Abstract
[Purpose] We focused on skeletal muscle mass index, one of the biomarkers of sarcopenia, and investigated the association between skeletal muscle mass index and the parameters of lung function and respiratory muscle strength. [Participants and Methods] After applying the exclusion criteria, we included, in this cross-sectional study, 120 community-dwelling older adults aged ≥65 years who required long-term care/support and underwent ambulatory rehabilitation under the long-term care insurance system in Japan. We measured the skeletal muscle mass index, forced vital capacity, forced expiratory volume in 1 second, peak expiratory flow rate, maximum expiratory pressure, and maximum inspiratory pressure. The data were analyzed using Pearson correlation coefficient and multiple regression analysis. [Results] The skeletal muscle mass index was positively correlated with only maximum expiratory pressure for both male and female participants by Pearson’s correlation coefficient. With the skeletal muscle mass index as a dependent variable, only the maximum expiratory pressure was significant for both male and female participants by the multiple regression analysis. [Conclusion] Therefore, the findings of this study suggested that compared with lung function tests, maximum expiratory pressure, which is an indicator of respiratory muscle strength, is related to muscle mass. Maximum expiratory pressure might be the most useful indicator for sarcopenia.
Key words: Maximum expiratory pressure, Sarcopenia, Skeletal muscle mass index
INTRODUCTION
Various definitions of sarcopenia were announced by several research groups1,2,3,4). Sarcopenia must be properly treated to prevent increases in the personal, social, and economic burdens5). Sarcopenia is diagnosed on the basis of decreases in muscle strength, muscle mass, and physical function1,2,3,4). These decreases are age-related, and the prevalence of sarcopenia increases with age6, 7). Respiratory muscle strength also decreases with age8). According to the report by the European Working Group on Sarcopenia in Older People (EWGSOP), the peak expiratory flow rate (PEFR), measured in lung function testing, is an indicator of muscle strength that can be analyzed for sarcopenia1). However, cutoff values were not described, and subsequent reports of the Asian Working Group for Sarcopenia (AWGS) and EWGSOP2 did not contain information regarding respiratory-related sarcopenia2,3,4).
Kera et al. proposed that PEFR was an effective predictor of sarcopenia with a cutoff value9, 10). Alternatively, the unit of measure for PEFR is liters per second. It represents flow velocity and indicates expiratory muscle in some cases9, 10). In addition, both vital capacity and forced vital capacity (FVC) in typical lung function tests are measured in liters. Because various muscles are involved in breathing, respiratory muscle strength is considered to include the strength of the diaphragm and other respiratory muscles. Consequently, respiratory muscle strength is generally determined as the pressure generated in each part of the lungs and thorax11). The maximum generated pressure is used as an index of respiratory muscle strength. Particularly, the maximum positive pressure generated during expiration is defined as the maximum expiratory pressure (PEmax), and the maximum negative pressure generated during inspiration is defined as the maximum inspiratory pressure (PImax). The American Thoracic Society (ATS) and European Respiratory Society (ERS) have also provided guidelines for respiratory muscle strength assessment12). However, only few studies have reported the effects of sarcopenia on respiratory muscle strength. Results of basic research suggest that skeletal muscle mass index (SMI) is related to the parameters of lung function and respiratory muscle strength in healthy young and older adults13,14,15). According to one report in Brazil, sarcopenia significantly reduced respiratory muscle strength16). However, the relationship between sarcopenia and respiratory muscle strength has not been described in an aging population of Japan.
Therefore, we focused on SMI alone, one of the biomarkers of sarcopenia, for basic research. This study aimed to clarify the association between SMI and parameters of lung function and respiratory muscle strength in older adults who were certified as needing long-term care or support based on the measurement of muscle mass using the bioelectrical impedance analysis (BIA) method with older adults in the standing position. We also aimed to explore respiratory parameters related to SMI. This study is a primary step for investigating the association between sarcopenia and respiratory muscle strength.
PARTICIPANTS AND METHODS
We conducted this cross-sectional study from March 2018 to August 2019. We included 196 older adults aged ≥65 years who were certified as needing long-term care or support. They lived in the community and were undergoing ambulatory rehabilitation in the long-term care insurance system at least once a week. Exclusion criteria were a diagnosis of dementia or respiratory disease as the main disease according to a primary doctor, the presence of a cardiac pacemaker, difficulty in measuring body composition during standing, difficulty in measuring lung function and respiratory muscle strength because of aphasia, and inability to perform all measurement testing. In addition, based on the report by Kera et al., participants with <70% of forced expiratory volume in 1 second (FEV1)/FVC ratio were excluded by airway obstructions9, 10). All participants provided comprehensive written informed consent before the study. This study was approved by the Ethics Review Committee of International University of Health and Welfare (approval number: 17-Io-189-7) and was conducted in accordance with the guidelines present in the Declaration of Helsinki.
A body composition analyzer (InBody 520, InBody, Japan), was used to measure muscle mass by means of multifrequency BIA. During measurement, participants were standing. As a parameter of skeletal muscle mass, SMI was calculated as the appendicular skeletal muscle mass divided by the square of height17). Height was measured in accordance with the protocol of Kubo et al18). An electronic spirometer (Autospiro AS-507, Minato, Japan) and attached unit (AAM377, Minato, Japan) were used to measure lung function and respiratory muscle strength. The parameters of lung function were FVC, FEV1, and PEFR. The parameters of respiratory muscle strength were PEmax and PImax. In accordance with the ATS and ERS guidelines for testing lung function, the following three acceptable modes were measured for each participant: FVC (which can measure FVC, FEV1, and PEFR); PEmax; and PImax12). FVC mode was measured first, followed by PEmax and PImax. The measurements were performed in the sitting position, and the best value was adopted. Physical therapists performed all measurements.
The associations between SMI and parameters of lung function and respiratory muscle strength were investigated with Pearson correlation coefficients and a multiple regression analysis. For multiple regression analysis, SMI was defined as a dependent variable, and all items of lung function and respiratory muscle strength, except FEV1, were defined as independent variables after consideration of multicollinearity. The Grubbs–Smirnov test was performed for each measurement, and participant data shown to be outliers were excluded from analysis. Clinical characteristics and data of male and female participants were compared in unpaired t-tests. Most statistical analyses were carried out with SPSS version 25 (IBM Japan, Tokyo, Japan); the Grubbs-Smirnov test was carried out using JSTAT for Windows (Nankodo, Tokyo, Japan). A p value of less than 0.05 was considered statistically significant.
RESULTS
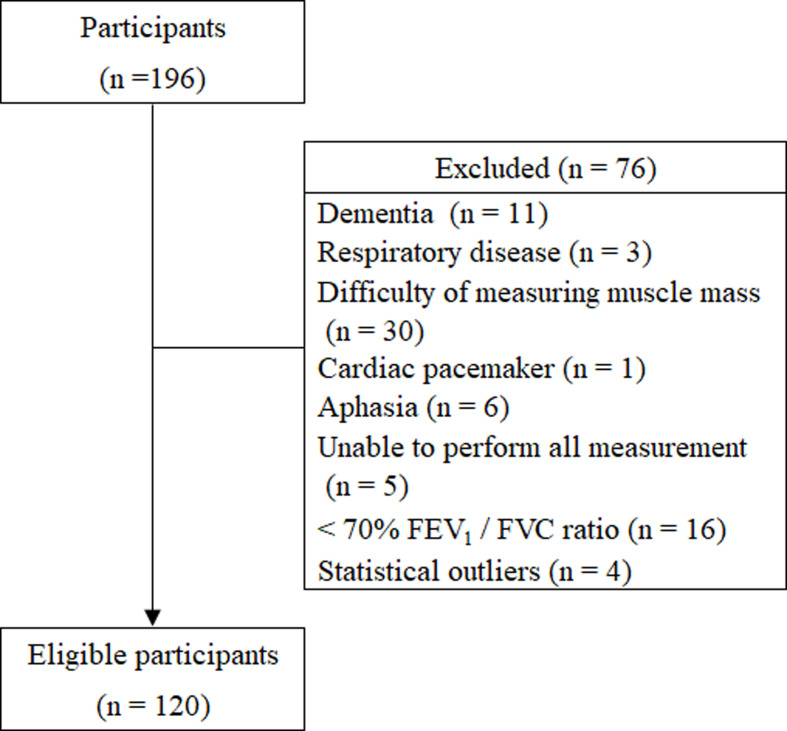
Figure 1 showed the flow chart of participant selection. A total of 120 eligible older adults (63 males, 57 females) participated in this study. The primary diagnosis of the eligible participants were as follows: 41 older adults with cerebrovascular disease, 14 with spine disease, 14 with fracture, 12 with osteoarthritis, 12 with cancer, 6 with Parkinson disease, 5 with progressive supranuclear palsy, 4 with circulatory disease, 3 with renal failure, 2 with spinocerebellar degeneration, 2 with depression, 2 with diabetes mellitus, 1 with rheumatoid arthritis, 1 with skin disease, and 1 with liver disease.
Fig. 1.
Flow chart of participant selection.
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity.
Table 1 showed the clinical characteristics of study participants and results of lung function and respiratory muscle strength measurements. Table 2 showed the associations between SMI and the parameters of lung function and respiratory muscle strength. According to Pearson correlation coefficients, SMI was positively correlated with PEmax (r=0.344) in male participants and with PEmax (r=0.393) in female participants. Multiple regression analysis demonstrated that the regression equation was significant for both male and female participants. With the SMI as a dependent variable, only PEmax was significant in male participants (β=0.396) and in female participants (β=0.375), according to the multiple regression analysis.
Table 1. Clinical characteristics of study participants.
| Characteristics | Male participants (n=63) | Female participants (n=57) | p value |
| (mean ± SD) | (mean ± SD) | ||
| Age (years) | 76.2 ± 7.5 | 80.4 ± 8.0 | 0.004* |
| Height (cm) | 162.3 ± 5.6 | 152.0 ± 5.3 | <0.001* |
| Body weight (kg) | 60.3 ± 7.7 | 50.1 ± 9.8 | <0.001* |
| BMI (kg/m2) | 22.9 ± 2.8 | 21.7 ± 3.9 | 0.060 |
| SMI (kg/m2) | 6.5 ± 0.9 | 5.6 ± 0.7 | <0.001* |
| Lung function | |||
| FVC (L) | 2.3 ± 0.6 | 1.5 ± 0.4 | <0.001* |
| FEV1 (L) | 1.9 ± 0.5 | 1.3 ± 0.3 | <0.001* |
| PEFR (L/sec) | 4.7 ± 1.7 | 3.1 ± 1.1 | <0.001* |
| Respiratory muscle strength | |||
| PEmax (cm H2O) | 56.2 ± 19.0 | 39.1 ± 11.8 | <0.001* |
| PImax (cm H2O) | 39.1 ± 16.8 | 30.6 ± 13.1 | 0.003* |
*Unpaired t-test of male participants versus female participants, p<0.05.
SD: standard deviation; BMI: body mass index; SMI: skeletal muscle mass index; FVC: forced vital capacity; FEV1: one-second forced expiratory volume; PEFR: peak expiratory flow rate; PEmax: maximum expiratory pressure; PImax: maximum inspiratory pressure.
Table 2. Association between SMI and parameters of lung function and respiratory muscle strength.
| Parameter | Male participants (n=63) | Female participants (n=57) | ||||||
| r† | p value† | β‡ | p value‡ | r† | p value† | β‡ | p value‡ | |
| Lung function | ||||||||
| FVC (L) | −0.028 | 0.827 | −0.216 | 0.178 | 0.216 | 0.107 | 0.172 | 0.295 |
| FEV1 (L) | −0.003 | 0.979 | – | – | 0.142 | 0.293 | – | – |
| PEFR (L/sec) | 0.217 | 0.088 | 0.245 | 0.136 | 0.248 | 0.063 | −0.017 | 0.924 |
| Respiratory muscle strength | ||||||||
| PEmax (cm H2O) | 0.344 | 0.006* | 0.396 | 0.009* | 0.393 | 0.003* | 0.375 | 0.014* |
| PImax (cm H2O) | 0.000 | 1.000 | −0.193 | 0.183 | 0.196 | 0.144 | 0.000 | 0.997 |
†Association between SMI and parameters of lung function and respiratory muscle strength according to Pearson’s test.
‡Association between SMI and parameters of lung function and respiratory muscle strength according to multiple regression analysis. *p<0.05.
The FEV1 was excluded as an independent variable after considering multicollinearity.
SMI: skeletal muscle mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second; PEFR: peak expiratory flow rate; PEmax: maximum expiratory pressure; PImax: maximum inspiratory pressure.
DISCUSSION
After applying the exclusion criteria, our study has included 120 eligible older adults who required long-term care or support. In several cases, the ability to stand has declined in older adults requiring long-term care or support. Our study provided useful data because muscle mass was measured using the more accurate BIA method that required standing, not an estimated formula used previously16).
Because respiratory muscle strength differs by gender, we examined our findings with regard to gender8, 19). According to the report by EWGSOP, PEFR is an indicator of muscle strength in the evaluation of sarcopenia1). In this study, Only PEmax was significantly correlated with SMI according to Pearson correlation analysis and the multiple regression analysis for both male and female participants. In other words, the parameter most clearly related to SMI in lung function and respiratory muscle strength was PEmax, not PEFR for older adults who required long-term care or support. This result suggested that PEmax for these older adults, which was defined as an index of respiratory muscle strength, was more related to muscle mass than was PEFR, which was an index of lung function tests. Both PEmax and PEFR can be indicators of respiratory muscle strength. PEmax represents the intraoral pressure when “the only maximum expiratory effort” was performed after maximal inspiration. For older adults requiring long-term care/support, PEmax is much simpler and easier to perform than PEFR, which is measured by repeated inhalation and exhalation. We also assumed that FVC and FEV1 rather show the overall function of a pulmonary condition via the compliance of the lung–thorax system20).
Conversely, although our results showed a significant association between SMI and PEmax, we found no significant association between SMI and PImax. In previous studies, investigators reported associations between SMI and PImax in young adults, between SMI and PEmax in young men, and between SMI and PImax in healthy adults13,14,15). Therefore, there has been no consensus about which type of respiratory muscle, expiratory or inspiratory, is related to muscle mass. Resting expiratory function was passively maintained by elastic contraction forces in the lung and chest wall during inspiration, and the diaphragm’s main role is in inspiration21, 22). With regard to forced breathing, the activity of the transversus abdominis, obliques, and rectus abdominis muscles was observed during expiration, and the activity of the scalene and sternocleidomastoid muscles was observed during inspiration21). Our study suggested that SMI was associated with PEmax of respiratory muscle strength during expiration in older adults with a certification of requiring long-term care or support. Therefore, our findings also suggested that aging weakens the respiratory muscles, especially those involved in expiration. The results of several other studies have supported our opinion23,24,25,26,27). With regard to changes in respiratory muscles with aging, several investigators reported no or less change in muscle mass and no change in muscle fiber types in diaphragmatic muscle and inspiratory external intercostal muscles23,24,25,26). In addition, a postmortem study of respiratory muscles in older adults revealed that type II fibers exhibited atrophy in abdominal muscles but not in the diaphragm27). The expiratory muscles might be more affected by aging with long-term care/support as compared with other parameters. However, respiratory muscle strength is considered to include the strength of the diaphragm and other respiratory muscles. Further research is needed to determine the effects of specific muscles.
This study has some limitations. First, this study was a basic research for investigating the association between sarcopenia and PEmax & PImax. We focused on SMI alone, which is a biomarker of sarcopenia. A previous study has reported the relationship between pulmonary function and physical performance28). Second, it was difficult to establish the exclusion criteria for older adults who had various comorbid diseases and a complex medical history. For example, community-dwelling individuals with stroke showed a decrease in the strength of respiratory muscles29). The factors associated with the disease could not be excluded in this study. Third, muscle mass and pulmonary function are affected by smoking, and we excluded participants with <70% FEV1/FVC ratio (refer to previous study)9, 10, 30). In addition, many older adults were excluded from our study. In particular, 30 participants could not undergo muscle mass measurement in the standing position. However, the AWGS and EWGSOP recommend the BIA method for measuring muscle mass1,2,3,4). We had to exclude these patients to determine the relationship between sarcopenia and respiratory function. As part of our future study, we will also consider measuring muscle mass by the BIA method in the supine position. In the future, we will investigate the effect of the presence or absence of sarcopenia on PEmax and PImax, including physical performance in cross-sectional and longitudinal studies as well as multicenter studies.
In conclusion, the EWGSOP considered PEFR an indicator of muscle strength for sarcopenia, but we found that PEmax was more related to SMI in community-dwelling older adults undergoing ambulatory rehabilitation in the long-term care insurance system. This result indicated an association between sarcopenia and PEmax.
Funding
None.
Conflict of interest
The author declares no conflict of interest.
REFERENCES
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. European Working Group on Sarcopenia in Older People: European working group on sarcopenia in older people. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing, 2010, 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LK, Liu LK, Woo J, et al. : Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc, 2014, 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2: Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing, 2019, 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LK, Woo J, Assantachai P, et al. : Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc, 2020, 21: 300–307.e2. [DOI] [PubMed] [Google Scholar]
- 5.Mijnarends DM, Luiking YC, Halfens RJ, et al. : Muscle, health and costs: a glance at their relationship. J Nutr Health Aging, 2018, 22: 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makizako H, Shimada H, Doi T, et al. : Age-dependent changes in physical performance and body composition in community-dwelling Japanese older adults. J Cachexia Sarcopenia Muscle, 2017, 8: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada M, Nishiguchi S, Fukutani N, et al. : Prevalence of sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc, 2013, 14: 911–915. [DOI] [PubMed] [Google Scholar]
- 8.Enright PL, Kronmal RA, Manolio TA, et al. Cardiovascular Health Study Research Group: Respiratory muscle strength in the elderly. Correlates and reference values. Am J Respir Crit Care Med, 1994, 149: 430–438. [DOI] [PubMed] [Google Scholar]
- 9.Kera T, Kawai H, Hirano H, et al. : Relationships among peak expiratory flow rate, body composition, physical function, and sarcopenia in community-dwelling older adults. Aging Clin Exp Res, 2018, 30: 331–340. [DOI] [PubMed] [Google Scholar]
- 10.Kera T, Kawai H, Hirano H, et al. : Definition of respiratory sarcopenia with peak expiratory flow rate. J Am Med Dir Assoc, 2019, 20: 1021–1025. [DOI] [PubMed] [Google Scholar]
- 11.Cook CD, Mead J, Orzalesi MM: Static volume-pressure characteristics of respiratory system during maximal efforts. J Appl Physiol, 1964, 19: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society/European Respiratory Society: ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med, 2002, 166: 518–624. [DOI] [PubMed] [Google Scholar]
- 13.Ro HJ, Kim DK, Lee SY, et al. : Relationship between respiratory muscle strength and conventional sarcopenic indices in young adults: a preliminary study. Ann Rehabil Med, 2015, 39: 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin HI, Kim DK, Seo KM, et al. : Relation between respiratory muscle strength and skeletal muscle mass and hand grip strength in the healthy elderly. Ann Rehabil Med, 2017, 41: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawaya Y, Ishizaka M, Kubo A, et al. : Correlation between skeletal muscle mass index and parameters of respiratory function and muscle strength in young healthy adults according to gender. J Phys Ther Sci, 2018, 30: 1424–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohara DG, Pegorari MS, Oliveira Dos Santos NL, et al. : Respiratory muscle strength as a discriminator of sarcopenia in community-dwelling elderly: a cross-sectional study. J Nutr Health Aging, 2018, 22: 952–958. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner RN, Koehler KM, Gallagher D, et al. : Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol, 1998, 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 18.Kubo A, Keiri H: Estimating height from forearm and lower leg lengths of elderly persons. Rigakuryoho Kagaku, 2007, 22: 115–118(In Japanese, English abstract). [Google Scholar]
- 19.Black LF, Hyatt RE: Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis, 1969, 99: 696–702. [DOI] [PubMed] [Google Scholar]
- 20.Neder JA, Andreoni S, Lerario MC, et al. : Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res, 1999, 32: 719–727. [DOI] [PubMed] [Google Scholar]
- 21.De Troyer A: Actions of the respiratory muscles. In: Physiological Basis of Respiratory Disease. Hamilton: B C Decker, 2005, pp 263–275. [Google Scholar]
- 22.Mead J, Smith JC, Loring SH: Volume displacements of the chest wall and their mechanical significance. In: The Thorax, 2nd ed. New York: Marcel Dekker, 1995, pp 565–586. [Google Scholar]
- 23.Krumpe PE, Knudson RJ, Parsons G, et al. : The aging respiratory system. Clin Geriatr Med, 1985, 1: 143–175. [PubMed] [Google Scholar]
- 24.Caskey CI, Zerhouni EA, Fishman EK, et al. : Aging of the diaphragm: a CT study. Radiology, 1989, 171: 385–389. [DOI] [PubMed] [Google Scholar]
- 25.Polkey MI, Harris ML, Hughes PD, et al. : The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med, 1997, 155: 1560–1564. [DOI] [PubMed] [Google Scholar]
- 26.Tolep K, Higgins N, Muza S, et al. : Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med, 1995, 152: 677–682. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno M: Human respiratory muscles: fibre morphology and capillary supply. Eur Respir J, 1991, 4: 587–601. [PubMed] [Google Scholar]
- 28.Landi F, Salini S, Zazzara MB, et al. : Relationship between pulmonary function and physical performance among community-living people: results from Look-up 7+ study. J Cachexia Sarcopenia Muscle, 2020, 11: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teixeira-Salmela LF, Parreira VF, Britto RR, et al. : Respiratory pressures and thoracoabdominal motion in community-dwelling chronic stroke survivors. Arch Phys Med Rehabil, 2005, 86: 1974–1978. [DOI] [PubMed] [Google Scholar]
- 30.Moon JH, Kong MH, Kim HJ: Implication of sarcopenia and sarcopenic obesity on lung function in healthy elderly: Using Korean national health and nutrition examination survey. J Korean Med Sci, 2015, 30: 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]