Abstract
New Orleans’ first case of coronavirus disease 2019 (COVID-19) was reported on March 9, 2020, with a subsequent rapid increase in the number of cases throughout the state of Louisiana. Traditional educational efforts were no longer viable with social distancing and stay-at-home orders; therefore, virtual didactics were integrated into our curriculum. Due to an exponential increase in the number of patients with acute kidney injury requiring kidney replacement therapy, the nephrology sections at Louisiana State University School of Medicine and Tulane University School of Medicine adapted their clinical workflows to accommodate these increased clinical volumes by using prolonged intermittent kidney replacement therapies and acute peritoneal dialysis, as well as other strategies to mitigate nursing burnout and decrease scarce resource use. Telehealth was implemented in outpatient clinics and dialysis units to protect vulnerable patients with kidney disease while maintaining access to care. Lessons learned from this pandemic and subsequent response may be used for future responses in similar situations.
Index Words: AKI, COVID-19, New Orleans, ESKD
Introduction
New Orleans’ first case of coronavirus disease 2019 (COVID-19) was reported on March 9, 2020, approximately 2 weeks after Mardi Gras festivities culminated on February 25. This has led to speculation that Mardi Gras greatly accelerated what would become an explosive outbreak of COVID-19 in the New Orleans area.1 Within weeks, most of the city’s restaurants and bars shuttered their doors, effectively shutting down the tourism and hospitality industry on which the local economy depends.
By the time Governor John Bel Edwards issued a statewide stay-at-home order on March 23, Louisiana had more than 1,000 confirmed cases of COVID-19, with one of the highest per capita infection rates in the nation.2 Less than 2 weeks later, on April 3, the number of cases had increased to more than 10,000. With more than two-thirds of cases occurring in the New Orleans area, our local health care system became inundated with acutely ill patients.3,4 In an unprecedented response effort, health care providers from all backgrounds worked collaboratively to care for patients under extremely challenging and often bleak circumstances. Those of us working in nephrology faced the dual challenge of increasing our surge capacity to meet the demands for kidney replacement therapy (KRT) in the inpatient setting while simultaneously implementing measures in the outpatient dialysis setting to ensure the safety of patients and staff.
In many ways, COVID-19 has been a uniquely deadly disease for the New Orleans area, which at one point had the highest per capita death rate in the United States.2 Certainly our nephrology experience has been that kidney involvement from COVID-19 is associated with increased mortality, particularly when KRT is required.5 However, we would also support the observation made in the broader medical community that racial and social disparities have played a significant role in disproportionate mortality rates, in New Orleans in particular.6 This resounds from our prior experience with Hurricane Katrina in which socioeconomic risk factors led to increased vulnerability for poor outcomes.7 Although we collectively work to eradicate these disparities, we must ensure that they are properly addressed in future pandemic and disaster response plans to improve outcomes for vulnerable populations.
The following review details our experience dealing with COVID-19 in our 2 academic medical centers in New Orleans in regard to our educational and clinical experiences. Lessons learned from this pandemic and subsequent response may be used for future responses and in other geographic areas that experience a similar situation.
Fellowship and Teaching
The COVID-19 pandemic has required a major restructuring of both clinical and educational efforts within our respective nephrology departments. As reports emerged of COVID-19 spreading throughout the United States, both Louisiana State University (LSU) and Tulane medical schools enforced a mandatory quarantine period for individuals traveling from parts of Asia back to New Orleans. One faculty member at LSU was required to quarantine for 14 days on arrival to the United States, which necessitated urgent rearrangement of the faculty schedule. Additionally, several fellows required sick leave due to suspected or confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. To maintain sufficient inpatient service coverage, outpatient duties for both faculty and fellows were minimized to the extent possible, including clinics and dialysis rounds.
With more time spent on inpatient services and up to an 80% increase (generally 15-20 patients increased to 35-45 patients) in the number of nephrology consults across our institutions, there was significant concern for burnout in both faculty and fellows. Based on prior studies showing that courses in mindfulness-based stress reduction decrease clinician burnout, we introduced wellness and mindfulness sessions into our weekly didactic conferences and also held weekly Zoom (Zoom Video Communications) check-ins with fellows to ensure that their concerns were heard and addressed.8 One such session included a clinical psychologist from LSU School of Medicine educating the section on breathing exercises to decrease stress. After meeting with internal medicine residency leadership, we were able to recruit additional residents to assist fellows on the consult services. We also doubled our weekend inpatient coverage to include 2 fellows and 2 attendings where needed to reduce workload. As censuses increased across hospitals, faculty and fellows spent additional hours providing care to patients. The rotation structure and call schedule were otherwise unchanged.
With regard to educational efforts, all didactic sessions were transitioned into web-based audiovisual meetings using the Zoom platform. The originally planned curriculum largely was unchanged to minimize disruption in content delivery. With most of our inpatient consults being related to COVID-19, both fellows and faculty welcomed the opportunity to learn and teach about traditional nephrology topics.
Due to all medical students being dismissed from clinical services, 3 students who had previously been assigned to nephrology consult services or clinics no longer had the ability to experience nephrology. Knowing that students who are not exposed to nephrology are less likely to choose nephrology as a career, we implemented Zoom on inpatient rounds and in telehealth clinics so that students could engage with the team virtually.9 Before rounds on the consult service, students were assigned patients to perform chart reviews, and they presented those patients during rounds virtually to the team with a laptop that included webcam videoconference ability. The students were able to discuss the results of diagnostic tests and review assessments and plans with the fellow and attending. Although they were unable to do physical examinations, this still offered students the ability to participate in learning during their nephrology consult service block.
Acute Kidney Injury in COVID-19
The factors for developing acute kidney injury (AKI) with SARS-CoV-2 infection vary by region. Reports from China indicate a very low incidence of elevated creatinine levels or chronic kidney disease (CKD), while elsewhere the incidence is much higher—as high as 47% in the United States.10 In all reports, baseline elevation in creatinine levels was associated with a higher incidence of AKI. The overall incidence of AKI is reported to be 37% in the United States, with 14% of hospitalized patients requiring dialysis.11
At University Medical Center New Orleans, 256 SARS-CoV-2–positive patients were admitted between March 9 and March 30. Of these, 123 (48%) developed AKI (38% with stage 1, 23% with stage 2, and 39% with stage 3 by KDIGO [Kidney Disease: Improving Global Outcomes] criteria). Eighteen (7%) had moderate or severe underlying CKD (at least a creatinine level of 3.0 mg/dL at baseline, including end-stage kidney disease [ESKD]) before admission. Thirty-nine (15%) of all admitted SARS-CoV-2–positive patients who developed AKI required KRT. Seventy-four (29%) of the admitted SARS-CoV-2–positive patients required mechanical ventilation in the intensive care unit (ICU). Of these ventilated ICU patients, 59 (79.7%) developed AKI, with 27 (36.5%) requiring KRT. Sixteen (59.2%) ventilated patients receiving KRT died.
At Tulane Medical Center, 302 SARS-CoV-2–positive patients were admitted (41 in ICU) from March 1, 2020, to July 20, 2020. Of these, 113 patients (37%) developed AKI (43% with stage 1, 21% with stage 2, and 36% with stage 3). Twenty-four patients (8%) had moderate or severe underlying CKD (at least creatinine level of 3.0 mg/dL at baseline including ESKD) before admission. Thirty-six (12%) of the admitted SARS-CoV-2–positive patients required mechanical ventilation in the ICU. Of these ventilated ICU patients, 29 (80%) developed AKI, with 21 (58%) requiring dialysis. Sixteen (76%) ventilated patients receiving dialysis died. The presence of AKI severe enough to require dialysis is often seen in the setting of multisystem organ failure and “cytokine storm,” which may not always be the case for patients with ESKD. Our results in New Orleans support underlying CKD as a risk factor for AKI, need for KRT, and increased mortality.
The cause of AKI in COVID-19 appears to be multifactorial. As with any infection, acute tubular necrosis is prominent. However, there is also prominent interstitial infiltration of lymphocytes and macrophages.12 Interestingly, C5b-9 (the membrane attack complex of the alternate complement pathway) has been seen on tubules in autopsy specimens.13 The alternate complement pathway has been noted in SARS and a trial of eculizumab is under way in acute respiratory distress syndrome with COVID-19.14 The kidney expresses the receptor for SARS-CoV-2, angiotensin-converting enzyme 2 is present on the proximal tubule brush border and glomeruli.15 However, how the virus would gain entry from the tubule luminal side is unknown, as is the mechanism of necessary virus cleavage (a process known as priming) though the enzyme furin (but not the TMPRSS2) is present.16 SARS-CoV-2 virions have been found in kidney tubules and podocytes,12 though this has been questioned.17,18 Viral RNA has also been detected in the proximal tubule.19
This has generated the hypothesis that direct toxicity of the virus on kidney tubule cells is important. Other studies suggest a podocytopathy. In a case report from our institution, a patient developed nephrotic-range proteinuria and fairly new onset of severe AKI.20 The biopsy showed collapsing focal and segmental glomerulosclerosis and was homozygous for an APOL1 (apolipoprotein L1) risk allele. Subsequently the patient’s COVID-19 nasopharyngeal swab was positive. This raises the possibility that COVID-19, like HIV, can be the “second hit” that triggers focal segmental glomerulosclerosis in susceptible patients.21,22 Other factors proposed in the cause of COVID-19 AKI include tissue hypoxia, coagulopathy, and rhabdomyolysis12; we have also anecdotally observed rhabdomyolysis.
Inpatient Experience
During the course of the pandemic, nearly all hospitals in the New Orleans area saw a tremendous surge in the demand for KRT for both patients with AKI and ESKD. When comparing data between March 15, 2020, and April 15, 2020, with the same period in 2019, there was a 47.3% total increase in inpatient dialysis treatments across the city, including a 261.5% increase in continuous KRT treatments and 7.4% increase in peritoneal dialysis (PD) treatments.
In anticipation of this surge, early attempts to increase capacity included spacing out intermittent hemodialysis (HD) treatments in patients with ESKD with significant residual kidney function, shortening treatment times to 2 to 3 hours, and facilitating discharge to outpatient facilities when medically appropriate.
Initially, all persons under investigation (PUIs) and confirmed positive patients were treated in a 1:1 isolation setting requiring acute HD nurses to spend many hours performing these treatments. A 71.1% increase in 1:1 HD treatments was seen during the time frame noted. Eventually, due to the inability to keep up with the demand of 1:1 HD treatments, both patients with AKI and those with ESKD requiring HD with confirmed COVID-19 were cohorted to the last shift of the day following regular and PUI shifts. Droplet precautions were maintained in the dialysis unit and staff wore full personal protective equipment according to Centers for Disease Control and Prevention (CDC) recommendations.
The University Medical Center New Orleans also provides dialysis services for undocumented immigrants and some other uninsured patients who come to the emergency department. During the surge, this placed an added burden on the already stressed hospital dialysis unit. This also led to the concern of exposure to SARS-CoV-2 for patients who were dialyzing in the hospital acute unit.
ICU Dialysis Considerations
Providing KRT to COVID-19–infected patients in the ICU has been challenging. With our first cases of AKI requiring KRT, it became clear that our standard therapy of continuous venovenous HD would not be sustainable for 2 main reasons. First, the hypercoagulability that has been widely observed in COVID-19 led to such excessive clotting of hemofilters that it was impossible to keep patients on dialysis long enough to provide a therapeutic benefit. Second, we could not meet the clinical demand for KRT by running our 6 NxStage machines continuously for each patient (Table 1). After clotting persisted despite a trial of a systemic anticoagulation with heparin, continuous venovenous HD was abandoned for a period in favor of intermittent HD with vasopressor support. Although this significantly reduced both the time spent at the bedside and personal protective equipment use for ICU nursing staff, it also led to suboptimal volume management in some patients who had significant obligate intake. It also imposed a significant burden on our HD nurses given the large numbers of patients needing 1:1 HD. Following the release of American Society of Nephrology guidelines on March 21 for the management of KRT in hospitalized patients, prolonged intermittent KRT was suggested for select ICU patients.23 By using a combination of prolonged intermittent KRT and HD modalities, with HD reserved for the more hemodynamically stable patients, we were able to provide KRT to more patients on a daily basis. We also noted significantly less clotting with prolonged intermittent KRT due to the use of higher blood flows in conjunction with systemic anticoagulation with heparin.
Table 1.
University Medical Center New Orleans ICU and Dialysis Volumes Before COVID-19 and During COVID-19 Peak
| Before March 9 | Peak of COVID-19 Surge | |
|---|---|---|
| ICU staffed bed capacity | 44 | 70 |
| HD machines | 12 | 14 |
| PD cyclers | 3 | 8 |
| CKRT machines | 3 | 6 |
| Dialysis nurses | 10 | 14 |
Abbreviations: CKRT, continuous kidney replacement therapy; COVID-19, coronavirus disease 2019; HD, hemodialysis; ICU, intensive care unit; PD, peritoneal dialysis.
Acute PD
As COVID-19 cases increased, the demand for intermittent HD and continuous KRT outpaced our ability to continue supplying those therapies. For this reason, alternative strategies such as using acute PD for AKI were implemented in the hospital. A number of studies from Brazil and Saudi Arabia showed acceptable rates of clearance and ultrafiltration using acute PD to treat AKI.24, 25, 26 The LSU acute PD protocol was developed, implemented, and distributed among nephrologists around the New Orleans metropolitan area using the guidelines of the International Society of Peritoneal Dialysis.27 A surgeon or interventional radiologist was identified at each hospital with experience in placing PD catheters. Rather than placing PD catheters in the operating room, surgeons placed PD catheters at the bedside in the ICU on ventilated patients using a bedside mini-laparotomy technique with cut down to rectus muscle and subsequent tunneling of the catheter. Patient selection was another factor to initiate acute PD. Patients who were mechanically ventilated and required prone positioning were considered poor candidates for acute PD, though it has been reported previously.28 Patients with acute abdominal infection, severe hyperkalemia, severe volume overload, or a history of extensive abdominal surgery were also considered poor candidates. Using low dwell volumes in the supine position, automated cycler PD was initiated. ICU patients received 24 hours of therapy, whereas patients on the ward received 12 hours of therapy. Ultimately, 13 patients used acute PD for AKI.
Outpatient Experience
Because New Orleans was one of the first cities in the United States to experience an outbreak of COVID-19, we had to improvise before the large dialysis organizations (LDOs) provided significant guidance. Similar to the strategies used by colleagues in Seattle,29 we began by screening patients for symptoms and isolating or cohorting them within their usual dialysis shift. Subsequently, upon recommendations from 1 LDO, 1 dialysis unit was designated to provide dialysis services for all PUIs and another unit for all confirmed COVID-19–infected patients, each on separate shifts that had previously been open. At another LDO, 1 shift was identified to dialyze all PUIs as well as confirmed patients, moving all other patients to other shifts. Regardless, neither plan was ideal because of the potential for PUIs and positive patients to be mixed before the advent of rapid testing. Since rapid testing has been made available, we have been able to isolate and dialyze a PUI and by the next treatment know their actual COVID-19 status.
With regard to patients receiving home modalities, we felt a duty to keep them safe at home without the need for unnecessary exposure. Within 1 week of the first COVID-19 case in Louisiana, we implemented telehealth visits with ∼80% of our home dialysis patients. We were able to consolidate all dialysis unit visits (for laboratory draws, adequacy, and medication administration) to 1 visit per month and have been successful in maintaining this frequency of visits. These lead to minimal SARS-CoV-2 exposure for our home dialysis patients.
Patients with CKD were also transitioned to telehealth. Routine visits for patients with CKD stages 1-3 were largely postponed after a telephone call to check in with them. Patients with more advanced CKD or issues requiring more urgent evaluation were scheduled for either a telehealth or in-person visit according to their preference.
Another major challenge in caring for patients with CKD and ESKD was obtaining and troubleshooting new dialysis vascular accesses, including arteriovenous fistulas and grafts. Early in the COVID-19 outbreak, hospital administrators mandated that elective surgeries be avoided to curb the spread of the virus based on guidance from the Centers for Medicare & Medicaid Services (CMS).30 This left us unable to get arteriovenous fistula/graft placements for our patients with CKD and ESKD. On March 26, CMS released a statement clarifying that planned procedures to establish long-term vascular access are essential procedures.31 PD catheter placement and HD catheters are considered urgent and therefore are being placed. If the clinical ramifications of the current pandemic persist for a prolonged period and vascular access procedures are further postponed, there will be a large backlog of patients who never received adequate vascular access for outpatient HD. The health implications of initiating countless new dialysis patients with catheters could be extensive from an infectious and vascular standpoint.
Louisiana Response and Plateau of Wave One
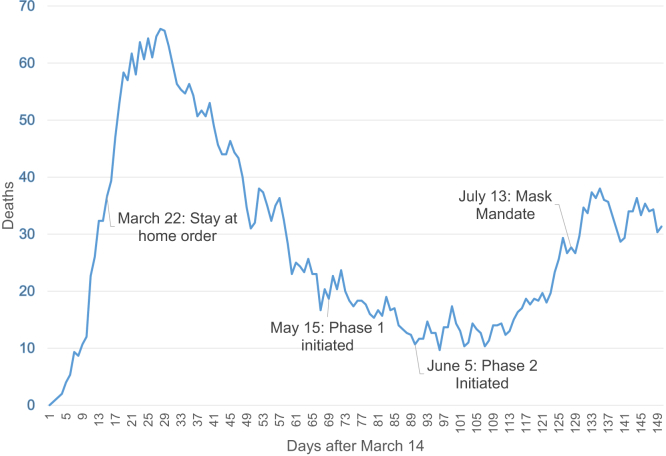
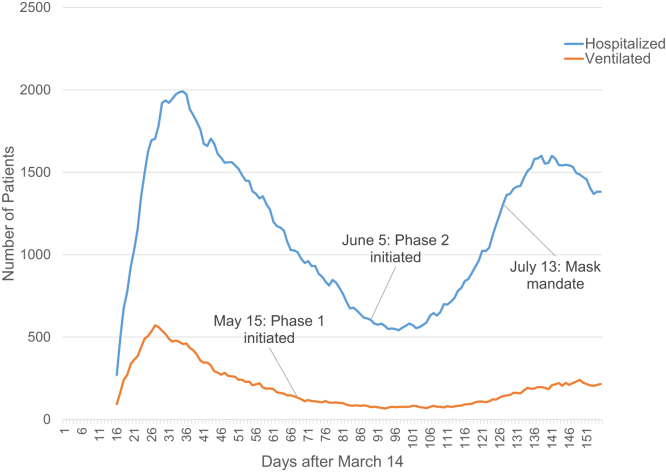
Louisiana very rapidly became one of the epicenters of viral impact in the United States. The implementation of drive-through testing centers began the week of March 13, although the number of tests was limited at first. Our Department of Health maintained excellent and transparent communication regarding rates of infection as well as access to testing and treatment, and we also received daily correspondence from our academic institutions, our governor, the CDC, and the LDOs. Orleans and nearby Jefferson parishes began seeing evidence of a plateauing of cases by April 15. The daily death rate rapidly increased in the initial period after the first positive patient was identified, with a downward trend over the next few weeks, but a small increase as a second peak of deaths occurred (Fig 1). Concurrent with the daily death rate, we also saw a rapid increase in hospitalized patients followed by a downward trend with a second smaller peak. Mechanical ventilation followed a similar trend but the second peak was much flatter (Fig 2).
Figure 1.
Daily number of deaths in Louisiana due to coronavirus disease 2019 (COVID-19). First reported death was on March 14 and the figure demonstrates days after March 14.
Figure 2.
Louisiana statewide number of coronavirus disease 2019 (COVID-19) hospitalized (blue) and ventilated (red) patients. The figure demonstrates rates on days after March 14.
Conclusion
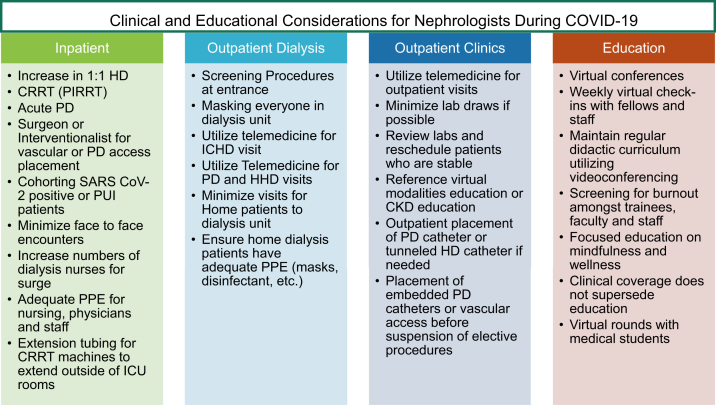
It would be an understatement to describe this COVID-19 pandemic as anything short of the most significant event to involve the medical community in more than a generation. Likewise, our experience within the nephrology community has been extremely challenging. The notion that dialysis is an essential service and requires patients to frequently trust us by risking exposure to the virus is not to be taken lightly; we have a responsibility to ensure the safety of our patients receiving maintenance dialysis and avoid hospitalizations. The surge of incident AKI episodes within our institutions was a burden on our resources, and this required frequent communication between institutions and services. There were many changes and disruptions to an academic program, including transitioning conferences to a virtual format, cancelling other conferences outright, COVID-19 illness among our trainees, and the need to make concessions to avoid burnout due to coverage and patient load (Fig 3). Ultimately, what allowed us to sustain our practice within this first wave of virus cases was the state’s order for Louisianans to stay at home, social distancing, better sanitation, and the work of the Louisiana Department of Health. There are still yet many unknowns but we must find opportunities in this lull to start answering them.
Figure 3.
Clinical and educational considerations for nephrologists during coronavirus disease 2019 (COVID-19). Abbreviations: CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; HD, hemodialysis; ICHD, in-center hemodialysis; ICU, intensive care unit; PD, peritoneal dialysis; PIRRT, prolonged intermittent renal replacement therapy; PPE, personal protective equipment; PUI, person under investigation; SARS CoV-2, severe acute respiratory syndrome coronavirus 2.
Article Information
Authors’ Full Names and Academic Degrees
Mihran Naljayan, MD, MHA, Farshid Yazdi, MD, MSPH, Sarah Struthers, MD, Moh’d Sharshir, MD, Amanda Williamson, MBA, and Eric E. Simon, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received May 10, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form September 13, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Reckdahl K., Robertson C., Faust R. The New York Times; 2020. New Orleans faces a virus nightmare, and Mardi Gras may be why.https://www.nytimes.com/2020/03/26/us/coronavirus-louisiana-new-orleans.html Accessed June 22, 2020. [Google Scholar]
- 2.Wagner G. COVID-19 scenarios for Louisiana. https://github.com/gary-wagner/louisiana_covid19 Published April 3, 2020. Updated 2020. Accessed April 22, 2020.
- 3.Louisiana Department of Health COVID-19. http://ldh.la.gov/Coronavirus/ Updated 2020. Accessed April 17, 2020.
- 4.The Atlantic Monthly Group The Covid Tracking Project. https://covidtracking.com/ Updated 2020. Accessed April 18, 2020.
- 5.Mohamed M., Lukitsch I., Torres-Ortiz A. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney 360. 2020;1(7):614–622. doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losh J., Plyer A. Demographics of New Orleans and early COVID-19 hot spots in the U.S. https://www.datacenterresearch.org/covid-19-data-and-information/demographic-data/ Published March 25, 2020. Updated 2020. Accessed April 18, 2020.
- 7.Zoraster R.M. Vulnerable populations: Hurricane Katrina as a case study. Prehosp Disaster Med. 2010;25(1):74–78. doi: 10.1017/s1049023x00007718. [DOI] [PubMed] [Google Scholar]
- 8.Dobkin P.L., Bernardi N.F., Bagnis C.I. Enhancing clinicians’ well-being and patient-centered care through mindfulness. J Contin Educ Health Prof. 2016;36(1):11–16. doi: 10.1097/CEH.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 9.Nair D., Pivert K.A., Baudy A., 4th, Thakar C.V. Perceptions of nephrology among medical students and internal medicine residents: a national survey among institutions with nephrology exposure. BMC Nephrol. 2019;20(1):146. doi: 10.1186/s12882-019-1289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gralinski L.E., Sheahan T.P., Morrison T.E. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5) doi: 10.1128/mBio.01753-18. e01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. National Library of Medicine Eculizumab (soliris) in Covid-19 infected patients (SOLID-C19) https://clinicaltrials.gov/ct2/show/NCT04288713 Updated 2020. Accessed August 22, 2020.
- 15.Mizuiri S., Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4(1):74–82. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batlle D., Soler M.J., Sparks M.A. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calomeni E., Satoskar A., Ayoub I., Brodsky S., Rovin B.H., Nadasdy T. Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. 2020;98(1):233–234. doi: 10.1016/j.kint.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller S.E., Brealey J.K. Visualization of putative coronavirus in kidney. Kidney Int. 2020;98(1):231–232. doi: 10.1016/j.kint.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puelles V.G., Lutgehetmann M., Sperhake J.P. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.d.A. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H., Larsen C.P., Hernandez-Arroyo C.F. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol. 2020;31(8):1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma Y., Nasr S.H., Larsen C.P., Kemper A., Ormsby A.H., Williamson S.R. COVID-19-associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2(4):493–497. doi: 10.1016/j.xkme.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Society of Nephrology Recommendations on the care of hospitalized patients with COVID-19 and kidney failure requiring renal replacement therapy. https://www.asn-online.org/g/blast/files/AKI_COVID-19_Recommendations_Document_03.20.2020.pdf Published March 20, 2020. Accessed April 18, 2020.
- 24.Al-Hwiesh A., Abdul-Rahman I., Finkelstein F. Acute kidney injury in critically ill patients: a prospective randomized study of tidal peritoneal dialysis versus continuous renal replacement therapy. Ther Apher Dial. 2018;22(4):371–379. doi: 10.1111/1744-9987.12660. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel D.P., Caramori J.T., Martim L.C., Barretti P., Balbi A.L. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int Suppl. 2008;108:S87–S93. doi: 10.1038/sj.ki.5002608. [DOI] [PubMed] [Google Scholar]
- 26.Ponce D., Berbel M.N., Regina de Goes C., Almeida C.T., Balbi A.L. High-volume peritoneal dialysis in acute kidney injury: indications and limitations. Clin J Am Soc Nephrol. 2012;7(6):887–894. doi: 10.2215/CJN.11131111. [DOI] [PubMed] [Google Scholar]
- 27.Cullis B., Abdelraheem M., Abrahams G. Peritoneal dialysis for acute kidney injury. Perit Dial Int. 2014;34(5):494–517. doi: 10.3747/pdi.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klisnick A., Souweine B., Filaire M. Peritoneal dialysis in a patient receiving mechanical ventilation in prone position. Perit Dial Int. 1998;18(5):536–538. [PubMed] [Google Scholar]
- 29.Watnick S., McNamara E. On the frontline of the COVID-19 outbreak: keeping patients on long-term dialysis safe. Clin J Am Soc Nephrol. 2020;15(5):710–713. doi: 10.2215/CJN.03540320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. https://www.cms.gov/newsroom/press-releases/cms-releases-recommendations-adult-elective-surgeries-non-essential-medical-surgical-and-dental Published March 18, 2020. Accessed April 20, 2020.
- 31.White D. Critical clarification from CMS: PD catheter and vascular access placement is essential. https://www.kidneynews.org/policy-advocacy/leading-edge/critical-clarification-from-cms-pd-catheter-and-vascular-access-placement-is-essential Published March 26, 2020. Accessed April 18, 2020.