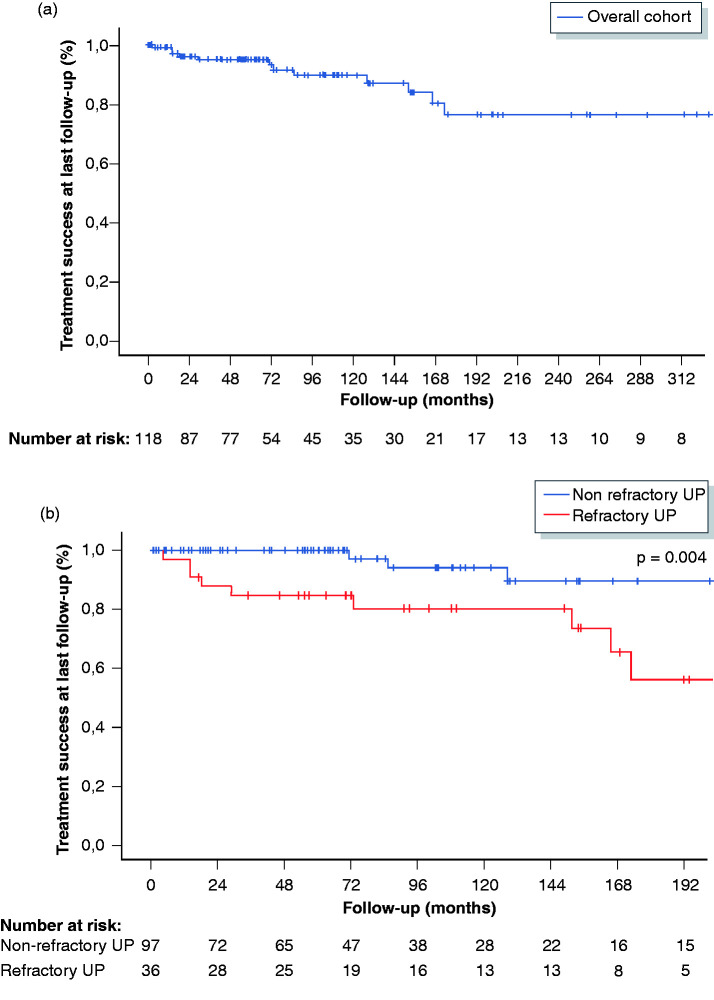
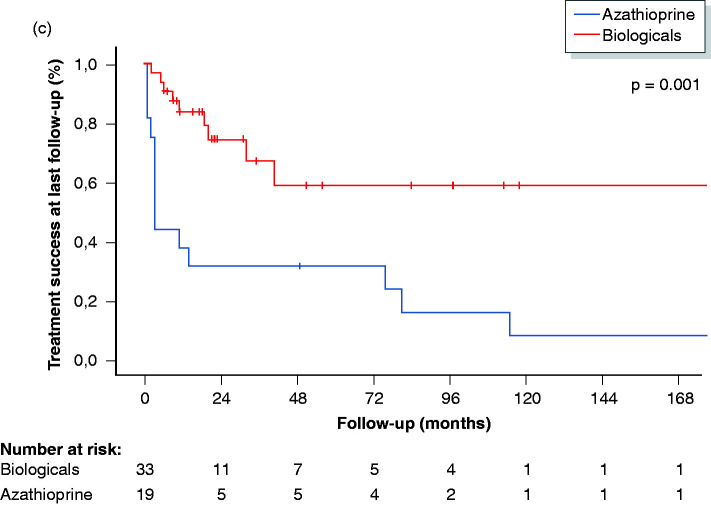
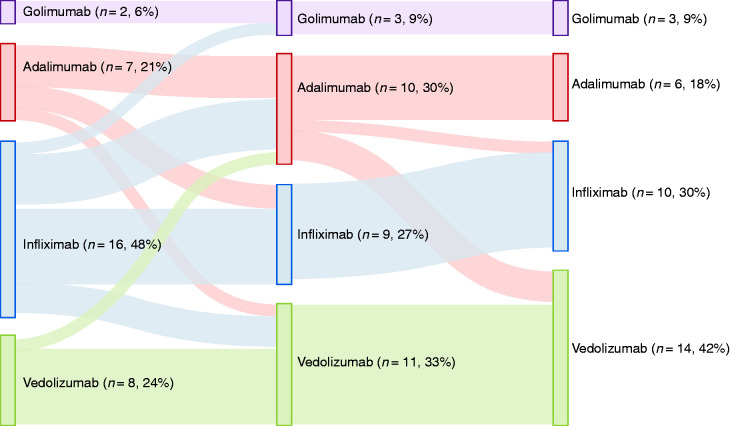
A total of 33/118 (28%) patients required biological therapy. The median delay to starting biologicals was 76 months (IQR 17–144 months). The median duration of treatment with anti-TNF was 18 months (IQR 9–33 months), and the median duration of treatment with vedolizumab was 11 months (IQR 6–19 months). In 76% (25/33), the first biological was an anti-TNF antagonist (16 infliximab, 7 adalimumab and 2 golimumab), and in 24% (8/33), the first biological was vedolizumab. Of the 33 patients, 14 (42%) achieved remission with their first biological (9 patients on anti-TNF and 5 patients on vedolizumab). Treatment success was present in 5/16 patients on intravenous infliximab in comparison to 4/9 patients on subcutaneous anti-TNF agents. In total, nine patients were switched to a second anti-TNF (2 infliximab, 6 adalimumab and 1 golimumab) and four to vedolizumab. Response to the second biological was observed in 38% (5/13) of patients. Five patients eventually cycled through a third biological, and four (80%) had treatment success at last follow-up. Taken together, therapy with anti-TNF was given to a total of 26 patients (first-choice biological in 25 patients, and second-choice biological after failure of vedolizumab in one patient) and was successful in 50% (13/26) after a median follow-up of 21 months (IQR 9–43 months). In total, 15 patients started with vedolizumab (first-choice biological in eight patients, second-choice biological after failure of one anti-TNF agent in four patients and third choice after failure of two anti-TNF agents in three patients), and 10 (67%) reported treatment success, with a median follow-up of 11 months (IQR 6–19 months). Clinical response rates were significantly higher for patients treated with biologicals (23/33; 70%) compared to patients treated with azathioprine (2/19; 11%; p = 0.001). Figure 2 shows the treatment success rates at last follow-up in the overall cohort, in patients with refractory and non-refractory UP and in patients on azathioprine and biologicals. Consecutive use of biological therapies is shown in Figure 3.
Figure 2.
Treatment success (%) at last follow-up in the overall cohort (a), in patients with non-refractory and refractory UP (B) and in patients on biologicals and azathioprine (c). UP: ulcerative proctitis.
Figure 3.
Consecutive use of biological therapies.
Proctocolectomy
Only one patient in the entire cohort needed proctocolectomy after therapy failure with oral steroids, azathioprine and infliximab.
Endoscopic data
A total of 56% (46/82) of patients with non-therapy-refractory UP and 56% (20/36) of patients with therapy-refractory UP underwent endoscopic assessment within the last 7 months of follow-up. An endoscopic response was observed in 68% (45/66) of patients: in 74% (34/46) of patients with non-therapy-refractory UP and in 55% (11/20) of patients with therapy-refractory UP.
Safety
Eight patients reported adverse events on azathioprine, leading to drug withdrawal in seven patients. Gastro-intestinal intolerance was the most common complaint (n = 3), followed by pancreatitis (n = 1), arthralgia and myalgia (n = 1), headache (n = 1) and delayed wound healing (n = 1). The adverse event was unknown in one patient. Twelve patients developed adverse events under anti-TNF, necessitating cessation of therapy. Five patients developed high-titre anti-drug antibodies during therapy. Other adverse events included anti-TNF-induced lupus-like syndrome (n = 1), psoriasiform dermatitis (n = 1), urticaria (n = 1), arthralgia (n = 1), neuritis optica (n = 1) and recurring vaginal fungal infections (n = 1). In one patient, the type of adverse event was not specified. Vedolizumab was generally well tolerated, and only one patient stopped therapy due to arthralgia, myalgia and psoriasiform dermatitis. No opportunistic infections, tuberculosis, colorectal dysplasia, malignancies or lymphoma were observed during follow-up.
Discussion
This study assessed the long-term outcomes of a specific subgroup of UC patients, namely those with UP. Although with a limited extent of inflammation, UP can be a disabling condition with distressing symptoms and can greatly decrease quality of life.4,9
In our study, 31% (36/118) of patients were refractory to conventional therapy with 5-ASA and steroids. Azathioprine was successful as monotherapy in only 11% (2/19) of patients. These results are comparable with a retrospective multicentre study from Mallet et al.20 Among 1279 patients with UC who were screened at three referral centres, 25 patients had refractory UP and were treated with azathioprine. Although 40% of patients had a short-term clinical response, only five (20%) had treatment success at the end of follow-up, with a median follow-up of 46 months.
A total of 28% of our patients eventually required treatment escalation to biological agents. The long-term outcome for biologicals was better, as 70% (23/33) had treatment success at last follow-up. Anti-TNF therapy was successful in 50% of patients. A small French retrospective multicentre study showed a short-term clinical improvement in 85% (11/13) of patients and maintenance of a complete response in 69% (9/13) of patients.24 Another French retrospective multicentre study analysed the efficacy of anti-TNF therapy in patients with refractory UP. This study included 104 patients, of whom 50% (52/104) were treated with infliximab, 39% (41/104) with adalimumab and 11% (11/104) with golimumab. Clinical remission at last follow-up was achieved in 64% of the patients, with a median follow-up of 24 months.21 Table 2 shows a summary of these studies where long-term follow-up of biologicals in patients with therapy refractory UP is analysed. In our study, vedolizumab was successful in 10/15 (67%) patients. Analogous to this, in the study by Pineton De Chambrun et al., 20 patients were treated with vedolizumab after failure of anti-TNF, of whom 70% (14/20) achieved clinical remission.21
Table 2.
Long-term follow-up of biologicals in patients with therapy-refractory UP.
| Study | Number of patients with refractory UP | Biological | Long-term remission | Median follow-up (months) |
|---|---|---|---|---|
| Bouguen et al.24 | n = 13 | Anti-TNF (infliximab) | 69% (9/13) | 17 |
| Pineton De Chambrun et al.21 | n = 104 | Anti-TNF (infliximab, adalimumab, golimumab) | 64% (67/104) | 24 |
| This study | n = 26 | Anti-TNF (infliximab, adalimumab, golimumab) | 50% (13/26) | 21 |
| This study | n = 15 | Vedolizumab | 67% (10/15) | 11 |
anti-TNF: anti-tumour necrosis factor; UP, ulcerative proctitis.
Ustekinumab has recently been approved for the treatment of moderate to severe UC following the positive results of the Phase III UNIFI study.18 Tofacitinib, an oral small-molecule Janus kinase inhibitor, is also effective in inducing and maintaining remission in UC.19 There are no reports on the efficacy of ustekinumab and tofacitinib in UP, as patients with disease limited to the rectum were excluded, but these may offer other potentially effective treatment options in the future.
Rectal therapies offer a number of advantages, including direct delivery of the drug to the site of inflammation and reduced systemic drug exposure with favourable safety profiles and rapid response to treatment.25 Several topical compounds have been tested, including alicaforsen, cyclosporin, epidermal growth factor, nicotine, rebapamide, rosiglitazone, tacrolimus and short-chain fatty acid enemas.9 However, most agents were evaluated in only a very small number of patients with distal forms of UC. Efficacy of topical tacrolimus has been demonstrated in a randomized, placebo-controlled, double-blinded trial using a rectal tacrolimus ointment in patients with a maximum extent of inflammation of 25 cm.22 A total of 73% (8/11) of patients reached clinical response, and 45% (5/11) achieved clinical remission after 8 weeks with tacrolimus compared to 10% (1/10) and 0% (0/10) with placebo. A recent German retrospective study analysed the efficacy of tacrolimus suppositories in patients with refractory distal UC and UP.26 A total of 52% of patients with UP reached clinical remission. In both studies, patient numbers were small, and concomitant medications including immunosuppression were allowed.22,26
During follow-up, one patient in our cohort underwent proctocolectomy with ileo-anal pouch anastomosis.
Our study has limitations. First, it was a retrospective study with no comparator group. The evaluation of treatment success was based on clinical and endoscopic features judged by the treating physicians. Second, our centre is a tertiary referral hospital with the intrinsic bias of having a selected population and a higher proportion of refractory UP. Third, some therapies were only available more recently, with consequently shorter periods of follow-up. This may bias the interpretation of treatment success.
Conclusion
In this large single-centre study on patients with UP, one third of patients were refractory to conventional therapies and needed immunomodulators or biological therapies. Whereas long-term outcomes on azathioprine were poor, our findings indicate that biologicals (anti-TNF and vedolizumab) may be effective in inducing and maintaining clinical and endoscopic remission in patients with refractory UP. Given the importance of achieving rapid disease control in these patients to reduce symptoms, improve quality of life and prevent disease progression, accelerated step up to biologicals may be preferred. The combined efficacy–safety results with the newer biologicals such as vedolizumab are promising.
Acknowledgements
This article formed a poster presentation at the European Crohn’s and Colitis Organisation 2020 and at Belgian Week of Gastroenterology 2020.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.S. reports consultancy fees (Janssen) and speaker fees (Nestle Health Sciences, Abbvie and Takeda). M.F. reports financial support for research (Amgen, Biogen, Janssen, Pfizer and Takeda), consultancy fees (Abbvie, Biogen, Boehringer-Ingelheim, Ferring, Janssen, MSD, Pfizer, Sandoz, Takeda and Thermo Fisher Scientific) and speaker fees (Abbvie, Amgen, Biogen, Boehringer-Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, Sandoz and Takeda). S.V. reports financial support for research (MSD, Abbvie, Takeda, Janssen and Pfizer), consultancy fees (Abbvie, MSD, Takeda, Ferring, Genentech/Roche, Shire, Pfizer, Inc., Galapagos, Mundipharma, Hospira, Celgene, Second Genome, Progenity, Lilly, Arena, Gilead and Janssen) and speaker fees (Abbvie, MSD, Takeda, Ferring, Hospira, Pfizer, Janssen and Tillots). E.D., A.M. and RG declare no conflict of interest.
Ethics approval
All patients included in the analysis had given written consent to participate in the Institutional Review Board approved IBD Biobank (B322201213950/S53684. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Informed consent
All patients included in the analysis had given written consent.
ORCID iDs
Evelyne Dubois https://orcid.org/0000-0001-5923-2154
Séverine Vermeire https://orcid.org/0000-0001-9942-3019
References
- 1.Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012; 380: 1606–1619. [DOI] [PubMed] [Google Scholar]
- 2.Gajendran M, Loganathan P, Jimenez G, et al. A comprehensive review and update on ulcerative colitis. Dis Mon 2019; 65: 100851. [DOI] [PubMed] [Google Scholar]
- 3.Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut 1996; 39: 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meucci G, Vecchi M, Astegiano M, et al. The natural history of ulcerative proctitis: a multicenter, retrospective study. Am J Gastroenterol 2000; 95: 469–473. [DOI] [PubMed] [Google Scholar]
- 5.Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis 2007; 13: 481–489. [DOI] [PubMed] [Google Scholar]
- 6.Kim B, Park SJ, Hong SP, et al. Proximal disease extension and related predicting factors in ulcerative proctitis. Scand J Gastroenterol 2014; 49: 177–183. [DOI] [PubMed] [Google Scholar]
- 7.Lie MRKL, Kanis SL, Hansen BE, et al. Drug therapies for ulcerative proctitis: systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20: 2157–2178. [DOI] [PubMed] [Google Scholar]
- 8.Gecse KB, Lakatos PL. Ulcerative proctitis: an update on the pharmacotherapy and management. Expert Opin Pharmacother 2014; 15: 1565–1573. [DOI] [PubMed] [Google Scholar]
- 9.Pineton De Chambrun G, Tassy B, Kollen L, et al. The treatment of refractory ulcerative colitis. Best Pract Res Clin Gastroenterol 2018; 32–33: 49–57. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren S, Löfberg R, Bergholm L, et al. Effect of budesonide enema on remission and relapse rate in distal ulcerative colitis and proctitis. Scand J Gastroenterol 2002; 37: 705–710. [DOI] [PubMed] [Google Scholar]
- 11.Pica R, Paoluzi OA, Iacopini F, et al. Oral mesalazine (5-ASA) treatment may protect against proximal extension of mucosal inflammation in ulcerative proctitis. Inflamm Bowel Dis 2004; 10: 731–736. [DOI] [PubMed] [Google Scholar]
- 12.Calafat M, Lobatón T, Mañosa M, et al. Therapeutic requirements in active ulcerative proctitis: a single-centre study. Gastroenterol Hepatol 2017; 40: 663–668. [DOI] [PubMed] [Google Scholar]
- 13.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114: 384–413. [DOI] [PubMed] [Google Scholar]
- 15.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 16.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011; 60: 780–787. [DOI] [PubMed] [Google Scholar]
- 17.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 18.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019; 381: 1201–1214. [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 20.Mallet AL, Bouguen G, Conroy G, et al. Azathioprine for refractory ulcerative proctitis: a retrospective multicenter study. Dig Liver Dis 2017; 49: 280–285. [DOI] [PubMed] [Google Scholar]
- 21.Pineton De Chambrun G, Amiot A, Bouguen G, et al. Efficacy of tumor necrosis factor antagonist treatment in patients with refractory ulcerative proctitis. Clin Gastroenterol Hepatol 2020; 18: 620–627. [DOI] [PubMed] [Google Scholar]
- 22.Lawrance IC, Baird A, Lightower D, et al. Efficacy of rectal tacrolimus for induction therapy in patients with resistant ulcerative proctitis. Clin Gastroenterol Hepatol 2017; 15: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 23.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouguen G, Roblin X, Bourreille A, et al. Infliximab for refractory ulcerative proctitis. Aliment Pharmacol Ther 2010; 31: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 25.Cohen RD, Dalal SR. Systematic review: rectal therapies for the treatment of distal forms of ulcerative colitis. Inflamm Bowel Dis 2015; 21: 1719–1736. [DOI] [PubMed] [Google Scholar]
- 26.Jaeger SU, Klag T, Hoeger K, et al. Tacrolimus suppositories in therapy-resistant ulcerative proctitis. Inflamm Intest Dis 2018; 3: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]