Abstract
Background
The lack of scientific evidence regarding the effectiveness of 5-aminosalicylate in patients with Crohn’s disease is in sharp contrast to its widespread use in clinical practice.
Aims
The aim of the study was to investigate the use of 5-aminosalicylate in patients with Crohn’s disease as well as the disease course of a subgroup of patients who were treated with 5-aminosalicylate as maintenance monotherapy during the first year of disease.
Methods
In a European community-based inception cohort, 488 patients with Crohn’s disease were followed from the time of their diagnosis. Information on clinical data, demographics, disease activity, medical therapy and rates of surgery, cancers and deaths was collected prospectively. Patient management was left to the discretion of the treating gastroenterologists.
Results
Overall, 292 (60%) patients with Crohn’s disease received 5-aminosalicylate period during follow-up for a median duration of 28 months (interquartile range 6–60). Of these, 78 (16%) patients received 5-aminosalicylate monotherapy during the first year following diagnosis. Patients who received monotherapy with 5-aminosalicylate experienced a mild disease course with only nine (12%) who required hospitalization, surgery, or developed stricturing or penetrating disease, and most never needed more intensive therapy. The remaining 214 patients were treated with 5-aminosalicylate as the first maintenance drug although most eventually needed to step up to other treatments including immunomodulators (75 (35%)), biological therapy (49 (23%)) or surgery (38 (18%)).
Conclusion
In this European community-based inception cohort of unselected Crohn’s disease patients, 5-aminosalicylate was commonly used. A substantial group of these patients experienced a quiescent disease course without need of additional treatment during follow-up. Therefore, despite the controversy regarding the efficacy of 5-aminosalicylate in Crohn’s disease, its use seems to result in a satisfying disease course for both patients and physicians.
Keywords: Population-based cohort, 5-aminosalicylates, disease course
Introduction
Crohn’s disease (CD) is a chronic, disabling inflammatory bowel disease (IBD) of unknown aetiology. Patients require anti-inflammatory treatments such as corticosteroids, immunomodulators and biological therapies, and sometimes surgery, in order to induce and maintain remission. The disease course varies among patients with some developing potentially disabling complications over time including strictures and fistulas, while others experience an indolent disease course with limited need for medication.1
While 5-aminosalicylate (5-ASA) remains a fundamental treatment for ulcerative colitis, its use in CD remains questionable. Several systematic reviews have concluded that the role of 5-ASA in CD to either induce remission or prevent relapses is no better than placebo or, at best, remains uncertain.2–4 Accordingly, international guidelines do not recommend the use of 5-ASA in CD,5 except in some specific clinical situations such as postoperative prophylactic treatment among patients with low risk of relapse. However, the scientific controversy regarding the effectiveness of 5-ASA in CD patients stands in sharp contrast to clinical practice. In recent population-based cohorts, more than half of CD patients received 5-ASA at some point in their disease course.6–8 Whether the high use of 5-ASA in clinical practice indicates that a subgroup of patients with mild disease might benefit from these drugs, or whether most patients on 5-ASA quickly will need treatment escalation remains unknown. To date, population-based data regarding the use of 5-ASA in CD are lacking.
The Epi-IBD study is a prospective population-based inception cohort investigating the occurrence and disease course of IBD in Europe.9The aim of the present study was (a) to assess the use of 5-ASA in CD, and (b) to investigate the disease course of a subgroup of patients who were treated with 5-ASA as maintenance monotherapy during the first year of disease.
Materials and methods
Study population and design
Details of the Epi-IBD cohort have been described previously.9 In short, the Epi-IBD cohort is a prospective population-based inception cohort of IBD patients diagnosed in 2010 from 29 centres from eight Eastern and 13 Western European countries. The study population consists of 1289 IBD patients, 488 with CD, 717 with ulcerative colitis (UC), and 84 with IBD unclassified (IBDU). All patients are recruited within well-described geographical areas covering a total background population of 9.7 m people (2.6 m in Eastern Europe and 7.1 m in Western Europe).
All participating centres were university-affiliated and offered the full range of care including infusions with biologics. Requirement for centres to participate included having a well-defined primary catchment area with up-to-date population data. They were also required to have an established network of gastroenterologists, colorectal surgeons and general practitioners (GPs) within the uptake area, who were contacted twice during the 1-year inclusion period to ensure complete coverage and recruitment of patients. Case ascertainment methods, diagnostic criteria, inclusion period and patient data were all standardised.
Patients were followed prospectively from diagnosis for 5 years, or until the date of their emigration or death. We collected data regarding demographics, disease activity, medical therapy including dose, date of initiation and date of cessation, surgery, hospitalization, disease classification, cancers and deaths prospectively in the Web-based Epi-IBD database.10 To assess the 5-year outcome of the cohort, a follow-up period of 5 years (with a three-month margin either side of the end date) was chosen.
Measures to secure data validity included built-in control and validation tests as well as locked diagnostic criteria in the database. Furthermore, manual data standardization and random audits of case ascertainment and data quality9 were performed as part of the study.
Classifications and definitions
Centres included all patients diagnosed with IBD according to the Copenhagen Diagnostic Criteria11,12 between 1 January–31 December 2010, aged ≥15 years and living in the predefined catchment areas at the time of diagnosis. Patients experiencing a change in their diagnosis during follow-up were reclassified as having the new diagnosis from the date of the original diagnosis, as previously described.6,13
Disease location and behaviour for CD were defined according to the Montreal Classification.14 Progression in disease behaviour was defined as the development of strictures (B2) or fistula (B3) in patients with non-stricturing, non-penetrating phenotype (B1) at diagnosis. Patients were categorised according to the most intense level of disease behaviour observed in them during the study period. Surgery included resection because of intestinal inflammation or a CD-related complication such as a stenosis, fistula or perforation. Hospitalization was defined as either admission for CD-related surgery (surgical hospitalization) or other CD-related complications (medical hospitalization). We analysed each of the previously mentioned outcomes as well as a composite endpoint of either surgery, hospitalization and/or disease progression.
Patients were defined as users of various IBD drugs on the first day of administration, regardless of treatment duration. Treatment was categorised according to five levels of ascending therapeutic potency: oral 5-ASA, corticosteroids, immunomodulators (azathioprine, 6-mercaptopurine, cyclosporine or methotrexate), biologicals (infliximab or adalimumab) and surgery (major abdominal surgery due to CD). Topical treatments with 5-ASA were excluded. Immunomodulators (azathioprine, 6-mercaptopurine, cyclosporine and methotrexate) were grouped as >90% of patients treated with immunomodulators received thiopurines. Disease activity of CD was measured using the Harvey Bradshaw Index (HBI).15 A HBI score of <5 was defined as remission, 5–7 as mildly active disease, 8–16 as moderately active disease, and ≥16 as severely active disease.16
We defined a subgroup of CD patients that only received 5-ASA as maintenance monotherapy during their first year following diagnosis, either following a single course of prednisolone or budesonide, or as initial treatment. These patients were compared with all other CD patients in terms of disease outcomes (treatment escalation, disease progression, hospitalisation, surgery).
Statistical analysis
Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). Possible associations between multiple covariates and belonging to the group of CD patients being treated with only 5-ASA in the first year were analysed by logistic regression analysis. The following covariates were included in the statistical model: age at diagnosis (continuous variable), sex, geographic region (Western vs Eastern Europe), disease behaviour, disease location, smoking status at diagnosis(currently, never/former), diagnostic delay (time from debut of symptoms to diagnosis, continuous variable). Differences in disease outcomes (surgery, hospitalisation) between the aforementioned subgroup of CD patients and the rest of the CD cohort were visualised using Kaplan-Meier plots and analysed with the log-rank test.
Differences regarding time to events were compared using the Wilcoxon two-sample test. Continuous variables are expressed as median (interquartile range (IQR)) unless otherwise stated. Groups were compared using chi-squared test or Fischer exact test, where appropriate. A p-value of <0.05 was considered statistically significant.
Ethical considerations
Ethical committees at each centre approved the study according to local regulations. The Danish Ethical Committee approved the study 21 September 2009 (H-4-42009-115). Written, informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
A total of 488 CD patients from the Epi-IBD cohort were included in this study of which 292 (60%) patients had received 5-ASA at least once during the 5-year follow-up period for a median treatment time frame of 28 months (IQR 6–60). A total of 16 (5%) patients were initially diagnosed with UC or IBDU, but had their diagnosis changed to CD during follow-up. Patients receiving 5-ASA at some point during follow-up had shorter diagnostic delay, less complicated CD, and the proportion of patients being diagnosed in Eastern European centres was higher compared to those that did not. Patient characteristics are shown in Table 1 and the number of CD patients included in each country is shown in the Supplementary Material.
Table 1.
Characteristics of Crohn’s disease patients from the Epi-IBD cohort.
| Patients receiving 5-ASA monotherapy within the first year of follow-up (n = 128) | Patients receiving 5-ASA at any time during follow-up (n = 292) | All Crohn’s disease patients (n = 488) | ||||
|---|---|---|---|---|---|---|
| Age, years (IQR) | 37 (25–58)a | 34 (24–50) | 33 (23–49) | |||
| Males, n (%) | 62 (48%) | 145 (50%) | 244 (50%) | |||
| Diagnostic delay, months (IQR) | 3.5 (1.0–11.3) | 3.9 (1.2–11.5)b | 4.2 (1.7–12.0) | |||
| Extraintestinal manifestations at diagnosis, n (%) | 21 (16%) | 51 (17%) | 79 (16%) | |||
| Length of follow-up, months (IQR) | 60 (26–63)a | 63 (51–63) | 63 (39–63) | |||
| Geographic region, n (%) | ||||||
| Eastern Europe | 35 (27%)a | 75 (26%)b | 84 (17%) | |||
| Western Europe | 93 (73%) | 217 (74%) | 404 (83%) | |||
| Smoking at diagnosis, n (%) | ||||||
| Never | 56 (49%) | 110 (40%) | 183 (40%) | |||
| Current | 37 (33%) | 106 (39%) | 171 (37%) | |||
| Former | 21 (18%) | 57 (21%) | 103 (23%) | |||
| Disease behaviour at diagnosis, n (%) | ||||||
| B1: non-stricturing, non-penetrating | 111 (87%)a | 210 (75%)b | 347 (71%) | |||
| B2: stricturing | 14 (11%) | 52 (19%) | 100 (21%) | |||
| B3: penetrating | 3 (2%) | 17 (6%) | 41 (8%) | |||
| Perianal disease | 6 (5%) | 16 (5%) | 46 (9%) | |||
| Disease location at diagnosis, n (%) | ||||||
| L1: terminal ileum | 35 (28%) | 72 (25%) | 128 (27%) | |||
| L2: colon | 44 (35%) | 95 (33%) | 134 (28%) | |||
| L3: terminal ileum + colon | 27 (21%) | 65 (23%) | 111 (23%) | |||
| L4: upper GI | 5 (4%) | 16 (5%) | 30 (6%) | |||
| L1–L3 + L4 | 16 (13%) | 40 (14%) | 76 (16%) | |||
| Cumulative frequency and duration of medical treatment | ||||||
|
n (%) |
Months, median (IQR) |
n (%) |
Months, median (IQR) |
n (%) |
Months, median (IQR) |
|
| Oral 5-ASAs | 128 (100%) | 34 (12–60) | 292 (100%) | 28 (6–60) | 290 (60%) | 28 (6–60) |
| Budesonide | 18 (14%) | 4 (2–10) | 74 (25%) | 6 (3–12) | 135 (28%) | 4 (3–10) |
| Prednisolone | 50 (39%) | 3 (2–4) | 178 (61%) | 3 (2–7) | 294 (60%) | 3 (2–6) |
| Immunomodulators | 14 (11%) | 21 (15–38) | 160 (55%) | 33 (12–55) | 311 (64%) | 37 (12–58) |
| Biological therapy | 3 (2%) | 11 (2–13) | 66 (23%) | 25 (11–45) | 144 (30%) | 32 (13–49) |
5-ASA: 5-aminosalicylate; IQR: inter-quartile range; GI: gastrointestinal tract.
ap < 0.05, comparison with the rest of the cohort (n = 360).
bp < 0.05, comparison with the rest of the cohort (n = 196).
Of the 292 patients, 164 patients (56%; 34% of the total CD cohort) received 5-ASA at some point during follow-up, including as monotherapy, but were escalated to higher treatment steps. Only two of those patients had not received 5-ASA already during their first year following diagnosis: one following surgery and one as first treatment but after 15 months delay due to patient preference. Overall, in 110 (68%) patients, 5-ASA was the first treatment initiated following diagnosis, while 48 (29%) patients initially received prednisolone or budesonide, four (2%) had received immunomodulators, one (0.5%) patient had received adalimumab and one (0.5%) had an intestinal resection. All patients eventually needed to step up therapy within the first year of disease (with the exception of the two patients mentioned previously): the highest treatment step needed during follow-up was immunomodulators in 75 (46%) patients, biological therapy in 49 (30%) patients and surgery in 38 (23%) patients.
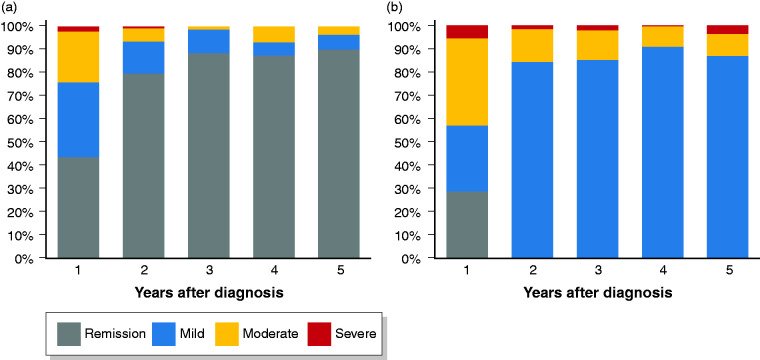
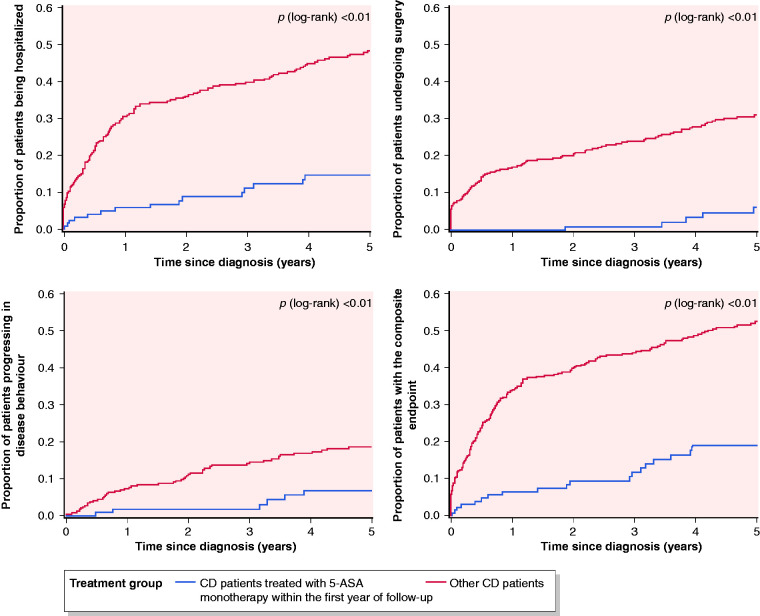
A total of 128 CD patients (44%; 26% of the total CD cohort) received 5-ASA as their only medical maintenance treatment during the first year following diagnosis. Of those, 78 (61%) patients received only 5-ASA monotherapy while 50 (39%) patients were initially treated with prednisolone or budesonide before starting 5-ASA monotherapy. These patients were treated with 5-ASA for a median duration of 34 months (IQR 12–60 months). Few patients had complicated disease behaviour at diagnosis and the proportion of patients treated was higher in the Eastern cohort compared to the rest of the cohort (Table 1). A total of 6 out of 34 (16%) patients that were smokers at the time of diagnosis stopped smoking during follow-up, which did not differ compared to the rest of the cohort (data not shown). The proportion of these 128 patients in clinical remission increased from 43% during the first year of disease to 90% in the fifth year of follow-up with only one patient experiencing severe clinical disease activity (Figure 1). Compared to the rest of the cohort, the distribution of patients within the categories of clinical disease activity was significantly different in year 1–3. During follow-up, rates of surgery and hospitalisation in these patients were significantly lower compared to the rest of the cohort (Figure 2(a) and (b)). Five patients (4%) needed surgery after a median of 3.8 years IQR (3.4–4.1 years) and 15 patients (12%) were hospitalised for CD after a median of 1.4 years (IQR 0.2–3.0 years). Similarly, significantly fewer patients experienced disease progression (Figure 2(c)) as only seven (6%) patients progressed to stricturing (n=6) or penetrating (n = 1) disease after a median of 3.3 years (IQR 0.8–3.9). When combining all three endpoints, 19 (15%) patients CD treated with 5-ASA had surgery, hospitalization and/or experienced progression in disease behaviour compared to 175 (49%, p<0.01) of the remaining CD cohort (Figure 2(d))
Figure 1.
Distribution of disease activity measured by the Harvey-Bradshaw Index among (a) patients with Crohn’s disease treated with 5-aminosalicylate (5-ASA) monotherapy within the first year of follow-up (n = 128) and (b) the rest of the cohort (n = 360) during 5 years of follow-up.
Figure 2.
Cumulative probability for (a) hospitalization, (b) surgery, (c) disease progression and (d) the composite endpoint of all three outcomes in patients with Crohn’s disease (CD) in a European inception cohort.
5-ASA: 5-aminosalicylate.
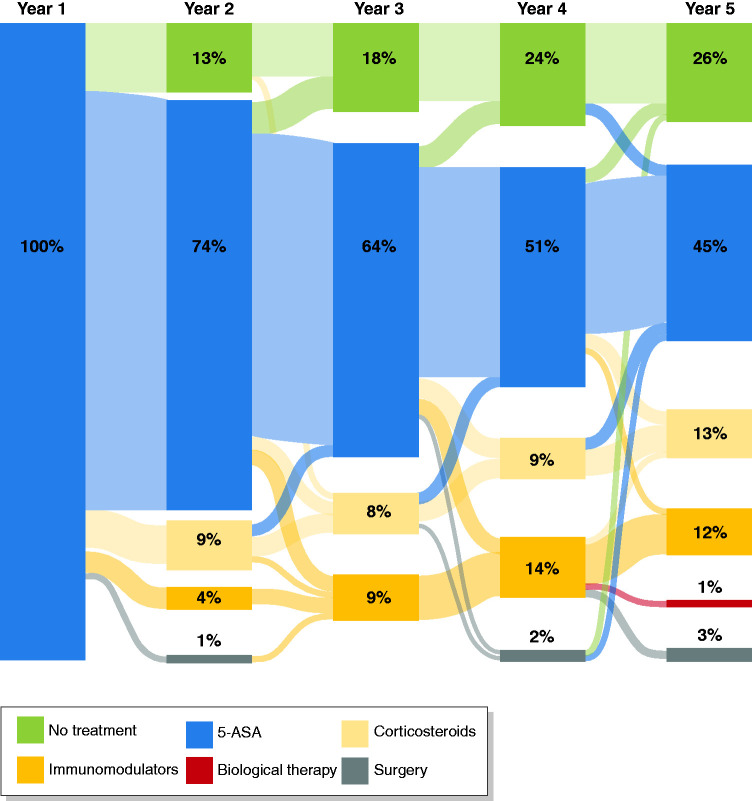
Patient characteristics associated with belonging to the subgroup of CD patients treated with only 5-ASA as maintenance therapy during the first year are shown in Table 2. Older age, non-stricturing/non-penetrating disease behaviour and residing in Western Europe were identified as significant factors. During follow-up, patients (n = 95 (74% of the 5-ASA-only group), representing 19% of the total cohort) did not need a higher treatment step than 5-ASA. The cumulative prevalence of medical treatments is shown in Table 1 and the pathways between treatment steps is shown in Figure 3. In all but one patient, 5-ASAs were continued during the step up to immunomodulators and/or biological therapy. A total of 15 (12%) of these patients were started on immunomodulators, biological therapy and/or needed surgery during follow-up. In a Cox regression analysis, only disease location (terminal ileum vs colon odds ratio (OR) 24.8, 95% confidence interval (CI) 2.0–304.0) was associated with the risk of treatment escalation.
Table 2.
Factors associated with belonging to the group of Crohn’s disease, that received 5-aminosalicylate (5-ASA) monotherapy within the first year of follow-up in the Epi-IBD cohort.
| Patients receiving 5-ASA monotherapy within the first year of follow-up (n = 128) | |
|---|---|
|
Odds ratio (95% confidence interval) |
|
| Age at diagnosis (per year)a | 1.014 (1.001–1.032) |
| Sex | |
| Male | Reference |
| Female | 0.992 (0.643–1.530) |
| Diagnostic delay (per day) | 0.932 (0.592 –1.467) |
| Geographic regiona | |
| Western Europe | Reference |
| Eastern Europe | 0.377 (0.222–0.638) |
| Smoking status at diagnosis | |
| Currently | Reference |
| Former/never | 0.725 (0.455–1.155) |
| Disease behavioura | |
| B1: non-stricturing, non-penetrating | Reference |
| B2: stricturing | 0.537 (0.143–2.023) |
| B3: penetrating | 0.175 (0.052–0.597) |
| Disease location | |
| L1: terminal ileum | 0.879 (0.482–1.602) |
| L2: colon | Reference |
| L3: terminal ileum + colon | 1.242 (0.685–2.253) |
| L4: upper GI (± L1–L3) | 1.264 (0.660–2.419) |
| Extra-intestinal manifestations at diagnosis | |
| Yes | Reference |
| No | 0.987 (0.556–1.753) |
GI: gastrointestinal tract.ap < 0.05.
Figure 3.
Pathways between treatment stepsa during follow-up of patients with Crohn’s disease treated with 5-aminosalicylate (5-ASA) monotherapy within the first year of follow-up
aPatients receiving combination therapy are categorised according to the highest treatment step.
Discussion
In this European population-based inception cohort of unselected CD patients, we found that despite the unclear efficacy of 5-ASA in CD the use of this group of drugs remains high with more than half of CD patients treated with 5-ASA during their disease course. Most patients received 5-ASA immediately following diagnosis or after an initial course of steroids and then stepped up to other drugs. However, a subgroup of patients remained on 5-ASA for the whole observation period. This subgroup of CD patients was older, had less complicated disease behaviour at diagnosis and exhibited a mild disease course with only very few patients experiencing disease progression or surgery. There was no association between colonic disease location and the use of 5-ASA. To our knowledge, this is the first study of a cohort of unselected CD patients to demonstrate these findings.
Systematic reviews have shown no convincing benefit of oral 5-ASA over placebo either in induction or in maintenance of medically induced remission in CD3,4 and there seems to be no additional benefit in colonic CD.17 Accordingly, most guidelines do not recommend 5-ASA for induction or maintenance treatment of CD.5,18 Nonetheless, 5-ASA is used in a large proportion of patients. In a primary care cohort from the UK more than 60% of patients had 5-ASA prescriptions within 5 years of diagnosis, and in fact the proportion of patients with at least one prescription for oral 5-ASAs within a year of diagnosis increased during 1990–2010.19 Within the Swiss IBD cohort 60% of patients were treated with 5-ASA during their disease course.20 Neither age nor disease phenotype was associated with the use of 5-ASA, in contrast to our findings. In a population-based cohort from Olmsted County, Minnesota, USA, more than half of all CD patients had been exposed to 5-ASA,21 similar to findings from a recent Swedish population-based cohort.22
One possible explanation for the high use of 5-ASA in CD patients is their relatively safe side-effect profile, which is of particular importance in elderly IBD patients. Older patients tended to be treated more often with 5-ASA in our cohort, and others studies have also found that the use of 5-ASA is particularly high in the group of elderly IBD patients. For example, 77% of elderly onset CD patients had received 5-ASA within 5 years after diagnosis in a French population-based cohort.7 Finally, in a Swedish nationwide study 29% of elderly CD patients used 5-ASA.23 Hence, it is likely that such prescribing patterns are driven by physician hesitancy to use more potent and toxic drugs considering challenges posed by clinical comorbidities, the potential for polypharmacy and drug interactions.
Another possible explanation is that the available studies on 5-ASA in CD have showed conflicting results, are underpowered or difficult to interpretable. Therefore, there might also be a perception of efficacy of 5-ASA in CD amongst physicians.24 For example, in the study from the Swiss IBD cohort, physicians judged 5-ASA to have been clinically successful in nearly half of CD patients.20 In a survey of German gastroenterologists, one-third of respondents would use 5-ASA as maintenance therapy in colonic and ileo-colonic CD25 and in a survey of Australian gastroenterologists 96% of respondents answered that they had used 5-ASA in CD patients.26 Finally, in a Danish cohort of 537 consecutive CD patients, 31% of patients with primarily non-stricturing, non-penetrating behaviour and colonic location were treated with 5-ASA monotherapy for a relapse.27 Of these, 36% of patients experienced complete or partial remission 1 year after initiation while 23% of patients were categorised as 5-ASA dependent and were deemed unable to stop or reduce 5-ASAs without experiencing a relapse by their physician.
Physicians tend to prescribe medication in accordance with what they believe their patients expect.28,29 The majority of CD patients in our cohort being treated with 5-ASA did so right after diagnosis and quickly moved on to other drugs during follow-up. Interestingly, many patients continued taking 5-ASA together with immunomodulators or biologics despite recent studies suggesting that this is of no benefit.30 We observed a higher use of 5-ASA in CD patients originating from Eastern European countries compared to those from Western European countries. We previously demonstrated that patients do not differ in terms of socio-demographic and disease related characteristics31 but choices regarding medical treatment are strongly linked to extra-medical considerations such as reimbursement rules and drug availability and therefore the differences observed between Western and Eastern Europe might be caused by variations between national healthcare systems.
Finally, we observed that at approximately one in six patients with CD within our cohort experienced a quiescent disease course with low disease activity as well as low rates of progression and surgery. This finding is in line with that of a previous Danish population-based cohort from 1962–1987, where available treatment options included only 5-ASA, prednisolone and surgery. In that cohort, 13% of patients experienced no further flare following diagnosis.1 More recently, in a Dutch population-based cohort of patients diagnosed 1991–2004, almost 30% of patients were categorised as quiescent.32 Therefore, in population-based cohorts a significant portion of CD patients seems to have a mild disease course with limited need to immunosuppressive therapy. Better identification of these patients is important both in terms of our understanding of the disease as well as to stratify patients at diagnosis and avoid over-treating these patients with drugs with potential severe adverse events. We identified older age and being diagnosed without strictures or fistulising disease at diagnosis to be indicative of a mild disease course. Similarly, in a Norwegian population-based cohort older age as well as colonic ileo-colonic disease location, Anti-Saccharomyces cerevisiae antibodies (ASCA)-negativity and no need for prednisolone at diagnosis were associated with a low probability of disease progression.33
Strengths of the Epi-IBD cohort include the prospective inclusion and follow-up of incident and unselected IBD patients diagnosed within well-defined geographic areas. We standardised diagnostic criteria, case ascertainment methods as well as the recorded data, and several measures previously described31 ensured that all centres performed a population-based cohort study with good data quality and validity. As patients were unselected and represent the whole spectrum of disease severity; the choices of treatment in this cohort occurred in a real-life clinical setting.
Limitations of the study include the heterogeneity of the participating centres in terms of healthcare systems, which are likely to have influence on treatment decisions. In addition, the distribution of participating centres is skewed, with more centres located in Western Europe. As the Eastern European centres are in mostly low-incident areas,34 the majority of patients in this study originate in Western Europe. Furthermore, the use of 5-ASA served as a proxy for mild disease without being able to take objective markers of inflammation including endoscopic severity or levels of calprotectin into consideration. Also, cross-sectional imaging data was not available, which could have been used to assess the level of intestinal damage which in previous studies has been shown to be present in patients with silent CD.35 Finally, it is possible that some CD patients treated with 5-ASA and in long-term remission could be misdiagnosed with CD. We have previously reported that patients in our cohort were investigated with endoscopy and/or imaging infrequently during the observation period.6 Choices regarding investigations were, however, made according to the treating physicians’ discretion and also patient preference which would explain the infrequent use of colonoscopy in a setting of long-term remission and mild disease.
To conclude, in this prospective population-based cohort of unselected patients with CD, the use of 5-ASA was high but, in most cases, served as the first treatment initiated following diagnosis and was later changed to other drugs for maintenance treatment. A substantial group of patients, however, received only 5-ASA as maintenance treatment during 5 years of follow-up and experienced a quiescent disease course. Our findings suggest that despite the controversy regarding the efficacy of 5-ASA in CD, in some patients 5-ASA has a role and results in a satisfying disease course for both patients and physicians.
Summarise the established knowledge on this subject
5-Aminosalicylate (5-ASA) remains a fundamental treatment for ulcerative colitis but its use in Crohn’s disease (CD) remains questionable.
Despite the scientific controversy regarding the effectiveness of 5-ASA in CD patients, many patients are exposed to 5-ASA during their disease course.
Population-based data regarding the use of 5-ASA in CD are lacking.
What are the significant and/or new findings of this study?
The use of 5-ASA in CD was high with more than half of patients being treated with 5-ASA during their disease course.
Most patients received 5-ASA immediately following diagnosis or after an initial course of steroids and then stepped up to other drugs.
A subgroup of patients remained on 5-ASA for the whole observation period. This subgroup of CD patients was older, had less complicated disease behaviour at diagnosis and exhibited a mild disease course with only very few patients experiencing disease progression or surgery.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620945949 for The use of 5-aminosalicylate for patients with Crohn’s disease in a prospective European inception cohort with 5 years follow-up – an Epi-IBD study by Johan Burisch, Daniel Bergemalm, Jonas Halfvarson, Viktor Domislovic, Zeljko Krznaric, Adrian Goldis, Jens F Dahlerup, Pia Oksanen, Pekka Collin, Luisa de Castro, Vicent Hernandez, Svetlana Turcan, Elena Belousova, Renata D'Incà, Alessandro Sartini, Daniela Valpiani, Martina Giannotta, Ravi Misra, Naila Arebi, Dana Duricova, Martin Bortlik, Kelly Gatt, Pierre Ellul, Natalia Pedersen, Jens Kjeldsen, Karina W Andersen, Vibeke Andersen, Konstantinos H Katsanos, Dimitrios K Christodoulou, Shaji Sebastian, Luisa Barros, Fernando Magro, Jóngerð MM Midjord, Kári R Nielsen, Riina Salupere, Hendrika AL Kievit, Gediminas Kiudelis, Juozas Kupčinskas, Mathurin Fumery, Corinne Gower-Rousseau, Ioannis P Kaimakliotis, Doron Schwartz, Selwyn Odes, Laszlo Lakatos, Peter L Lakatos, Ebbe Langholz, Pia Munkholm and for the Epi-IBD group in United European Gastroenterology Journal
Acknowledgements
The authors are grateful to Alberto Fernandez (Spain), Luciano Sanroman (Spain), Marko Brinar (Croatia), Niksa Turk (Croatia), Zsuzsanna Vegh (Hungary), Claus Ålykke (Denmark), Limas Kupcinskas, (Lithuania) and Laimas Jonaitis (Lithuania) for their contribution to the study including the inclusion and follow-up of patients as well as collecting patient data. Specific author contributions: JB: study design, data acquisition, data analysis and writing up of the first draft of the paper; PM: study design, data analysis and study supervision. All authors: data acquisition, critical review of the draft manuscript. JB had full access to the data in the study and takes full responsibility for its veracity and statistical analysis. All authors approved the final version for publication.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J Burisch: consulting fees from Celgene, Janssen-Cilag, AbbVie, Tillots Pharma and Ferring; lecture fees from Abbvie, Pfizer, MSD, Pharmacosmos and Takeda Pharma A/S; unrestricted grant support from Takeda Pharma, Tillots Pharma and MSD; V Andersen: consulting fees from Janssen and MSD, and advisory board fee from MSD; PL Lakatos: speaker and/or advisory board for AbbVie, Falk Pharma GmbH, Ferring, Genetech, Janssen, Kyowa Hakko, Kirin Pharma, Mitsubishi Tanabe Pharma Corporation, MSD, Pfizer, Pharmacosmos, Roche, Shire and Takeda. Unrestricted research grant from AbbVie, MSD and Pfizer; Z Krznaric: speaker and/or advisory board member for AbbVie, Janssen, Celltrion, Sandoz, Fresenius, Mylan, MSD, Pfizer, Shire and Takeda; D Duricova: lecture fees from Takeda, Janssen, Pfizer; M Bortlik: consulting fees from Takeda, Janssen-Cilag, Tillots and Pfizer. Lecture fees from Takeda, Janssen-Cilag, AbbVie, Ferring and Sandoz; N Arebi: consulting fees from Takeda and Janssen; E Langholz: grants from MSD, Abbvie, Janssen Pharmaceuticals and Ferring Pharmaceuticals. Personal fees from Takeda; D Schwartz: consultancy/advisory for Takeda, AbbVie, Janssen, Check-cap, Rafa, Neopharm, Pfizer. Speaker for Takeda, AbbVie, Janssen, Neopharm; M Fumery: speaker or lecture fees from Abbvie, MSD, Takeda, Ferring, Janssen, Tillots, Gilead, Celgene, Biogen and Boehringer; R Salupere: lecture fees from AbbVie, Takeda, MSD and Pfizer; V Hernandez: speaker for and has received travel support or research funding from MSD, AbbVie, Ferring, FAES Farma, Shire Pharmaceuticals, Dr Falk Pharma, Tillotts Pharma, Otsuka Pharmaceutical, Pfizer, Takeda, Jansen, KernPharma Biologics, Gebro Pharma, Adacyte, Sandoz and Biogen; P Munkholm: consulting fees from Takeda, Janssen-Cilag, AbbVie, MSD, Tillotts and Ferring; lecture fees from Abbvie, MSD; Takeda Pharma; unrestricted grant from Ferring and Calpro. All other authors report no competing interests.
Funding
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by unrestricted grants from Kirsten og Freddy Johansens Fond as well as from Nordsjællands Hospital Forskningsråd. The study sponsors have made no contributions to the study design, analysis, data interpretation or publication.
Ethics approval
Ethical committees at each centre approved the study according to local regulations. The Danish Ethical Committee approved the study 21 September 2009 (H-4-42009-115). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed Consent
Written, informed consent was obtained from each patient included in the study.
ORCID iDs
Johan Burisch https://orcid.org/0000-0002-3312-5139
Viktor Domislovic https://orcid.org/0000-0002-3715-5730
Alessandro Sartini https://orcid.org/0000-0003-1573-6451
Fernando Magro https://orcid.org/0000-0003-2634-9668
Supplemental material
Supplemental material for this article is available online.
References
- 1.Munkholm P, Langholz E, Davidsen M, et al. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol 1995; 30: 699–706. [DOI] [PubMed] [Google Scholar]
- 2.Ford AC, Kane S V, Khan KJ, et al. Efficacy of 5-aminosalicylates in Crohn’s disease: Systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 617–629. [DOI] [PubMed] [Google Scholar]
- 3.Lim W-C, Wang Y, MacDonald JK, et al. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev 2016; 7: CD008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akobeng AK, Zhang D, Gordon M, et al. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane Database Syst Rev 2016; 9: CD003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J Crohns Colitis 2020; 14: 4--22. [DOI] [PubMed] [Google Scholar]
- 6.Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: An Epi-IBD study. Gut 2019; 68: 423–433. [DOI] [PubMed] [Google Scholar]
- 7.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: A population-based cohort study. Gut 2014; 63: 423–432. [DOI] [PubMed] [Google Scholar]
- 8.Niewiadomski O, Studd C, Hair C, et al. Prospective population-based cohort of inflammatory bowel disease in the biologics era: Disease course and predictors of severity. J Gastroenterol Hepatol 2015; 30: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 9.Burisch J. Crohn’s disease and ulcerative colitis: Occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan Med J 2014; 61: B4778. [PubMed] [Google Scholar]
- 10.Burisch J, Cukovic-Cavka S, Kaimakliotis I, et al. Construction and validation of a Web-based epidemiological database for inflammatory bowel diseases in Europe. An EpiCom study. J Crohn’s Colitis 2011; 5: 342–349. [DOI] [PubMed] [Google Scholar]
- 11.Munkholm P. Crohn’s disease–occurrence, course and prognosis. An epidemiologic cohort-study. Dan Med Bull 1997; 44: 287–302. [PubMed] [Google Scholar]
- 12.Langholz E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull 1999; 46: 400–415. [PubMed] [Google Scholar]
- 13.Burisch J, Katsanos KH, Christodoulou DK, et al. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort–an Epi-IBD study. J Crohns Colitis 2019; 13: 198–208. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19: S5–S36. [DOI] [PubMed] [Google Scholar]
- 15.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980; 1: 514. [DOI] [PubMed] [Google Scholar]
- 16.Best WR. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis 2006; 12: 304–310. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: A systematic review of isolated colonic Crohn’s disease: The third IBD? Gut 2017; 66: 362–381. [DOI] [PubMed] [Google Scholar]
- 18.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68(Suppl 3): s1--s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chhaya V, Saxena S, Cecil E, et al. Steroid dependency and trends in prescribing for inflammatory bowel disease – a 20-year national population-based study. Aliment Pharmacol Ther 2016; 44: 482–494. [DOI] [PubMed] [Google Scholar]
- 20.Schoepfer A, Bortolotti M, Pittet V, et al. The gap between scientific evidence and clinical practice: 5-Aminosalicylates are frequently used for the treatment of Crohn’s disease. Aliment Pharmacol Ther 2014; 40: 930–937. [DOI] [PubMed] [Google Scholar]
- 21.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010; 139: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhulina Y, Udumyan R, Tysk C, et al. The changing face of Crohn’s disease: A population-based study of the natural history of Crohn’s disease in Örebro, Sweden 1963-2005. Scand J Gastroenterol 2016; 51: 304–313. [DOI] [PubMed] [Google Scholar]
- 23.Everhov ÅH, Halfvarson J, Myrelid P, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology 2018; 154: 518–528.e15. [DOI] [PubMed] [Google Scholar]
- 24.Ma C, Ascoytia C, McCarrier KP, et al. Physicians’ perspectives on cost, safety, and perceived efficacy determine aminosalicylate use in Crohn’s disease. Dig Dis Sci 2018; 63: 2555–2563. [DOI] [PubMed] [Google Scholar]
- 25.Klag T, Stange EF, Wehkamp J. Management of Crohn’s disease – are guidelines transferred to clinical practice? United Eur Gastroenterol J 2015; 3: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gearry RB, Ajlouni Y, Nandurkar S, et al. 5-Aminosalicylic acid (mesalazine) use in Crohn’s disease: A survey of the opinions and practice of Australian gastroenterologists. Inflamm Bowel Dis 2007; 13: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 27.Duricova D, Pedersen N, Elkjaer M, et al. 5-Aminosalicylic acid dependency in Crohn’s disease: A Danish Crohn Colitis Database study. J Crohn’s Colitis 2010; 4: 575–581. [DOI] [PubMed] [Google Scholar]
- 28.Linde K, Fässler M, Meissner K. Placebo interventions, placebo effects and clinical practice. Philos Trans R Soc Lond B Biol Sci 2011; 366: 1905–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lado E, Vacariza M, Fernández-González C, et al. Influence exerted on drug prescribing by patients’ attitudes and expectations and by doctors’ perception of such expectations: A cohort and nested case-control study. J Eval Clin Pract 2008; 14: 453–459. [DOI] [PubMed] [Google Scholar]
- 30.Ungaro RC, Limketkai BN, Jensen CB, et al. Stopping mesalamine therapy in patients with Crohn’s disease starting biologic therapy does not increase risk of adverse outcomes. Clin Gastroenterol Hepatol 2020; 18: 1152--1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burisch J, Pedersen N, Čuković-Čavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: The ECCO-EpiCom inception cohort. Gut 2014; 63: 588–597. [DOI] [PubMed] [Google Scholar]
- 32.Wintjens D, Bergey F, Saccenti E, et al. OP002 Assessment of disease activity patterns during the first 10 years after diagnosis in a population-based Crohn’s disease cohort shows a quiescent disease course for a substantial proportion of the population. J Crohn’s Colitis 2018; 12: S001–S003. [Google Scholar]
- 33.Solberg IC, Cvancarova M, Vatn MH, et al. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn’s disease (the IBSEN Study). Inflamm Bowel Dis 2014; 20: 60–68. [DOI] [PubMed] [Google Scholar]
- 34.Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohn’s Colitis 2013; 7: 322–337. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharya A, Rao BB, Koutroubakis IE, et al. Silent Crohn’s disease predicts increased bowel damage during multiyear follow-up: The consequences of under-reporting active inflammation. Inflamm Bowel Dis 2016; 22: 2665–2671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620945949 for The use of 5-aminosalicylate for patients with Crohn’s disease in a prospective European inception cohort with 5 years follow-up – an Epi-IBD study by Johan Burisch, Daniel Bergemalm, Jonas Halfvarson, Viktor Domislovic, Zeljko Krznaric, Adrian Goldis, Jens F Dahlerup, Pia Oksanen, Pekka Collin, Luisa de Castro, Vicent Hernandez, Svetlana Turcan, Elena Belousova, Renata D'Incà, Alessandro Sartini, Daniela Valpiani, Martina Giannotta, Ravi Misra, Naila Arebi, Dana Duricova, Martin Bortlik, Kelly Gatt, Pierre Ellul, Natalia Pedersen, Jens Kjeldsen, Karina W Andersen, Vibeke Andersen, Konstantinos H Katsanos, Dimitrios K Christodoulou, Shaji Sebastian, Luisa Barros, Fernando Magro, Jóngerð MM Midjord, Kári R Nielsen, Riina Salupere, Hendrika AL Kievit, Gediminas Kiudelis, Juozas Kupčinskas, Mathurin Fumery, Corinne Gower-Rousseau, Ioannis P Kaimakliotis, Doron Schwartz, Selwyn Odes, Laszlo Lakatos, Peter L Lakatos, Ebbe Langholz, Pia Munkholm and for the Epi-IBD group in United European Gastroenterology Journal