Abstract
Promoters serve a critical role in establishing baseline transcriptional capacity through the recruitment of proteins, including transcription factors. Previously, a paucity of data for cis-regulatory elements in plants meant that it was challenging to determine which sequence elements in plant promoter sequences contributed to transcriptional function. In this study, we have identified functional elements in the promoters of plant genes and plant pathogens that utilize plant transcriptional machinery for gene expression. We have established a quantitative experimental system to investigate transcriptional function, investigating how identity, density and position contribute to regulatory function. We then identified permissive architectures for minimal synthetic plant promoters enabling the computational design of a suite of synthetic promoters of different strengths. These have been used to regulate the relative expression of output genes in simple genetic devices.
INTRODUCTION
Transgenic techniques are used to investigate the function of plant genes and to develop new products for agriculture and industry. Biotech crops, typically containing at least one transgene, are now planted on over 190 million hectares each year (1), and plants are finding new roles as platforms for biomanufacturing pharmaceuticals (2,3). For many years, the majority of transgenic events involved only a single gene of interest and a selectable marker gene. However, recent advances in DNA assembly techniques pioneered by the nascent field of synthetic biology have enabled the facile construction of multigene constructs for plants (4). Researchers are now able to apply these tools to design and deploy synthetic genetic circuits and reconstruct heterologous biochemical pathways in plant systems (5,6). However, the realization of synthetic genetic circuits that function as expected requires the ability to precisely and predictably regulate gene expression. Spatiotemporal quantities of endogenous gene products are regulated through numerous mechanisms including transcript elongation (7), antisense transcription (8) and several post-transcriptional and translational processes (9,10). Although these mechanisms could be leveraged to fine-tune the expression of transgenes, information flow from synthetic genetic circuits is initiated by transcription and, therefore, control of transcription is considered the simplest way to balance the expression of transgenes within a synthetic genetic circuit (11). To achieve this, regulatory elements with predicable characteristics are highly desirable. However, suites of promoters with different levels of expression for plants are not widely available. The promoters used are often several kilobases in length and their functional elements have only rarely been identified and characterized. Many plant scientists and biotechnologists still rely on a small set of natural promoters first isolated in the 1980s. In particular, constitutive promoters from plant-infecting DNA viruses and from the opine biosynthetic genes found on tumour-inducing (Ti) plasmids of Agrobacterium tumefaciens that recruit the host cell's transcriptional machinery (12). These include the 35s promoter from the double-stranded DNA virus, cauliflower mosaic virus (CaMV), which is reported to have at least partial function in numerous plant species as well as in bacteria (13), fungi (14,15) and vertebrates (16,17).
Deletion and rearrangement studies performed in the 80s and 90s, identified several key functional elements in the promoters of plant-infecting viruses and bacteria, revealing synergistic interactions between cis-elements (18–20). Later, engineered variants were made by swapping domains to achieve promoters of similar strengths with reduced sequence homologies (21). However, progress towards rational-design of synthetic promoters was limited by a lack of comprehensive data for plant transcription factor binding sites (TFBSs) as well as by the technical limitations of rearranging and rewriting DNA sequences before chemical gene synthesis was widely available. Consequently, although some functional elements were characterized and the possibility of designing synthetic plant promoters was discussed (22), these goals have yet to be fully realized. Progress has been made engineering plant promoters for tissue-specific or condition-inducible expression (23–25). In addition, a number of increasingly complex synthetic regulatory elements comprised of binding sites for orthogonal transcription factors (TFs) fused to a minimal core promoter have been used to enable inducible constitutive expression (26–29). However, a non-orthogonal promoter is required to drive expression of the TF, for which an endogenous plant or plant viral promoter is typically used (30).
In recent years, significant progress has been made in the design of synthetic regulatory elements for microorganisms, initially with the rational design of ribosome binding sites (31), TFs (32) and enhancers (33) and, subsequently, promoters (34–37). Such studies were substantially enabled by comprehensive datasets of TFBSs as well as by the ability to deliver sizable and complex libraries of sequences to populations of cells, sorting, selecting and sequencing cells with desired expression profiles. Equivalent experiments are challenging in plants due to the limitations of DNA-delivery technologies and a paucity of cell-lines. However, genome sequencing technologies have recently shed light on epigenetic states and chromatin accessibility in plant genomes (38) and have identified candidate binding-motifs for many plant TFs (39–41). However, genomic datasets alone cannot be used to predict the intrinsic regulatory functions of DNA sequences and assessing the contribution of sequence motifs to regulatory activity is considered essential for characterizing function (42). Genome engineering technologies are enabling the functions of specific cis-regulatory elements (CREs) to be dissected (43). Rationally engineered suites of synthetic plant promoters of different strengths have yet to be reported. Here we describe a series of investigations to identify and functionally characterize plant CREs, revealing how complexity and the relative positions of CREs contribute to regulatory functions. We use these data to predict the performance of computationally designed minimal synthetic constitutive promoters and demonstrate predictable behaviour in dicotyledonous plants in transient expression and when integrated as stable transgenes. Thus, we present suites of minimal synthetic plant promoters of varied strengths, activated by either endogenous or orthogonal TFs and demonstrate how these can be used to control the relative expression of output genes in simple genetic circuits.
MATERIALS AND METHODS
Identification of candidate transcription factor binding sites (TFBSs)
The position weight matrices (PWMs) from the Arabidopsis thaliana (Arabidopsis) cistrome dataset (http://neomorph.salk.edu/dev/pages/shhuang/dap_web/pages/browse_table_aj.php) (39) and the plant TF database (44) were used to create a motif file for the command line version of FIMO (MEME suite) (45). This was used to scan FASTA files of promoter sequences with a threshold P-value of 0.0001. Candidate TFBSs were mapped back to the promoter sequences. Expression data for TF-encoding genes across multiple Arabidopsis tissues was obtained from the Expression Atlas (http://www.ebi.ac.uk/gxa) (46).
Construction of plasmids
All constructs were designed in Benchling (San Francisco, CA, USA), synthesized as double-stranded DNA fragments (Twist Biosciences, San Francisco, CA, USA) and cloned into a universal acceptor plasmid (pUAP1 (47) or pUPD2 (48)), to produce standardized Level 0 phytobricks, conforming to the plant common syntax standard (47). Expression cassettes and multigene constructs were assembled using the Type IIS DNA assembly protocol described in (49). Synthetic and control promoter parts were assembled with the omega 5′ untranslated region from tobacco mosaic virus (5UTR-ΩTMV; pICH41402, Addgene #50285), the coding sequence of firefly luciferase (LucF; pEPAS0CM0008, Addgene #154594), a C-terminal FLAG tag (pICSL50007, Addgene #50308) and a 3′ untranslated region and terminator sequence (3UTR) from A. tumefaciens octopine synthase (AtuOCS) (pICH41432, Addgene #50343). A calibrator construct (pEPYC1CB0197, Addgene #154654) for ratiometric quantification was assembled from A. tumefaciens nopaline synthase (AtuNOS) promoter (pICH42211, Addgene #50255), 5UTR-ΩTMV, the coding sequence of NanoLuc luciferase (LucN, pEPYC0CM0133, Addgene #154595) and AtuOCS terminator. For stable plant transformation, synthetic and control promoters were assembled with the 5′UTR from cowpea mosaic virus (CPMV), a chimeric coding sequence consisting of an N′-terminal HiBit (pEPYC0CM0258, Addgene #154593) the uidA coding sequence, encoding β-glucuronidase (GUS; pICSL80016, Addgene #50332) and a C′-terminal yellow fluorescent protein (YFP; pICSL50005, Addgene #117536) and AtuOCS terminator. This reporter cassette was assembled with a plant selectable marker cassette conferring resistance to kanamycin (pEPYC1CB0308, Addgene #154624) Synthetic and control promoters were additionally fused to either a transcription activator like effector (TALE) or a synthetic TF comprised of a Gal4 activation domain (GB0900, received from the Orzaez laboratory) and a PhiC3 binding domain (GB_UD_32AB, received from the Orzaez laboratory). A table of all 91 minimal synthetic promoters tested in plant cells is provided in Supplementary Data 1. Tables with the details of all plasmids used and constructed for this study are provided in Supplementary Data 2 and all plasmids, together with their complete sequences, have been submitted to the Addgene repository.
Growth of plant material
Arabidopsis thaliana (Col-0), Nicotiana benthamiana, Brassica rapa and Hordeum vulgare (barley) plants were germinated and grown in potting medium (two-parts sieved compost to one-part sand) within controlled environment chambers with a 16 h photoperiod at 22°C with 120–180 μmol/m2/s light intensity. For the 2 days before leaves were harvested for the preparation of protoplasts, the photoperiod was reduced to 8 h.
Protoplast preparation and transfection
Protoplasts were prepared from the leaf tissues of A. thaliana, N. benthamiana, B. rapa and H. vulgare and as previously described (50) and diluted to 104–105/ml for transfection. A total of 4.5 μg purified plasmid DNA, comprising equal molar ratios of the plasmid containing the expression cassette for which expression was measured (test-p:ΩTMV:LucF:AtuNOSt) and a calibrating plasmid (pEPYC1CB0197; AtuNOSp:ΩTMV:LucN:AtuNOSt) were added to each designated well of a 2.2 ml 96 deep-well plate containing 200 μl protoplasts (104–105/ml) and mixed gently by shaking. PEG solution was freshly prepared by mixing 2 g PEG (poly(ethylene glycol), MW 4000 Da) with 2 ml 500 mM mannitol and 0.5 ml 1M CaCl2 and 220 μl was added to each well. After 5 min at room temperature, 1.2 ml W5 (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES pH5.6) was added and protoplasts were collected by centrifugation at 100 g for 2 min and resuspended in 100 μl W5 solution. Resuspended protoplasts were transferred to a round bottom 96-well plate (pre-prepared by incubation with 0.1% bovine serum albumin for 10 mins). Transfected protoplasts were incubated at 22°C with 100 μmol/m2/s2 light intensity for at least 16 h. For each batch of protoplasts, a control plasmid, pEPYC1CB0199 (AtuMASp: ΩTMV:LucF:AtuNOSt) and the calibrator (pEPYC1CB0197; AtuNOSp: ΩTMV:LucN:AtuNOSt) were used to transfect three aliquots of protoplasts.
Production of stable transformants
Transgenic Arabidopsis lines were produced by Agrobacterium-mediated transformation of floral tissues. Assembled plasmids were transformed into A. tumefaciens (GV3101) and liquid cultures were grown from single colonies in growth medium supplemented with 50 μg/ml rifampicin, 25 μg/ml gentamycin and 50 μg/ml kanamycin at 28°C. Agrobacterium tumefaciens cells were collected by centrifugation and resuspended to OD600 0.8 in 5% sucrose, 0.05% Silvet L-77 and sprayed onto Arabidopsis floral tissues. Plants were sealed in black plastic bags for 24 h. Seeds were collected from mature siliques and surface sterilized with 70% EtOH for 10 min followed by 3–5% sodium hypochlorite for 10 min. For selection of transgenics, sterilized seeds were germinated and grown on Murashige and Skoog medium supplemented with 75 μg/ml kanamycin with 16 h light 22°C.
Determination of transgene copy number by digital droplet PCR (ddPCR)
Samples of leaf tissue (0.1 g) were ground in liquid nitrogen. DNA was extracted using the cetyltrimethylammonium bromide (CTAB) extraction protocol described in (51) and 2 μg genomic DNA was digested with 20 units EcoRV for 2 h at 37°C. A total of 400 ng of digested genomic DNA was used in digital droplet polymerase chain reaction (ddPCR) reactions with QX200™ddPCR™EvaGreen®Supermix (Bio-Rad, Hercules, CA, USA) and oligonucleotide primers to the UidA transgene sequence (5′-CGGCGAAATTCCATACCTGTT and 5′-TCAGCCGATTATCATCACCGA) or a homozygous single-copy reference gene, AtADH1 (AT1G77120; 5′-ACTTCTCTCTGTCACACCGA and 5′- GGCCGAAGATACGTGGAAAC). Droplets were generated using the QX200™Droplet Generator (Bio-Rad), PCR reactions were run on the C1000 Touch™Thermal Cycler (Bio-Rad) and analysed on the QX200™Droplet Reader (Bio-Rad). Absolute transgene copy number was calculated using the QuantaSoft™software (Bio-rad) to analyse the ratio of droplets in which the target (UidA) was amplified to those in which the reference (AtADH1) was amplified.
Quantification of gene expression
Luciferase expression was detected using the Nano-Glo® Dual-Luciferase® reporter assay system (Promega, Madison, WI, USA). Protoplasts were homogenized in 30 μl passive lysis buffer (Promega) containing protease inhibitor (P9599, Sigma-Aldrich, Dorset, UK). Following incubation on ice for 15 min and centrifugation (100 × g, 2 min, 4°C), 30 μl supernatant was removed and mixed with 30 μl ONE-Glo™ EX Luciferase Assay Reagent (Promega) and incubated at room temperature for 10 min. LucF luminescence was detected using a Clariostar microplate reader (BMG Labtech, Aylesbury, UK) with a 10 s read time and 1 s settling time. Gain was set at 3600. LucN luminescence was detected from the same sample by adding 30 μl NanoDLR™ Stop & Glo® Reagent (Promega). After incubation for 10 min at room temperature, luminescence was detected as above. Normalized expression is reported throughout this manuscript as the ratio of luminescence from the test construct (LucF) to the calibrator (LucN; pEPYC1CB0197), normalized to the luminescence of the experiment control (LucF; pEPYC1CB0199/ LucN; pEPYC1CB0197).
Expression from stably integrated HiBit:GUS:YFP transgenes was quantified using the Nano-Glo® HiBiT Extracellular Detection System (Promega). A total of 10 mg leaf tissue was homogenized in 50 μl passive lysis buffer (Promega) containing protease inhibitor (P9599, Sigma-Aldrich). Homogenized leaf tissues were centrifuged at 18 000 g 10 min 4°C and 2 μl supernatant mixed with 48 μl Bradford reagent (ThermoFisher Scientific, Waltham, MA, USA). Protein concentration was estimated by absorbance at 595 nm and concentrations were normalized. A total of 5 μl normalized extract were diluted to 30 μl in passive lysis buffer and mixed with 30 μl Nano-Glo® HiBiT Extracellular Detection Reagent (Promega) and luminescence was detected as above. GUS expression was visualized by submerging 10-day-old seedlings in 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, 100 mM sodium phosphate buffer pH7.0, 10 mM ethylenediaminetetraacetic acid, 0.5 mg/ml X-Gluc (5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid cyclohexylammonium salt) for 24 h at room temperature. To remove chlorophyll, this was replaced with 70% EtOH followed by 100% EtOH for 8 h each. Images were taken using a Leica M205FA stereo microscope (Leica, Wetzlar, Germany). YFP expression was visualized using a SP5 (II) confocal microscope (Leica) with a 20× air objective, excitation 514 nm, emission 530 nm. Final images were prepared using Fiji ImageJ (52) (https://imagej.net/Fiji).
RESULTS
Constitutive promoters are comprised of multiple functional elements with the potential to bind numerous transcription factors
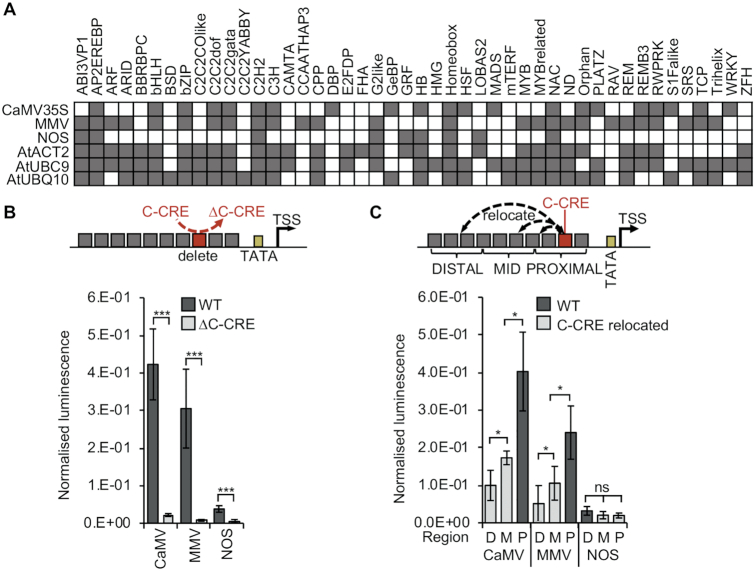
To identify candidate CREs for use in minimal synthetic constitutive promoters, we analysed promoters widely used for exogenous expression for the presence of candidate TFBSs. These included promoters from vascular plants as well as those from plant-infecting pathogens that recruit the plant's transcriptional machinery, including CaMV35S, A. tumefaciens nopaline synthase (AtuNOS) and Mirabilis Mosaic Virus (MMV). The data indicated that constitutive promoters have, in principle, the ability to bind multiple classes of TFs (Figure 1A). Analysis of Arabidopsis gene expression data indicated that few of the TFs predicted to bind to constitutive promoters show constitutive expression themselves (Supplementary Data 3). We also performed de novo motif identification using MEME. This analysis identified the presence of a CRE common to all 14 pathogen promoters (common-CRE or C-CRE) (Supplementary Data 4). In six of these promoters, the C-CRE contained a predicted binding site for a basic-leucine-zipper (bZIP) TF. These C-CREs can therefore be considered to be equivalent to the previously described activation sequence 1 (As-1), shown to directly bind members of the TGACG-motif binding (TGA) family of basic-leucine-zipper (bZIP) TFs (53–55). The other eight pathogen promoters were not predicted to bind bZIP TFs. However, it was previously shown that this region of AtuNOS is able to bind TGA4 in the presence of a cofactor, OBP5 (56). Consistent with early studies in which regions of promoters were sequentially deleted, quantitative ratiometric dual luminescence reporter assays (see materials and methods) revealed that specifically deleting individual C-CREs significantly reduced expression (Figure 1B). This was in contrast to the majority of candidate CREs, of which deletion did not significantly change expression (Supplementary Data 5). To investigate whether the position of the C-CREs within the promoter was essential, the element was relocated varying its proximity to the transcriptional start site (TSS). Whilst expression reduced when the C-CREs in CaMV35S and MMV were moved further from the TSS, relocating the C-CRE in AtuNOS, which is already located distally to the TSS, had a negligible impact (Figure 1C).
Figure 1.
Identification and characterization of plant CREs. (A) Identification of candidate CREs in constitutive promoters. Shaded squared indicate presence of TF binding site motifs. (B) Deletion, or, (C) Relocation of a CRE common to all pathogen promoters (C-CRE) significantly reduces expression. D = distal, M = mid, P = proximal. Error bars = 2 × standard error; n = 3; P-values were calculated using unpaired two-tailed Student's t-test; *P < 0.05, ***P < 0.001, ns = not significant.
Orthogonal tools with a range of expression levels
To identify a functional basic design for minimal synthetic plant promoters (MinSyn-P), we first built and tested synthetic promoters with a range of expression levels that respond to orthogonal TFs. The initial design was based on previously reported synthetic TALE-responsive synthetic elements to which single binding sites for TALES were added (28). These synthetic promoters consist of 19 bps of random sequence, followed by a second region of variable length (to which CREs are added), a TATA box sequence (TATATAA) and a 43 bp minimal core including TSS (Figure 2A). We successfully verified that this general design could be used to build promoters with a range of expression levels by adding different numbers of binding sites for either TALES or recently described synthetic Gal4:ΦC31 TFs (Vazquez-Vilar et al. (48) (Figure 2B).
Figure 2.
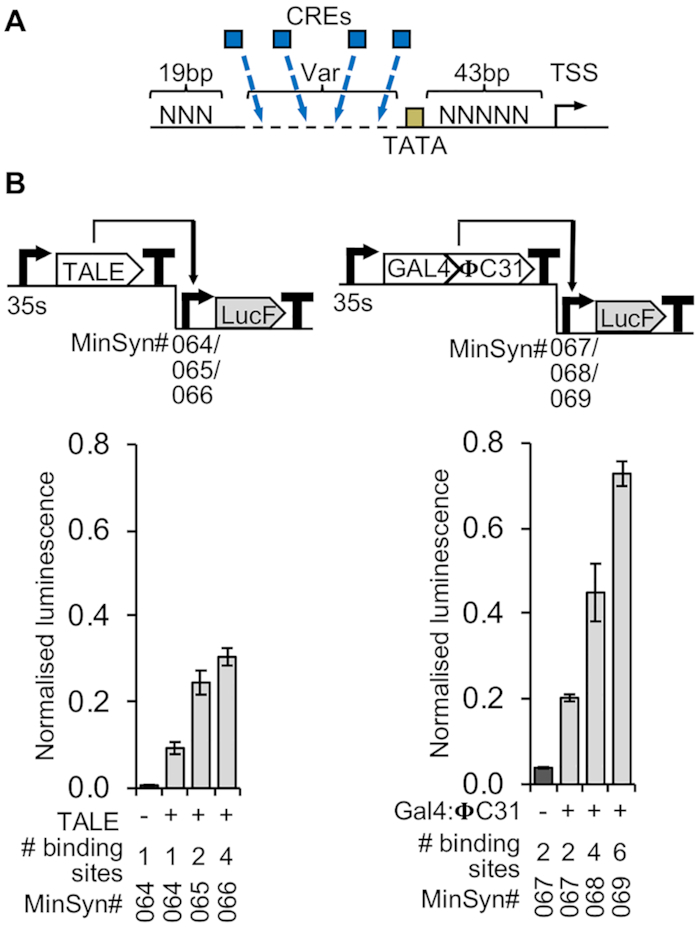
Minimal synthetic promoters (MinSyns) of different strengths regulated by orthogonal TFs. (A) Schematic showing the architecture of MinSyns, which consist of 19 bps of random sequence (NNN) followed by a region of variable length (Var) to which CREs (blue boxes) are added, a TATA box sequence, a 43 bp core sequence and TSS. (B) Orthogonal synthetic TFs must be co-expressed to regulate expression of MinSyns with cognate binding sites. Expression levels correspond to the number of binding sites in the variable region for transcription activator-like effectors (TALES) (left) or GAL4:ΦC31 transcriptional activators (right); n = 3.
Expression from minimal synthetic regulatory elements by passive cooperativity
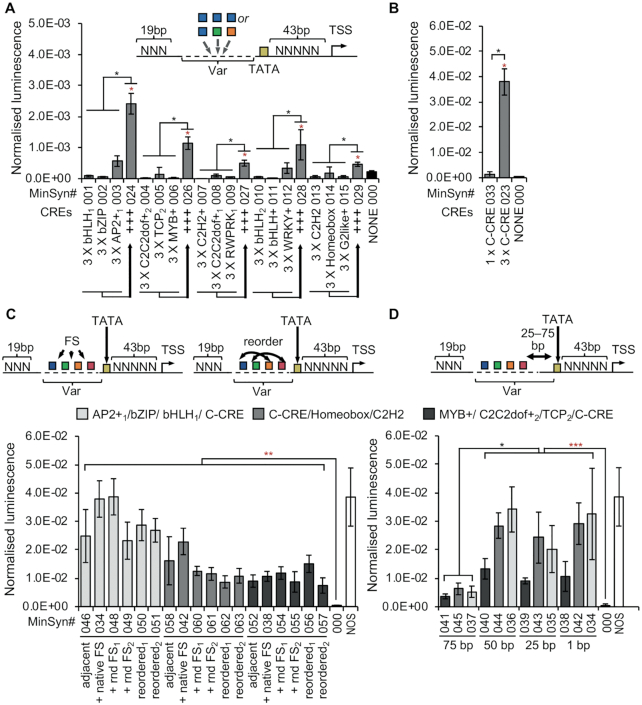
To define rules for the design of constitutive MinSyns that respond to endogenous TFs, experiments were progressed to test the function of candidate CREs identified from constitutive promoters (Figure 1). To do this, we first inserted three copies of the same CRE into the variable region of the MinSyn. Whilst it was expected that some candidate CREs might be false-positives and that others might either recruit repressors of transcription or would require a specific local sequence context, no expression was observed from any MinSyns containing only one type of CRE (Figure 3A), with the exception of MinSyns containing multiple copies of C-CREs (Figure 3B). To further investigate, we added random combinations of different CREs to the variable regions of MinSyns. In the majority of cases, this resulted in significant expression (Figure 3A). In a few cases, combinations of CREs did not result in significant expression (Supplementary Data 6). This is consistent with expectations that some CREs recruit transcriptional repressors whilst other may need to be correctly co-located to enable TFs to form functional heterocomplexes. To test if expression from MinSyns with multiple CREs was dependent on specific TF–TF interactions, the relative positions and spacing of CREs within the variable region of the MinSyns were altered (Figure 3C). In one set of variants, we added up to 20 bp of additional sequence between the CREs. To control for the effect of local sequence context, we made three variants for each set of CREs, two with random sequence and one with the native flanking sequence (FS) from the natural promoter from which the CRE was identified. In a second set of variants, the relative positions of the CREs were permutated. Neither changes to the relative position nor moderate increases in spacing had any significant effect on expression. To determine if the relative location of the CREs to the TATA box was critical and to assess if the minimal length of the MinSyns was limiting function, random sequence was inserted between the variable regions containing the CREs and the TATA box. In chromosomal DNA, DNA looping allows distal enhancer elements to interact with the proximal regions; however, as our design goal was minimal constitutive promoters, further extensions to accommodate such interactions were undesirable. Expression was significantly impacted when more than 50 bps of sequence was inserted between the first CRE and the TATA box (Figure 3D).
Figure 3.
Defining design features of minimal synthetic promoters. (A) Combinations of three different CREs resulted in significantly higher expression than three copies of the same CRE. (B) The C-CREs enables expression in the absence of other CREs. (C) Rearranging the relative positions of CREs by either inserting native or random FS or by reordering does not significantly change expression levels. (D) Relocating CREs more than 50 base pairs (bp) from the TATA box significantly reduces expression. Error bars = 2 × standard error; n = 3; P-values were calculated using unpaired two-tailed Student's t-test; *P < 0.05, **P < 0.01, ***P < 0.001; Red asterisks indicate a significant difference from MinSyn000, which has no CREs. Black asterisks difference from MinSyns indicated by solid black brackets.
Computational design of minimal synthetic promoters (MinSyn) with predictable strengths
We applied the knowledge gained from these experiments to develop a script to create a library of 1000 constitutive MinSyn (Supplementary Data 7) for which was predicted. For each MinSyn, the script selects a random number (N) between three and ten that defines the number of CREs in the variable region and creates a random DNA sequence of 5 to 30 bases to comprise the sequence of the variable region. It then selects a single CRE sequence from the pool of previously identified CREs. This pool includes two C-CREs predicted to directly bind TGA TFs and one C-CRE for which direct TGA-binding was not predicted. The first CRE is added to the random DNA sequence and the process repeated N times without replacement. Thus, each MinSyn contains between three and ten different CREs, each added to the variable region in the randomly selected order. From our initial experiments, we observed that the strength of the promoters was most affected by the inclusion of multiple CREs, of which the C-CREs had the most significant impact. C-CREs predicted to directly bind TGA TFs had the strongest effect when proximal (within 60 bps) to the TATA box (Figure 1C and Supplementary Data 8). In contrast, the relative position was less important for C-CREs not predicted to directly bind TGA TFs, with strength only decreasing when located more than 130 bps from the TATA box (Supplementary data 8). These observations were used to assign scores to each nucleotide base as follows: bases within CREs were each assigned a specific score, with bases within C-CREs assigned a higher score adjusted by a numerator reflecting proximity to the TATA box. If a MinSyn contained more than one C-CRE, the bases between the motifs were also adjusted by a numerator reflecting proximity of the mid-point to the TATA box. Scores for all bases were summed and divided by the total number of bases. To convert the score into a predicted strength, we applied the prediction to the existing set of tested MinSyns for which strength had been experimentally determined, thus defining a numerator. We were therefore able to formulate a predicted expression level for each promoter in the library. As expected for the profile of CREs in the pool, the majority of computational-designed promoters were predicted to have relatively weak expression. Twenty-four MinSyn sequences were selected from the library for synthesis and testing and the predicted and actual levels of expression were compared (Figure 4). For the whole population, the predicted and actual values showed good correlation (Figure 4, dashed line, R2 = 0.7076), however, there were some outliers. We reanalysed the sequences for the presence of known TFBSs that were not present in the pool used to create the library of MinSyns (e.g. those created unintentionally at sequence junctions of CREs). In 17 cases, additional known TFBS were identified but in most cases, there was insufficient data to determine how the TFs predicted to bind might affect expression (if they were activators or repressors). In three cases, the new motifs were predicted to bind additional TGA, NAC or cytokinin-response factor or transcriptional activators that would explain the deviation from the predicted activity (Figure 4, red data points). Four MinSyns were selected for further analysis (Figure 4, blue data points).
Figure 4.
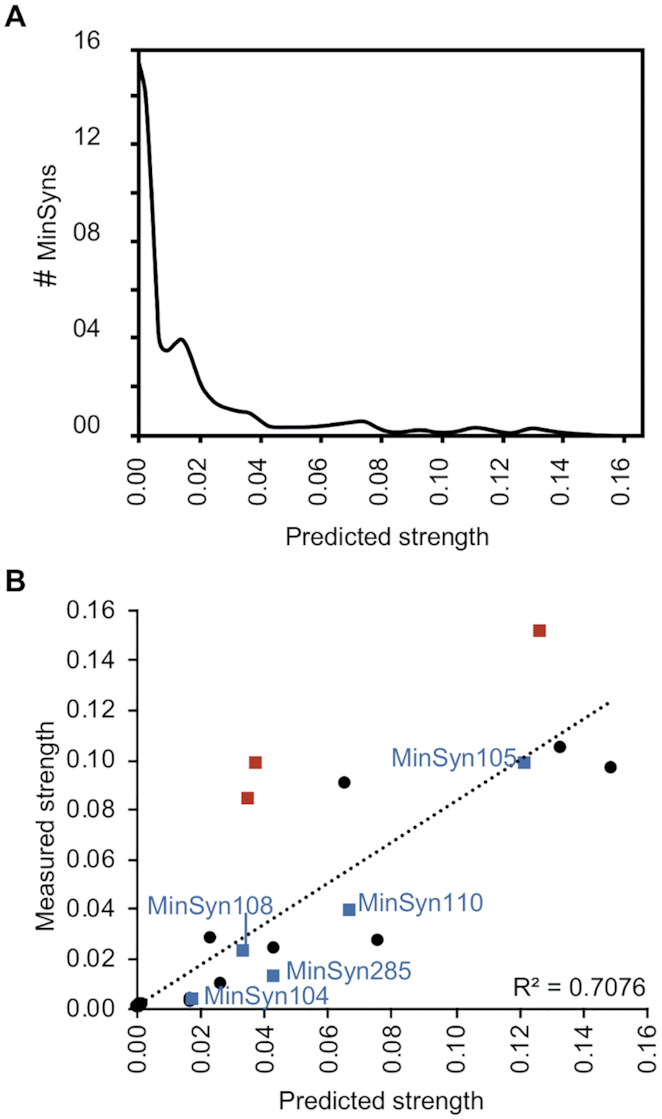
Computational design of minimal synthetic promoters (MinSyns). (A) Of a population of 1000 MinSyns, the majority were predicted to have relatively weak expression (B) Comparison of predicted and measured strengths of 24 computational-designed MinSyns. Red squares indicate MinSyns with unintended CREs formed at sequence junction that may explain deviance from predicted strength. Blue squares indicate MinSyns selected for further characterization (Figure 5).
MinSyns function in multiple species and as stable transgenes
Transient expression is used both for rapid experimentation and for production-scale protein expression in plants (3,57). Therefore, minimal promoters that perform as expected in transient expression are useful for several applications. However, for other applications, the ability to maintain expected levels of expression when stably integrated into the genome is desirable. Other studies have reported a strong correlation between the performance of transiently expressed and stably integrated transgenes (26). To investigate the performance of MinSyns in stably integrated transgenes, MinSyns of varying strengths were fused to multifunctional synthetic reporter protein-fusion enabling qualitative and quantitative detection of expression by luminescence, fluorescence and histochemical staining. Patterns of expression were assessed in five independent transgenic lines by GUS-staining and fluorescence microscopy (Figure 5A). Additionally, protein was extracted and expression quantified by detection of luminescence with data normalized to transgene copy number as determined by digital droplet PCR (Figure 5B). As expected, expression levels varied somewhat between independent lines (most likely the effect of local genomic context). However, the MinSyns expressed in most leaf and root tissues and the trends of expression levels observed in transient assays were maintained in stable lines (Figure 5B). We then compared the performance of MinSyns in two additional dicotyledenous species, B. rapa and N. benthamiana, in transient protoplast assays. The overall expression trend observed in these species was maintained, with expression levels in B. rapa being comparable to Arabidopsis, but expression levels in N. benthamiana being slightly higher (Figure 5C). Finally, we tested expression levels in the monocot, H. vulgare, observing that expression levels were minimal (Supplementary Data 9). This was not unexpected given the CREs were mined from the promoters of dicot-infecting viruses known to have low expression levels in monocots.
Figure 5.
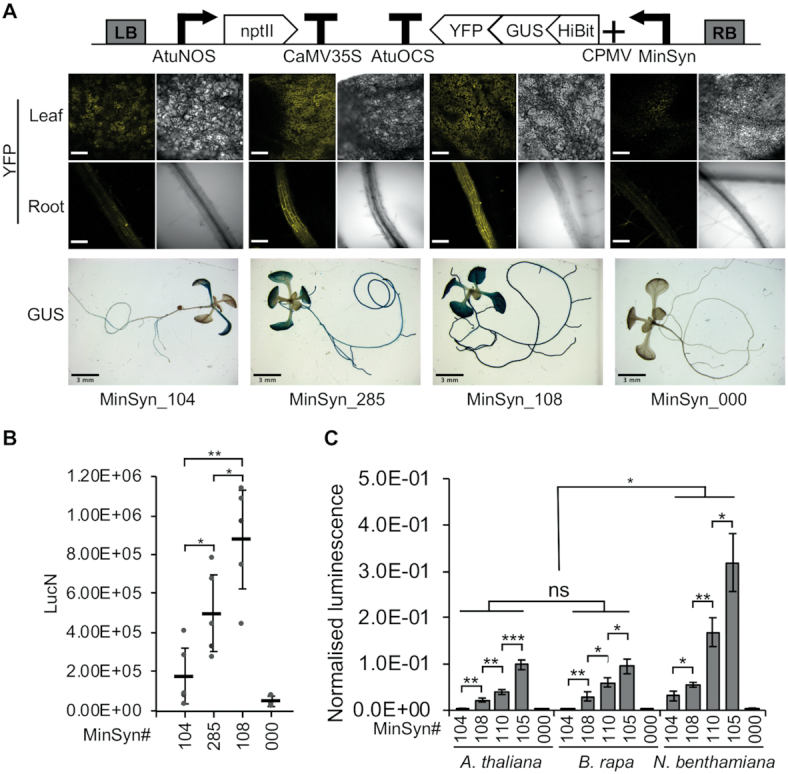
Characterization of minimal synthetic promoters (MinSyns). (A) Expression in Arabidopsis thaliana from stably integrated chimeric reporter cassettes was detected by fluorescence microscopy (yellow fluorescent protein; YFP), scale bar = 150 μm, and histochemical staining (β-glucuronidase; GUS), scale bar = 3 mm. (B) Expression levels from the same plants were quantified by detection of luminescence (LucN) from the Hi-Bit tag and normalized to transgene copy number. (C) Transient expression levels in mesophyll protoplasts of three plant species. Error bars = 2 × standard error; n = 5; P-values were calculated using unpaired two-tailed Student's t-test; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; ns = not significant.
Minimal synthetic elements for plants enable relative control of gene expression in synthetic genetic circuits
To demonstrate the utility of MinSyns in synthetic genetic circuits, we constructed simple multigene constructs in which all promoter elements were synthetic. Initially, we simply used a MinSyn to initiate transcriptional flow by controlling expression of an orthogonal TF, which activated expression of reporter (Figure 6A). Similar levels of expression were detected to circuits in which the TF was controlled by CaMV35s, which is widely used to initiate transcription in transgenic plants. We then demonstrated the ability to control the relative ratio of expression of two genes using two MinSyns with different numbers of cognate binding sites for an orthogonal TF to control expression of two reporters and a third MinSyn to control expression of the TF (Figure 6B).
Figure 6.
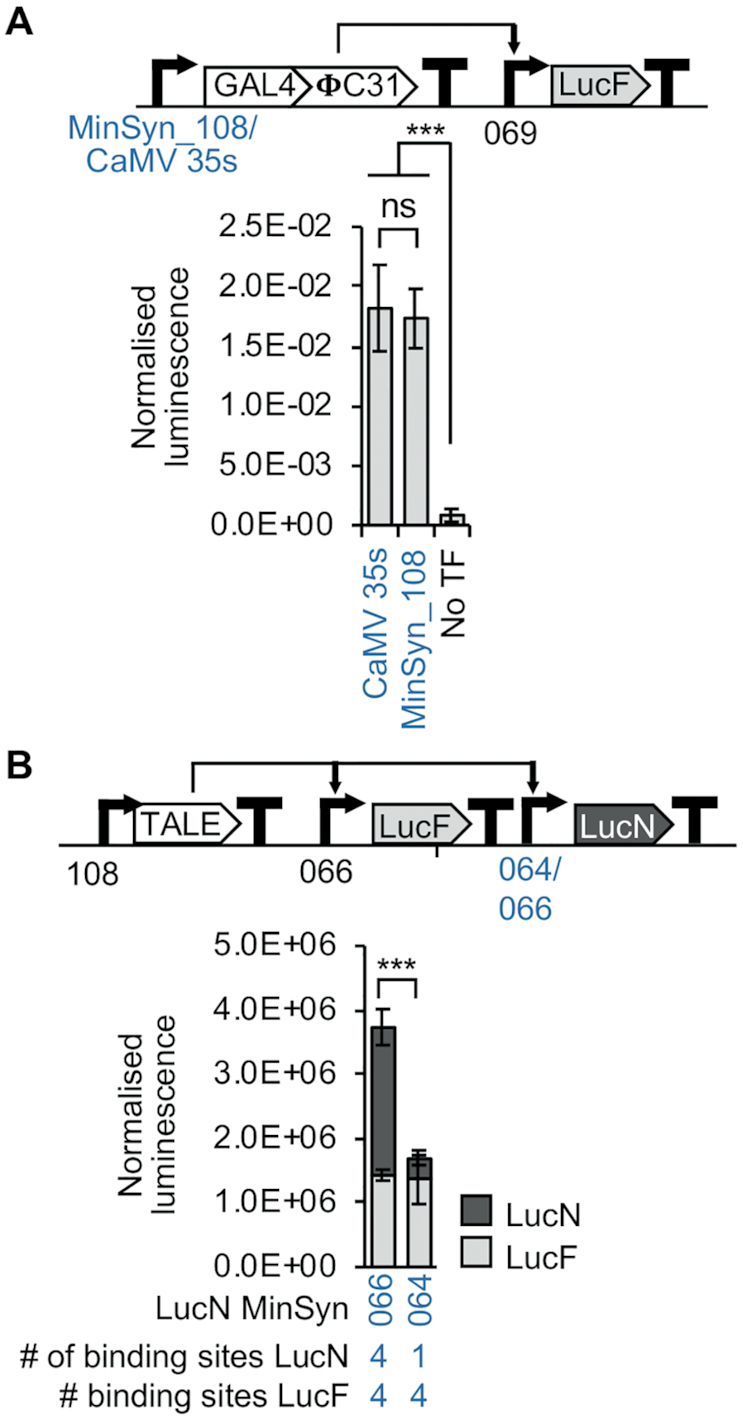
Initiation transcription from simple genetic devices with minimal synthetic promoters (MinSyns). (A) Constitutively expressed MinSyns drive expression of the orthogonal transcriptional factor GAL4:ΦC31, which regulates expression of a reporter. (B) The relative expression of two reporters is regulated using MinSyns with different numbers of binding sites for transcription activator-like effectors (TALES). Error bars = 2 × standard error; n = 3; P-values were calculated using unpaired two-tailed Student's t-test; ***P ≤ 0.001; ns = not significant.
DISCUSSION
Despite their dominance in plant research and biotechnology, comprehensive sequence analyses of even the most widely used constitutive promoters have not previously been reported. Analysis of expression levels of TF predicted to bind to these promoters, indicate that constitutive expression is unlikely to depend on steady-state presence of specific TFs across multiple cell types, but rather on the ability to utilize a wider range of TFs present in different cell types (Figure 1A and Supplementary Data 1). This is consistent with data obtained from early experiments in which the use of specific subdomains of CaMV35S resulted in tissue-specific expression (58). Promoters from numerous plant pathogens that have evolved to utilize the plants transcriptional machinery contain a common regulatory (C-CRE), likely to either directly or indirectly bind the TGA sub-class of bZIP TFs (53–56). This C-CRE has significant effect on the expression levels of both natural and synthetic promoters (Figures 1B-C and 3B) and was the only CRE able to promote detectable levels of expression without the presence of additional functional elements (Figure 3A and B). Several bZIP TFs are known to have a role in different disease and stress response pathways (59–61), which could therefore explain their dominance in pathogen regulatory elements. However, these promoters are known to confer broadly constitutive expression of stably integrated transgenes, including in healthy, non-stressed plants. Several bZIP TFs have been shown to function as pioneer TFs, able to displace nucleosomes in chromatin inaccessible to other TFs, thus enabling the assembly of other TFs (62,63). It has recently been hypothesized that some bZIP proteins inhibit chromatin compaction, initiating the formation of enhanceosomes (higher-order multicomponent TF–enhancer complexes) (64). Optimal positioning of pioneer TFs, in particular, has been suggested to be necessary for gene expression (65), which could explain the significant impact of relocation (Figure 1C). However, such roles have yet to be determined for plant TGA TFs. Further, although Transfer- DNAs (T-DNAs) integrated into plant nuclear genomes via Agrobacterium-mediated transformation might be packaged into chromatin, thus supporting a role for pioneer TFs in the promoters of the NOS, MAS and OCS opine biosynthetic genes from A. tumefaciens, the structures of caulimovirus DNA in plant nuclei are unknown.
Other than bZIP-binding C-CREs, multiple CREs needed to be combined to obtain significant expression from MinSyns (Figure 3A). These data indicate that, in the absence of the bZIP binding motif, multiple TFs are required for the proper recruitment of the transcriptional machinery. Previous studies have presented evidence that TF-complexes enable transcription either through direct protein–protein interactions or through the formation of enhanceosome complexes, but also without direct protein-protein interactions via a synergistic or collaborative binding process sometimes called passive cooperativity (66,67). Varying the relative positions and combinations of CREs within the MinSyns variable region revealed that direct protein-protein-interactions were unlikely (Figure 3C), therefore passive cooperativity is a reasonable hypothesis. This is consistent with experiments demonstrating that TFs can be substituted within enhancer complexes, enabling enhancer re-engineering by exchanging TF motifs (68). However, passive cooperativity is proposed to enable the displacement of nucleosomes. Whilst all core histones and the linker histone, H1, have been shown to associate with transiently delivered exogenous DNA in mammalian cells (albeit with aberrant stoichiometry) (69,70), this has not been investigated in plant cells.
Several synthetic promoters and cognate orthogonal TFs for plants, including those that can be induced by chemical signals, have been engineered for plant systems (26–28). In this study we aimed to expand on those efforts, creating regulatory elements of different strengths for use in the construction of larger genetic circuits, particularly biosynthetic pathways, in which it is desirable to control the relative expression levels of different proteins. We provide two options for such constructs: MinSyns of different strengths regulated by endogenous TFs (Figure 5C) or MinSyns of different strengths regulated by synthetic orthogonal TFs (Figures 2 and 6). Whilst the strength of MinSyns that bind orthogonal TFs correlates directly with the number of TF binding sites (Figures 2A and 6A), predicting the strength of constitutive MinSyns that utilize endogenous plant TFs was more challenging. The strength of the computationally designed MinSyns were broadly predictable (Figure 4) but predictability was undermined by the inadvertent introduction of additional TFBSs at sequence junctions. Similar issues were encountered during the creation of synthetic promoters for yeast (71,72), however the availability of complete datasets of yeast TFBSs allowed programming scripts to be modified to exclude these sequence motifs (71). We considered modifying the script for plant MinSyns to discard sequences in which additional elements were formed but, as the dataset for plant TFBSs is incomplete, we judged that the results would be unpredictable. A second option would be to include any newly created TFBSs in the prediction of strength. This also proved challenging, as relatively few plant TF-DNA interactions have been functionally characterized. In addition, the local context of binding sites has been shown to alter the activity of some TFs from repressors to activators (68,73), making it difficult to predict the impact on overall expression levels. Indeed, this phenomenon could also contribute to the difference between predicted and observed strengths of some MinSyns.
These investigations have enabled us to design a suite of minimal synthetic plant promoters of varied strengths, activated by either endogenous or orthogonal TFs, that provide numerous options for the construction of large and complex genetic circuits for dicotyledonous plants. The availability of promoters of different strengths provide plant scientists with new options for regulating the relative expression levels of different genes within synthetic pathways. Weak constitutive promoters are particularly well-suited for regulating the expression of TFs, including synthetic TFs to initiation expression from synthetic genetic circuits. We have characterized the performance of synthetic promoters as stable transgenes, finding that transient assays were broadly predictive of behaviour. In previous work, we have observed that permutations of other components such untranslated regions and terminator sequences also impacts the final expression levels of a synthetic transcriptional unit (74). In this work we have controlled for variance by maintaining the same sequences, allowing us to measure the intrinsic properties of the promoters. Further work will be required to determine if and how the properties of MinSyns are modulated when used in combination with different sequence elements.
DATA AVAILABILITY
Plasmids and sequences have been submitted to the Addgene repository, ID numbers #154477 to #154753 inclusive. Scripts are available at https://github.com/YaominCai/MinSyn_model.
Supplementary Material
ACKNOWLEDGEMENTS
Y.C., K.K. and N.P. conceived the study. Y.C. performed and analysed all experiments with plant CREs. K.K., H.T. and Y.C. performed and analysed experiments with orthogonal TFs. Y.C. and G.G. analysed and visualized gene expression data. A.S. and Y.C. designed and optimized the ratiometric transient protoplast assay. Y.C. and N.P. wrote the manuscript and all authors commented and approved. N.P. was responsible for fundraising and supervision. Plasmids containing Level 0 DNA parts: GB0900 (Gal4-AD) and GB_UD_32AB (PhiC31), GB0036 (35s terminator) were a kind gift from the Orzaez laboratory.
Notes
Present address: Henry Tidd, Rothamsted Research, Harpenden, Hertfordshire AL5 2JQ, UK.
Contributor Information
Yao-Min Cai, Engineering Biology, Earlham Institute, Norwich Research Park, Norfolk NR4 7UZ, UK.
Kalyani Kallam, Engineering Biology, Earlham Institute, Norwich Research Park, Norfolk NR4 7UZ, UK.
Henry Tidd, Engineering Biology, Earlham Institute, Norwich Research Park, Norfolk NR4 7UZ, UK.
Giovanni Gendarini, Engineering Biology, Earlham Institute, Norwich Research Park, Norfolk NR4 7UZ, UK.
Amanda Salzman, Engineering Biology, Earlham Institute, Norwich Research Park, Norfolk NR4 7UZ, UK.
Nicola J Patron, Engineering Biology, Earlham Institute, Norwich Research Park, Norfolk NR4 7UZ, UK.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
UK Research and Innovation; Biotechnology and Biological Sciences Research Council [BBS/E/T/000PR9819, BB/R021554/1, BBS/E/T/00PR9815]. Funding for open access charge: Institute Funding.
Conflict of interest statement. None declared.
REFERENCES
- 1. International Service for the Acquisition of Agribiotech Applications (ISAAA) Brief 54: Global Status of Commercialized Biotech/GM Crops: 2018. 2018; NY. [Google Scholar]
- 2. Fox J.L. First plant-made biologic approved. Nat. Biotechnol. 2012; 30:472. [Google Scholar]
- 3. Sainsbury F. Innovation in plant-based transient protein expression for infectious disease prevention and preparedness. Curr. Opin. Biotechnol. 2020; 61:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vazquez-Vilar M., Orzaez D., Patron N.. DNA assembly standards: Setting the low-level programming code for plant biotechnology. Plant Sci. 2018; 273:33–41. [DOI] [PubMed] [Google Scholar]
- 5. de Lange O., Klavins E., Nemhauser J.. Synthetic genetic circuits in crop plants. Curr. Opin. Biotechnol. 2018; 49:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andres J., Blomeier T., Zurbriggen M.D.. Synthetic switches and regulatory circuits in plants. Plant Physiol. 2019; 179:862–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Lijsebettens M., Grasser K.D.. Transcript elongation factors: shaping transcriptomes after transcript initiation. Trends Plant Sci. 2014; 19:717–726. [DOI] [PubMed] [Google Scholar]
- 8. Swiezewski S., Liu F., Magusin A., Dean C.. Cold-induced silencing by long antisense transcripts of an Arabidopsis polycomb target. Nature. 2009; 462:799–802. [DOI] [PubMed] [Google Scholar]
- 9. Martínez de Alba A.E., Elvira-Matelot E., Vaucheret H.. Gene silencing in plants: a diversity of pathways. Biochim. Biophys. Acta. 2013; 1829:1300–1308. [DOI] [PubMed] [Google Scholar]
- 10. Merchante C., Stepanova A.N., Alonso J.M.. Translation regulation in plants: an interesting past, an exciting present and a promising future. Plant J. 2017; 90:628–653. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H., Jiang T.. Synthetic circuits, devices and modules. Protein Cell. 2010; 1:974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koncz C., De Greve H., André D., Deboeck F., Van Montagu M., Schell J.. The opine synthase genes carried by Ti plasmids contain all signals necessary for expression in plants. EMBO J. 1983; 2:1597–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Assaad F.F., Signer E.R.. Cauliflower mosaic virus P35S promoter activity in Escherichia coli. Mol. Gen. Genet. 1990; 223:517–520. [DOI] [PubMed] [Google Scholar]
- 14. Rüth J., Hirt H., Schweyen R.J.. The cauliflower mosaic virus 35S promoter is regulated by cAMP in Saccharomyces cerevisiae. Mol. Gen. Genet. 1992; 235:365–372. [DOI] [PubMed] [Google Scholar]
- 15. Sun L., Cai H., Xu W., Hu Y., Lin Z.. CaMV 35S promoter directs β-glucuronidase expression in Ganoderma lucidum and Pleurotus citrinopileatus. Mol. Biotechnol. 2002; 20:239–244. [DOI] [PubMed] [Google Scholar]
- 16. Vlasák J., Šmahel M., Pavlík A., Pavingerová D., Bříza J.. Comparison of hCMV immediate early and CaMV 35S promoters in both plant and human cells. J. Biotechnol. 2003; 103:197–202. [DOI] [PubMed] [Google Scholar]
- 17. Seternes T., Tonheim T.C., Myhr A.I., Dalmo R.A.. A plant 35S CaMV promoter induces long-term expression of luciferase in Atlantic salmon. Sci. Rep. 2016; 6:25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ebert P.R., Ha S.B., An G.. Identification of an essential upstream element in the nopaline synthase promoter by stable and transient assays. Proc. Natl. Acad. Sci. U.S.A. 1987; 84:5745–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benfey P.N., Chua N.H.. The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science. 1990; 250:959–966. [DOI] [PubMed] [Google Scholar]
- 20. Fang R.X., Nagy F., Sivasubramaniam S., Chua N.H.. Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell. 1989; 1:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhullar S., Chakravarthy S., Advani S., Datta S., Pental D., Burma P.K.. Strategies for development of functionally equivalent promoters with minimum sequence homology for transgene expression in plants: cis-elements in a novel DNA context versus domain swapping. Plant Physiol. 2003; 132:988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venter M. Synthetic promoters: genetic control through cis engineering. Trends Plant Sci. 2007; 12:118–124. [DOI] [PubMed] [Google Scholar]
- 23. Ali S., Kim W.C.. A fruitful decade using synthetic promoters in the improvement of transgenic plants. Front. Plant Sci. 2019; 10:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu R., Duan L., Pruneda-Paz J.L., Oh D.H., Pound M., Kay S., Dinneny J.R.. The 6xABRE synthetic promoter enables the spatiotemporal analysis of ABA-mediated transcriptional regulation. Plant Physiol. 2018; 177:1650–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jameel A., Noman M., Liu W., Ahmad N., Wang F., Li X., Li H.. Tinkering cis motifs jigsaw puzzle led to root-specific drought-inducible novel synthetic promoters. Int. J. Mol. Sci. 2020; 21:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schaumberg K.A., Antunes M.S., Kassaw T.K., Xu W., Zalewski C.S., Medford J.I., Prasad A.. Quantitative characterization of genetic parts and circuits for plant synthetic biology. Nat. Methods. 2015; 13:94–100. [DOI] [PubMed] [Google Scholar]
- 27. Zuo J., Chua N.H.. Chemical-inducible systems for regulated expression of plant genes. Curr. Opin. Biotechnol. 2000; 11:146–151. [DOI] [PubMed] [Google Scholar]
- 28. Brückner K., Schäfer P., Weber E., Grützner R., Marillonnet S., Tissier A.. A library of synthetic transcription activator-like effector-activated promoters for coordinated orthogonal gene expression in plants. Plant J. 2015; 82:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belcher M.S., Vuu K.M., Zhou A., Mansoori N., Agosto Ramos A., Thompson M.G., Scheller H.V., Loqué D., Shih P.M.. Design of orthogonal regulatory systems for modulating gene expression in plants. Nat. Chem. Biol. 2020; 16:857–865. [DOI] [PubMed] [Google Scholar]
- 30. Liu W., Stewart C.N.. Plant synthetic promoters and transcription factors. Curr. Opin. Biotechnol. 2016; 37:36–44. [DOI] [PubMed] [Google Scholar]
- 31. Salis H.M., Mirsky E.A., Voigt C.A.. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 2009; 27:946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalil A.S., Lu T.K., Bashor C.J., Ramirez C.L., Pyenson N.C., Joung J.K., Collins J.J.. A synthetic biology framework for programming eukaryotic transcription functions. Cell. 2012; 150:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amit R., Garcia H.G., Phillips R., Fraser S.E.. Building enhancers from the ground up: a synthetic biology approach. Cell. 2011; 146:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schlabach M.R., Hu J.K., Li M., Elledge S.J.. Synthetic design of strong promoters. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Redden H., Alper H.S.. The development and characterization of synthetic minimal yeast promoters. Nat. Commun. 2015; 6:7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kotopka B.J., Smolke C.D.. Model-driven generation of artificial yeast promoters. Nat. Commun. 2020; 11:2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharon E., Kalma Y., Sharp A., Raveh-Sadka T., Levo M., Zeevi D., Keren L., Yakhini Z., Weinberger A., Segal E.. Inferring gene regulatory logic from high-throughput measurements of thousands of systematically designed promoters. Nat. Biotechnol. 2012; 30:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klemm S.L., Shipony Z., Greenleaf W.J.. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 2019; 20:207–220. [DOI] [PubMed] [Google Scholar]
- 39. O’Malley R.C., Huang S.S.C., Song L., Lewsey M.G., Bartlett A., Nery J.R., Galli M., Gallavotti A., Ecker J.R.. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell. 2016; 165:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smaczniak C., Angenent G.C., Kaufmann K.. SELEX-seq: a method to determine DNA binding specificities of plant transcription factors. Methods Mol. Biol. 2017; 1629:67–82. [DOI] [PubMed] [Google Scholar]
- 41. Ricci W.A., Lu Z., Ji L., Marand A.P., Ethridge C.L., Murphy N.G., Noshay J.M., Galli M., Mejía-Guerra M.K., Colomé-Tatché M. et al.. Widespread long-range cis-regulatory elements in the maize genome. Nat. Plants. 2019; 5:1237–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hernandez-Garcia C.M., Finer J.J.. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014; 217–218:109–119. [DOI] [PubMed] [Google Scholar]
- 43. Rodríguez-Leal D., Lemmon Z.H., Man J., Bartlett M.E., Lippman Z.B.. Engineering quantitative trait variation for crop improvement by genome editing. Cell. 2017; 171:470–480. [DOI] [PubMed] [Google Scholar]
- 44. Jin J., Tian F., Yang D.C., Meng Y.Q., Kong L., Luo J., Gao G.. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic. Acids. Res. 2017; 45:D1040–D1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grant C.E., Bailey T.L., Noble W.S.. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011; 27:1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Petryszak R., Keays M., Tang Y.A., Fonseca N.A., Barrera E., Burdett T., Füllgrabe A., Fuentes A.M.P., Jupp S., Koskinen S. et al.. Expression Atlas update—an integrated database of gene and protein expression in humans, animals and plants. Nucleic Acids Res. 2016; 44:D746–D752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patron N.N.J., Orzaez D., Marillonnet S., Warzecha W., Matthewman C., Youles M., Raitskin O., Leveau A., Farré G., Rogers C. et al.. Standards for plant synthetic biology: a common syntax for exchange of DNA parts. New Phytol. 2015; 208:13–19. [DOI] [PubMed] [Google Scholar]
- 48. Vazquez-Vilar M., Quijano-Rubio A., Fernandez-Del-Carmen A., Sarrion-Perdigones A., Ochoa-Fernandez R., Ziarsolo P., Blanca J., Granell A., Orzaez D.. GB3.0: a platform for plant bio-design that connects functional DNA elements with associated biological data. Nucleic Acids Res. 2017; 45:2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patron N. DNA assembly for plant biology. Curr. Protoc. Plant Biol. 2016; 1:604–616. [DOI] [PubMed] [Google Scholar]
- 50. Yoo S.D., Cho Y.H., Sheen J.. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2007; 2:1565–1572. [DOI] [PubMed] [Google Scholar]
- 51. Raitskin O., Schudoma C., West A., Patron N.J.. Comparison of efficiency and specificity of CRISPR-associated (Cas) nucleases in plants: an expanded toolkit for precision genome engineering. PLoS One. 2019; 14:e0211598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al.. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012; 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lam E., Benfey P.N., Gilmartin P.M., Fang R.X., Chua N.H.. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc. Natl. Acad. Sci. U.S.A. 1989; 86:7890–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lam E., Katagiri F., Chua N.H.. Plant nuclear factor ASF-1 binds to an essential region of nopaline synthase promoter. J. Biol. Chem. 1990; 265:9903–9913. [PubMed] [Google Scholar]
- 55. Lam E., Lam Y. kam P.. Binding site requirements and differential representation of TGA factors in nuclear ASF-1 activity. Nucleic Acids Res. 1995; 23:3778–37785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang B., Chen W., Foley R.C., Buttner M., Singh K.B.. Interactions between distinct types of DNA binding proteins enhance binding to ocs element promoter sequences. Plant Cell. 1995; 7:2241–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sainsbury F., Lomonossoff G.P.. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol. 2008; 148:1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benfey P.N., Ren L., Chua N.H.. Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J. 1990; 9:1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kesarwani M., Yoo J., Dong X.. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol. 2007; 144:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zander M., La Camera S., Lamotte O., Métraux J.-P., Gatz C.. Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses. Plant J. 2010; 61:200–210. [DOI] [PubMed] [Google Scholar]
- 61. Zander M., Thurow C., Gatz C.. TGA transcription factors activate the salicylic acid-suppressible branch of the ethylene-induced defense program by regulating ORA59 expression. Plant Physiol. 2014; 165:1671–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Biddie S.C., John S., Sabo P.J., Thurman R.E., Johnson T.A., Schiltz R.L., Miranda T.B., Sung M.H., Trump S., Lightman S.L. et al.. Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Mol. Cell. 2011; 43:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Collins C., Wang J., Miao H., Bronstein J., Nawer H., Xu T., Figueroa M., Muntean A.G., Hess J.L.. C/EBPα is an essential collaborator in Hoxa9/Meis1-mediated leukemogenesis. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:9899–9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hörberg J., Reymer A.. BZip transcription factors modulate DNA supercoiling transitions. 2019; bioRxiv doi:13 December 2019, preprint: not peer reviewed 10.1101/2019.12.13.875146. [DOI] [PMC free article] [PubMed]
- 65. Grossman S.R., Engreitz J., Ray J.P., Nguyen T.H., Hacohen N., Lander E.S.. Positional specificity of different transcription factor classes within enhancers. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E7222–E7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reiter F., Wienerroither S., Stark A.. Combinatorial function of transcription factors and cofactors. Curr. Opin. Genet. Dev. 2017; 43:73–81. [DOI] [PubMed] [Google Scholar]
- 67. Deplancke B., Alpern D., Gardeux V.. The genetics of transcription factor DNA binding variation. Cell. 2016; 166:538–554. [DOI] [PubMed] [Google Scholar]
- 68. Stampfel G., Kazmar T., Frank O., Wienerroither S., Reiter F., Stark A.. Transcriptional regulators form diverse groups with context-dependent regulatory functions. Nature. 2015; 528:147–151. [DOI] [PubMed] [Google Scholar]
- 69. Hebbar P.B., Archer T.K.. Altered histone H1 stoichiometry and an absence of nucleosome positioning on transfected DNA. J. Biol. Chem. 2008; 283:4595–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mladenova V., Mladenov E., Russev G.. Organization of plasmid dna into nucleosome-like structures after transfection in eukaryotic cells. Biotechnol. Biotechnol. Equip. 2009; 23:1044–1047. [Google Scholar]
- 71. Curran K.A., Crook N.C., Karim A.S., Gupta A., Wagman A.M., Alper H.S.. Design of synthetic yeast promoters via tuning of nucleosome architecture. Nat. Commun. 2014; 5:4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de Boer C.G., Vaishnav E.D., Sadeh R., Abeyta E.L., Friedman N., Regev A.. Deciphering eukaryotic gene-regulatory logic with 100 million random promoters. Nat. Biotechnol. 2020; 38:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Le D.D., Shimko T.C., Aditham A.K., Keys A.M., Longwell S.A., Orenstein Y., Fordyce P.M.. Comprehensive, high-resolution binding energy landscapes reveal context dependencies of transcription factor binding. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E3702–E3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Engler C., Youles M., Grüetzner R.. A golden gate modular cloning toolbox for plants. ACS Synth. Biol. 2014; 3:839–843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Plasmids and sequences have been submitted to the Addgene repository, ID numbers #154477 to #154753 inclusive. Scripts are available at https://github.com/YaominCai/MinSyn_model.