Abstract
Background
The transmission mode of severe acute respiratory syndrome coronavirus 2 is primarily known as droplet transmission. However, a recent argument has emerged about the possibility of airborne transmission. On June 17, there was a coronavirus disease 2019 (COVID-19) outbreak in Korea associated with long distance droplet transmission.
Methods
The epidemiological investigation was implemented based on personal interviews and data collection on closed-circuit television images, and cell phone location data. The epidemic investigation support system developed by the Korea Disease Control and Prevention Agency was used for contact tracing. At the restaurant considered the site of exposure, air flow direction and velocity, distances between cases, and movement of visitors were investigated.
Results
A total of 3 cases were identified in this outbreak, and maximum air flow velocity of 1.2 m/s was measured between the infector and infectee in a restaurant equipped with ceiling-type air conditioners. The index case was infected at a 6.5 m away from the infector and 5 minutes exposure without any direct or indirect contact.
Conclusion
Droplet transmission can occur at a distance greater than 2 m if there is direct air flow from an infected person. Therefore, updated guidelines involving prevention, contact tracing, and quarantine for COVID-19 are required for control of this highly contagious disease.
Keywords: Infectious Disease Transmission, SARS-CoV-2, COVID-19
Graphical Abstract

INTRODUCTION
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had spread throughout the world, and the total cases numbered more than 17 million and 680,000 deaths by COVID-19 as of August 3, 2020.1 On January 20, 2020, a Chinese traveler from Wuhan, China, was identified as the first COVID-19 case in Korea, after which only 30 cases of COVID-19 were reported until February 20 and were based on visitors to the country and contact with them. After an outbreak associated with a religious group in Daegu Metropolitan city, the number of new patients per day rapidly increased to a maximum of 813 from late February to late March. The government of Republic of Korea had increased the response level for public health emergency to level 3 (from level 0 to level 3) on February 23, and had implemented high-intensity social distancing until May 5.2 Furthermore, the government applied the K-quarantine model ‘3T policy (Test-Trace-Treat)’ system that included a rapid and exact test for COVID-19, investigation of the epidemic using information and communication technology (ICT), and an isolation and care program according to severity.3
On June 17, there was a new confirmed COVID-19 case (index case, case A) in Jeonju, Korea, considered as transmitted by droplets at 6.5 m away from the infector and 5 minutes exposure in a restaurant with air conditioning. It is important to know how SARS-CoV-2 is transmitted between people in various situations. We share these investigation results as a reference to update guidelines involving prevention, tracing, and quarantine for control of this pandemic infectious disease.
METHODS
Personal factor investigation
The epidemiological investigation was implemented according to the ‘Infectious Diseases Control and Prevention Act’ (Act number 16725) in Korea and the guidelines for response to COVID-19 by the Korea Disease Control and Prevention Agency (KDCA).4 Data comprising patient's personal statements by interview, medical institution usage history, credit card record, closed-circuit television (CCTV) images, cell phone location data, and other associated information were secured by an epidemiological investigation team.5 The Epidemic Investigation Support System (EISS) developed by KDCA was also used for location tracking of confirmed cases and hot-spot analysis.6 Nasopharyngeal specimens of cases and close contacts were collected and tested using real-time reverse transcription polymerase chain reaction (rRT-PCR) by Jeollabuk-do Institute of Health & Environment Research, and genome sequencing analysis for verifying association between cases was performed by the KDCA.7 In total, 10–100 ng of the extracted viral RNA with a maximum volume of 8.5 µL was subjected to target enrichment using a Truseq RNA library prep for enrichment (Illumina, San Diego, CA, USA) and Truseq RNA Enrichment (Illumina). Dual-index filtering and adapter trimming were conducted on the sequences using our in-house scripts. Hybridization probes were designed to cover the whole genome of SARS-CoV-2 using the Wuhan-Hu-1 strain.8 The biotinylated probes were 120 base pair in length with 3 × tiling (Celemics, Inc. Seoul, Korea). In total, 745 conserved probes were generated.
Environmental factor investigation
As a result of EISS analysis, one restaurant (restaurant A) was visited by case A on June 12 and was identified as the site of exposure. The first field investigation was started from June 19 based on assessment of CCTV, table locations, timeline, and movement route of case A and other people in the restaurant were verified. Also, the internal structure, distance between visitors, and exact locations of the ceiling type air conditioners were investigated. Air speed and direction at several specified positions were precisely measured using a portable anemometer (Kestrel 2500; Nielsen-Kellerman Co. Boothwyn, PA, USA) on June 24 and July 2. To measure air flow, we set the air conditioner at the same fan speed and direction of June 12. The chairs of cases and visitors were also occupied by people to simulate the same situation. A total of 39 environmental samples of inlets and outlets of air conditioners, table seat of case A, and nearby tables and chairs in consideration of air flow direction were collected on June 23 for testing of SARS-CoV-2 in the environment and were analyzed by rRT-PCR test.9
RESULTS
Personal factors associated with the outbreak
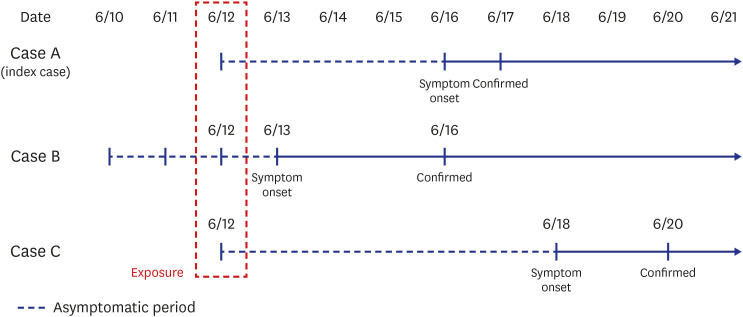
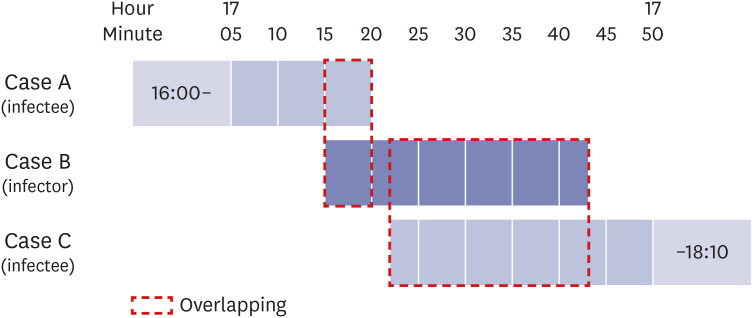
The symptoms of the index case (case A) started on June 16, and the probable period of exposure was assumed to be from June 2 to June 15 according to the incubation period of SARS-CoV-2. Because case A had no history of overseas travel and travel outside Jeonju, where there had been no confirmed case in the previous 2 weeks, we used the EISS of the KDCA to gather data from June 2 to June 15. The results showed only one (case B) of 538 confirmed domestic cases with a tracking map overlapping that of case A during that period. The site of overlap was a restaurant (restaurant A), where case A and case B were co-located for 5 minutes on June 12 based on CCTV images. Case B lives in Daejeon Metropolitan city within an hour's drive away from Jeonju, and visited Jeonju only on June 12. Therefore, we tentatively considered case B is the primary case (infector) and case A as the secondary case (infectee). When case B was in the restaurant, they came into close contact with 11 visitors and two employees who did not properly wear a mask. The epidemiological investigation team ordered these contacts to undergo the rRT-PCR test for SARS-CoV-2 on June 19 or 20, and to perform a minimum 14-day quarantine until June 26. Among the 13 close contacts during the quarantine period, one additional case (case C, visitor) was confirmed to have COVID-19 on June 20. Finally, case B spread COVID-19 to two infectees in this restaurant, for an attack rate of 15.4% (2/13). The exposure day was June 12, and the symptom onset of case B was June 13, resulting in a median incubation period and serial interval of 5 days and 4 days, respectively, consistent with previous reports.10,11,12 There was another case in that restaurant (case D). Case D is a companion of case B and visited this restaurant with case B on June 12. Case D had been exposed to another epidemic patient in Daejeon city on June 11 and showed a positive result for SARS-CoV-2 on June 16, with symptom onset the day before. Because case D could not spread COVID-19 to other people at that time, case D was excluded from this outbreak investigation (Figs. 1 and 2).
Fig. 1. The asymptomatic period and symptom onset of all three coronavirus disease 2019 cases.
Fig. 2. Timeline of coronavirus disease 2019 infector and infectees in the restaurant. Case A (index case) overlaps about 5 minutes (17:15–17:20) with case B (infector), who overlaps case C for about 21 minutes (17:22–17:43).
Environmental factors and mode of transmission
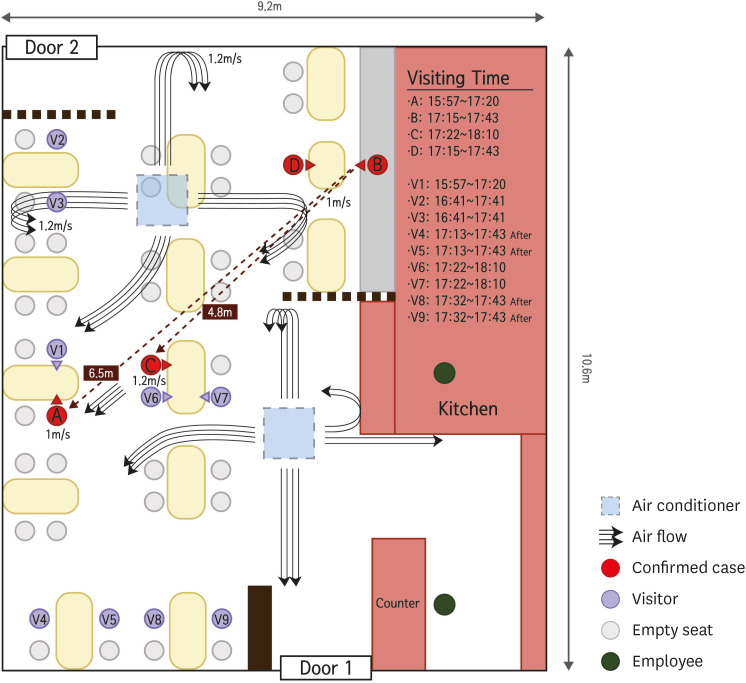
Restaurant A was located on the first floor of a six-story building totaling 96.6 square meters in size (9.2 × 10.5 m) without windows or a ventilation system. There were two doors in the restaurant, one at the front (door 1) and the other at the back (door 2). Two ceiling-type air conditioners were diagonally located at 3.2 m from the floor as shown in Fig. 3; they were fixed with wire and had been operating at the time the cases were in restaurant A. On CCTV, case A and his companion entered the restaurant at 16:00 on June 12 and finished their meals before case B (with case D) entered at 17:15 using door 2. Case B and his colleague sat at a table near door 2, at a 6.5-m distance from case A, who did not leave from his table or share his table with others. Cases A and B engaged in conversation with their respective companions without masks. At 17:20, case A went out of the restaurant A using door 1. In 2 minutes, case C and his companions (V6, V7) entered the restaurant A using door 1 and sat at another table 4.8 m distant from case B, where they remained for 21 minutes before case B left his table using door 1 at 17:43. The distance between case A and case B was 6.5 m, and the air flow direction at positions of both cases showed a maximum of 1.0 m/sec (3.6 km/hr) velocity measured by anemometer. The air flow between case B and case C showed a maximum of 1.2 m/sec (4.3 km/hr) over a 4.8 m distance. All positions such as guest tables, infectors, and infectees, ceiling air conditioners, and information for air speed and direction are shown in Fig. 3.
Fig. 3. Schematic diagram of the outbreak restaurant equipped with ceiling-type air conditioners. The arrowed solid streamlines represent the air flow directions in the restaurant. Curved air streamlines represent that air streams from the ceiling air conditioners are reflected from the wall or barrier, and move downward toward the floor.
The 39 environment samples for SARS-CoV-2 were all negative by rRT-PCR. The results of genome sequencing of the three patients were presented as all three cases' genomic types were GH type and identical for each other (data not shown).
Summary of epidemiological findings and implications: 1) Indoor air flow (maximum velocity, 1.0 m/sec) could have transmitted droplets from the infector (case B) to infectee (case A) within 6.5 m and 5 minutes of exposure and to a second infectee (case C) within 4.8 m and 21 minutes of exposure. 2) The attack rate among exposures at the restaurant was 15.4% (2/13; 95% CI, 8.3%–22.5%) and is higher than the secondary attack rate among the total close contacts (0.6%; 0.3%–1.0%) and only household contacts (7.6%; 3.7%–14.3%) but was similar to a call center exposure outbreak (15.1%; 10.8%–20.6%).13,14 3) COVID-19 transmission by droplets from an infector can occur over a greater than 2 m distance with a short period of exposure when combined with air flow. The guidelines on quarantine and epidemiological investigation must be updated to reflect these factors for control and prevention of COVID-19. 4) The EISS for tracing COVID-19 patients was very useful and shortened the time to locate the infection source. This system could suppress the regional epidemic scale of the virus and reduce the burden for testing, isolation, and treatment.
DISCUSSION
The SARS-CoV-2 virus is mainly transmitted through respiratory droplets emitted from an infector's coughing, sneezing, talking, and normal breathing and upon close contact between people.15 These droplets are typically divided into large and small sizes (also called aerosol) based on a diameter of 5 μm. The large particles (also called droplets) tend to settle within 1–2 m of their origin due to gravitational force, and the settling velocity is proportional to the particle diameter.16 Therefore, social distancing requires a minimum of 1–2 m to avoid contact with a virus-containing respiratory droplet. In the situation of no effective treatment drug or vaccine, the most important personal methods to prevent or control this pandemic of COVID-19 are social distancing, use of a mask (if social distancing cannot be maintained), and handwashing.
Recently, it has been suggested that COVID-19 can be spread through not only droplets or contact, but also airborne transmission. An experimental study showed that the COVID-19 virus in aerosol particles remained viable during 3 hours and 16 hours.17 Morawska and Milton, together with their co-authors, 239 scientists, strongly suggested the possibility of airborne transmission of COVID-19 based on several preprint findings, though there has been no peer review of this research.18 The last updated version of the WHO scientific brief reported on July 9, 2020 reported that airborne transmission by aerosols is rare, and SARS-CoV-2 is spread primary between people through droplets or close contact. However, the possibility of aerosol transmission in crowded indoor spaces has been suggested in combination with droplet transmission.19
In this outbreak, the distances between infector and infected persons were 4.8 and 6.5 m, both farther than the generally accepted 2 m droplet transmission range. This is some of the first evidence of airborne transmission. At the field investigation, we assumed the possibility of long-distance droplet movement by air flow. Dbouk and Drikakis reported results of computational fluid dynamics showing that most droplets settle within 1–2 m in the absence of airflow. However, with a 4 km/hr or 15 km/hr wind, droplets could travel 6 m after 5 or 1.6 seconds, respectively.20 In the presented case, the air flow from infector to infectee showed a 1.0–1.2 m/sec (3.6–4.3 km/hr) velocity, indicating the need for 6.5 seconds of contact to transmit droplets from the infector to the index case. Only the visitors (cases A and C) sitting in the air flow path of case B were infected with COVID-19, while other visitors (V2, V3) closer to the infector for a longer period of time but in the absence of direct air flow did not become infected. In addition, the visitors sitting at tables with cases A and C (V1, V6, and V7) were not infected with COVID-19 because they faced away from the infector’s face. These findings strongly suggest that this outbreak occurred by droplet transmission exceeding a 2 m distance and excluded contact and fomite transmission. This transmission pattern is similar with the outbreak of a restaurant with air conditioning in Guangzhou, China.21 In this article, the authors concluded that the most likely transmission was done by droplet and also emphasized the direction of air flow.
Without the K-quarantine model (test-trace-treat) and EISS, it may have been very difficult to establish an infection chain of this outbreak because the incubation period of COVID-19 has a wide range from 1 to 14 days, and the exposure occurred only once for 5 minutes at a 6.5 m distance. Based on this system, the infector was identified within 2 days after the index case was confirmed. This short period of identification could simplify identification of close contacts and reduce outbreak size by quarantining of all close contacts. In most COVID-19 outbreak situations, identification of the infection chain is difficult or almost impossible. An outbreak for which an infectious source cannot be determined may be due to failure of consideration of a short period of exposure or of clearly verifying the movements of the confirmed person. In addition, it suggests that some suspected airborne transmission reports may be misinterpreted based on lack of awareness of the long transmission mechanism of droplets.
The guideline for control of droplet transmission over a long distance (above 2 m) by air flow in indoor settings are similar to those of airborne transmission and comprise sufficient ventilation and social distancing (avoid overcrowding, maintain distance between people).22 However, if there is high possibility of transmission by aerosol or droplet transmission over a long distance, N95 respiratory or equivalent masks are needed not only in health care settings, but in any indoor environment. In Korea, the Ministry of Food and Drug Safety is concerned with approval of medical mask like KF99, KF94, and KF80 and developed a type of mask named KF-AD (anti-droplet) for COVID-19. Any such mask, including KF and surgical, that can protect against droplets should be sufficient for preventing droplet transmission.
According to this case, additional considerations need to occur for COVID-19 prevention and control. The first is that transmission in an indoor setting is possible at a distance greater than 2 m with a short period of exposure (five minutes), and selection of close contacts in contact tracing should be changed. When epidemiological field investigation of an indoor environment is needed, it is necessary to assess the seating arrangement and operation and location of fans (including ceiling fans) or air conditioners with wind direction and velocity. It is also necessary to ventilate frequently for management of indoor air or to apply a ventilation system or forced ventilation method if natural ventilation is not possible. Furthermore, the distance between tables at an indoor restaurant or cafeteria should be greater than 1–2 m, or installation of a wind partition should be considered based on air flow. In addition, in indoor settings such as restaurants, masks should be removed only during meals and should be worn before and after eating, while conversation during meals and loud talking or shouting should be avoided. In the long term, installation of separate rooms or bulkheads for indoor settings should be considered to prevent transmission of airborne and droplet infectious diseases.
There are some limitations to this study. First, we did not assess air flow using computational fluid dynamics. In addition, the air flow measurement can't reflect all the same situations because opening of doors and motions of cases and visitors were not reproduced. However, air flow direction and velocity were identified between the infector and infectees using an anemometer in the most similar environment as possible. Second, environmental samples were collected at 11 days after the inspector visit. Though all these results were negative, this was not proof against airborne transmission.
In conclusion, droplet transmission can occur at a distance greater than 2 m if there is direct air flow from an infected person in an indoor setting. Therefore, updated guidelines for quarantine and environmental management of COVID-19 are needed until approval of an effective treatment drug or vaccine.
ACKNOWLEDGMENTS
The authors appreciate the epidemiological investigation team of Jeonju and Jeollabuk-do and all researchers in Jeonbuk Center for Infectious Disease Control and Prevention. Also, we thank all members of the Department of Infectious Disease of Jeollabuk-do Institute of Health and Environment.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kwon KS, Park YJ, Lee JH.
- Data curation: Park JI.
- Investigation: Park JI, Jung DM, Ryu KW, Lee JH.
- Writing - original draft: Kwon KS, Park JI.
- Writing - review & editing: Kwon KS, Park JI, Park YJ, Jung DM, Ryu KW, Lee JH.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report-195. [Updated 2020]. [Accessed July 30, 2020]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200802-covid-19-sitrep-195.pdf.
- 2.Park K, Kim Y, Yeom H, Hwang I, Kwon J, Kim M, et al. Weekly report on the COVID-19 situation in the Republic of Korea (as of July 25, 2020) Public Health Wkly Rep. 2020;13(31):2264–2278. [Google Scholar]
- 3.Ministry of Economy and Finance. Flattening the curve on COVID-19 - how Korea responded to a pandemic using ICT. [Updated 2020]. [Accessed July 30, 2020]. http://english.moef.go.kr/pc/selectTbPressCenterDtl.do?boardCd=N0001&seq=4879.
- 4.National Law Information Center. Infectious Diseases Control and Prevention Act [Act number 16725] [Updated 2020]. [Accessed July 30, 2020]. http://www.law.go.kr/LSW/eng/engLsSc.do?menuId=2§ion=lawNm&query=infectious&x=0&y=0#liBgcolor5.
- 5.COVID-19 National Emergency Response Center, Epidemiology & Case Management Team, Korea Centers for Disease Control & Prevention. Contact transmission of COVID-19 in South Korea: novel investigation techniques for tracing contacts. Osong Public Health Res Perspect. 2020;11(1):60–63. doi: 10.24171/j.phrp.2020.11.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YJ, Cho SY, Lee J, Lee I, Park WH, Jeong S, et al. Development and utilization of a rapid and accurate epidemic investigation support system for COVID-19. Osong Public Health Res Perspect. 2020;11(3):118–127. doi: 10.24171/j.phrp.2020.11.3.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JM, Chung YS, Jo HJ, Lee NJ, Kim MS, Woo SH, et al. Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res Perspect. 2020;11(1):3–7. doi: 10.24171/j.phrp.2020.11.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pung R, Chiew CJ, Young BE, Chin S, Chen MI, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ki M Task Force for 2019-nCoV. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol Health. 2020;42:e2020007. doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park YJ, Choe YJ, Park O, Park SY, Kim YM, Kim J, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SY, Kim YM, Yi S, Lee S, Na BJ, Kim CB, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Scientific brief. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. [Updated 2020]. [Accessed July 12, 2020]. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
- 17.Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9 Suppl):S102–8. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morawska L, Milton DK. It is time to address airborne transmission of COVID-19. Clin Infect Dis. doi: 10.1093/cid/ciaa939. Forthcoming 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dbouk T, Drikakis D. On coughing and airborne droplet transmission to humans. Phys Fluids (1994) 2020;32(5):053310. doi: 10.1063/5.0011960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Gu J, Li K, Xu C, Su W, Lai Z, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Center for Disease Prevention and Control. Heating, ventilation and air-conditioning systems in the context of COVID-19 (22 June 2020) [Updated 2020]. [Accessed November 5, 2020]. https://www.ecdc.europa.eu/en/publications-data/heating-ventilation-air-conditioning-systems-covid-19.