Abstract
Purpose
This study was performed to investigate the effect of miRNA let-7a-5p on the proliferation, invasion, and migration of human hepatoma cells as well as to determine BZW2 expression in these cells.
Methods
Western blotting and real-time quantitative polymerase chain reaction were used to detect changes in the expression of miRNA let-7a-5p and BZW2 protein and gene, respectively. A luciferase reporter gene assay was used to examine whether BZW2 is the target gene of miR-let-7a-5p. The effect of miR-let-7a-5p on the invasion, migration, and proliferation of human hepatoma Bel-7404 and HepG2 cells was determined using the transwell invasion assay, scratch test, and CCK-8 assay, respectively. Flow cytometry was used to assess the effect of miR-let-7a-5p and BZW2 expression on apoptosis of hepatoma cells.
Results
The luciferase reporter gene assay identified BZW2 as the target gene of miR-let-7a-5p. Moreover, increased expression of miR-let-7a-5p was found to significantly decrease BZW2 expression; inhibit proliferation, invasion, and migration; and promote apoptosis of hepatoma cells.
Conclusion
miR-let-7a-5p can inhibit proliferation, invasion, and migration as well as promote apoptosis of hepatoma cells by decreasing BZW2 expression.
Keywords: liver cancer, cell migration, cell proliferation, apoptosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors that poses a serious threat to the health and life of people; moreover, HCC has a high mortality rate.1,2 At present, surgical resection and liver transplantation are primarily used for the treatment of liver cancer or hepatoma. Moreover, only 20% of the patients with hepatoma quality for surgical resection. In recent years, there has been a considerable improvement in the surgical procedures and treatment methods for hepatoma; however, factors such as low immune function, incomplete surgical resection, poor chemotherapy response, and strong drug resistance have resulted in high recurrence and metastasis of these tumors.3 The traditional method for the treatment of hepatoma is not ideal; moreover, the lack of early diagnosis indicators hinders the early diagnosis and treatment of liver cancer. Therefore, identifying the cause of hepatic malignant transformation or its surface-specific markers can help improve the understanding of tumor pathogenesis, early diagnosis, and targeted therapy using specific markers.4
The leucine zipper consists of a series of leucine heptad repeats in a coiled-coil α-helical structure that forms a dimer. Two leucine zippers form a dome-shaped structure, in which the zipper forms the backbone and the two basic regions symmetrically form the DNA-binding arm, a bZIP structural motif. The bZIP domain plays an important role in the development of cancer and epidermal cells.5,6 The BZW2 gene is well conserved among species and shares high sequence homology among species such as humans, mice, rats, zebrafish, orangutans, dogs, and chickens. The 1259-bp long human BZW2 gene encodes 419 amino acids.7 Bioinformatics has revealed that this gene is highly expressed in the heart muscle tissue. Furthermore, BZW2 has been shown to be closely related to malignant tumor metastasis.
Our previous study demonstrated for the first time that BZW2 is an oncogene for HCC.8 BZW2 is highly expressed in hepatoma cells and tissues, and its overexpression can inhibit the effect of rapamycin and promote phosphorylation of the PI3K/AKT/mTOR signaling pathway. Moreover, the recognition sequence of miRNA let-7a-5p was predicted in the 3′-UTR region of BZW2, suggesting that BZW2 expression might be regulated by miR-let-7a-5p.8 It is well known that there are several types of non-coding RNA molecules with different functions in eukaryotes.9–12 These RNA molecules do not express proteins but play important regulatory roles in gene transcription at the post-transcriptional and translational levels. MicroRNAs (miRs) are important non-coding RNA molecules that are highly conserved and play important roles in cell proliferation, growth, apoptosis, and differentiation by regulating the expression of its target genes.13,14 Simultaneously, miRs can promote the invasion and migration of cancer stem cells as well as regulate the tumor microenvironment. Studies have found that grape seed extract (GSE) has anti-cancer properties in the early development of liver cancer, induced cell apoptosis, and reduced cell proliferation15; and other studies have found that certain medicinal plant extracts have anti-proliferative activity and can be used as HCC treatment potentially effective anti-tumor agents.16 Using the second-generation gene sequencing technology, previous studies have demonstrated that the expression of BZW2 in hepatoma tissues is significantly higher than that in normal liver tissues. In addition, bioinformatics analysis showed that miR-let-7a-5p can bind to the 3′-UTR region of BZW2.
There have been literature reports on let-7 and tumor pathogenesis. For example, studies have found that the let-7 family is in a low-level state in a variety of tumor cells, and it can inhibit tumor growth and metastasis.17,18 In order to further investigate the changes in the expression of miRNA let-7a-5p in liver cancer tissues, we used the liver cancer expression profile data in the GEO database to analyze and found that the expression of miRNA let-7a-5p in liver cancer tissues decreased significantly, and the expression of BZW2 Significant increase. Studies have shown that overexpression of miRNA let-7a-5p can inhibit the expression of X-linked inhibitor of apoptosis protein (XIAP) and high mobility protein A2 (HMGA2), thereby inhibiting the development and proliferation of liver cancer.19 Through further analysis of qRT-PCR, we found that there is a significant negative correlation between miRNA let-7a-5p and BZW2 mRNA expression. We speculate that the decrease in the expression of miRNA let-7a-5p may partly explain the mechanism of the up-regulation of BZW2 expression in liver cancer tissues. Thus, the present study was conducted to investigate whether miR-let-7a-5p can affect the biological characteristics of hepatoma cells by regulating BZW2 expression.
Materials and Methods
Cell Culture and Reagents
Human hepatoma cell lines Bel-7404 and HepG2 were obtained from Keygentec Biotech Inc. (Nanjing, China). Cells were cultured in RPMI/DMEM containing 10% fetal bovine serum at 37 °C and 5% CO2. Fetal bovine serum and RPMI/DMEM were purchased from HyClone and BZW2 monoclonal antibody from Abcam (abl54193). Transwell chambers were purchased from Millipore (USA) and Matrigel from BD Biosciences (USA). Lipofectamine 2000 and miR-let-7a-5p mimic were purchased from Shanghai Genechem Co., Ltd., and TRIzol from Ambion (USA). The reverse transcription kit (FSQ-101) was purchased from Toyobo Corporation (Japan) and the PCR kit from Kapa (USA). Sorafenib and DMSO were purchased from Sigma. Sorafenib was dissolved in 100% DMSO, and a stock solution of 6 μM was prepared with DMEM. For in vitro studies, the final concentration of DMSO was set to 0.1%.
Protein Extraction and Western Blot Analysis
Western blotting was used to measure protein expression following target gene knockdown or overexpression and subsequently determine the interference effect on the target. Lysates were extracted from cultured cells using protein extraction reagent (Beyotime, Shanghai, China). The protein concentration was determined using the BCA method and proteins were denatured using the loading buffer. Protein samples (20 µg) were electrophoresed in 10% SDS-PAGE gels. The separated proteins were transferred to nitrocellulose membranes or polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), which were blocked with 5% non-fat milk and incubated overnight with rabbit anti‐BZW2 (1:2000, Sigma) and rabbit anti‐GAPDH (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) primary antibodies at 4 °C. On the following day, the membranes were incubated with goat anti-rabbit secondary antibody (1:5000, Sigma) for 2 h at 25 °C. The protein bands were visualized using the Pierce™ ECL Western Blotting Substrate Kit (#32106; Thermo Fisher Scientific, Waltham, MA, USA). All Western blotting experiments were performed three times.
RNA Extraction and Real‐Time Quantitative Polymerase Chain Reaction (qPCR)
Real-time qPCR was used to determine changes in BZW2 expression under various concentrations of miR-let-7a-5p. Total cellular RNA was extracted according to the instructions of TRIzol reagent kit and RNA concentration was determined using an ultra-micro spectrophotometer. The following primers were purchased from GeneCopoeia, Inc.: BZW2, sense: 5′-ATAGGAGCACACCCCTTGC-3′, anti-sense: 5′-CACTTTGTCGCCCATTATCC-3′; miR-let-7a-5p, sense: 5′-TACCCTGAGAAC-CGAATTTGTG-3′, anti-sense: 5′- CCCAAGCTTA-AAAACCTCCACCACGAAT-3′; and β-actin, sense: 5′-TGACTTCAACAGCGACACCCA-3′, anti-sense: 5′- CACCCTGTTGCTGTAGCCAAA-3′. The cDNA was reverse transcribed from 100 ng of total RNA according to the instructions of the Kapa PCR kit. The PCR conditions were as follows: 37 °C for 15 min and 98 °C for 5 min. mRNA expression was calculated using the equation RQ = 2-ΔΔCT. All qPCR experiments were performed three times.
Cell Transfection
Logarithmic phase hepatoma cells Bel-7404 and HepG2 were transfected with miR-let-7a-5p mimic and negative control, following the instruction of the Lipofectamine 2000 transfection kit. The qPCR assay was used to measure the expression of miR-let-7a-5p following transfection (Figure S1). The sequence of the shRNA targeting miR-let-7a-5p was 5′-TGAGGTAGTAGGTTGTATAGTT-3′. shControl and sh-miR-let-7a-5p lentiviral infection of Bel-7404 and HepG2 cells were monitored microscopically after 48 h. Wt-GLO-BZW2-3UTR sequence: 5′-GCTAGCCTTGGGACTCTACCTCTCA-3′. The nucleotides highlighted in red were mutated to design Mut-GLO-BZW2-3UTR. The partial sequence of this construct was 5′-GCTAGCCTTGGGACTTCGAAGTTCA-3′.
Luciferase Reporter Assays
The 3erase RepBZW2 was labeled with luciferase (Rluc-BZW2 3′-UTR WT/MUT) and co-transfected with miR-let-7a-5p into hepatoma cells seeded in 96-well plates to confirm whether BZW2 is the target gene of miR-let-7a-5p. After 24 h of transfection, cells were washed twice with phosphate-buffered saline (PBS) and treated with 50 µL 1× Dual-Glo Luciferase Assay Reagent. The 96-well plate was shaken at 20–25 °C for 15 min before recording luminescence intensity using the luminescence detector at 10 s (expression of Firefly luciferase). Thereafter, the reaction was terminated by adding 100 μL Stop & Glo reagent, and the luminescence intensity was recorded at 10 s after mixing (the expression level of Renilla luciferase).
Cell Invasion Assay
The transwell invasion assay was performed to determine the invasive ability of miR-let-7a-5p on hepatoma cells. All reagents and equipment were pre-cooled on ice. A transwell chamber evenly coated with 50 µL Matrigel (0.2 µg/µL) was placed in a 24-well plate and incubated at 37 °C for 15 min to solidify the gel. The cells were trypsinized and suspended in serum-free medium at 2.5×104 cells/mL. According to the standard procedure, 200 μL of the cell suspension was added to the upper chamber of the transwell unit, and 500 μL of the medium containing 10% FBS was added to the lower chamber. The transwell unit was subsequently placed in a 37 °C incubator for culture. Thereafter, cells were fixed in formaldehyde and stained with crystal violet for 15 min. A cotton swab was used to gently wipe the inner membrane of the transwell unit. Cells that passed through the filter membrane were enumerated under a microscope using four high-power fields (40×).
Cell Scratch Assay
A scratch test was performed to determine the effect of miR-let-7a-5p on the migration ability of hepatoma cells. Bel-7404 and HepG2 cells were seeded in 6-well plates and allowed to attain approximately 90% confluence. Thereafter, sterile 200-μL pipette tips were used to scratch the cell layer from the top under a microscope. The distance of the scratch wound was measured at 0, 24, 48, and 72 h, and images were acquired to calculate the migration rate of the cells.
Cell Proliferation Assay
The CCK-8 assay was used to examine the effect of miR-let-7a-5p on the proliferation of hepatoma cells. Bel-7404 and HepG2 cells were seeded into a 96‐well plate at a density of 1×104 per well and divided into the following groups: non-specific control (NC), miR-let-7a-5p control, miR-let-7a-5p mimic, shControl, and shRNA miR-let-7a-5p. There were four four replicate wells for each group. Cells were allowed to grow until they reached 50–60% confluency, following which 10 μL CCK-8 was added to each well. The absorbance (OD) of each well was measured at 24, 48, 72, and 96 h at 490 nm.
Cell Apoptosis Assay
Flow cytometry was used to determine the effect of miR-let-7a-5p on the apoptosis of hepatoma cells. Bel-7404 or HepG2 cells were seeded in 6-well plates. The medium from each well of the 6-well plate was collected in a 15 mL conical tube. While cells in suspension were collected by centrifugation at 2000 rpm for 5 min, adherent cells were collected by digestion with trypsin without EDTA. The collected cells were centrifuged at 1000 rpm for 3 min, washed twice with PBS, and re-centrifuged at 1000 rpm for 3 min. Next, 1×106 cells/mL were suspended in the binding buffer to which Annexin-V and PI were added and gently mixed. The cells were incubated at room temperature for 15 min, following which 400 μL binding buffer was added to each sample tube.
Statistical Analysis
The data are expressed as 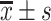 . The mean values between the two groups were compared using t-test. Differences among multiple groups were examined using analysis of variance. Statistical analysis was performed using the SPSS 18.0 software. Results with P<0.05 were considered statistically significant.
. The mean values between the two groups were compared using t-test. Differences among multiple groups were examined using analysis of variance. Statistical analysis was performed using the SPSS 18.0 software. Results with P<0.05 were considered statistically significant.
Results
Protein and mRNA Expression of BZW2 in Hepatoma Cells
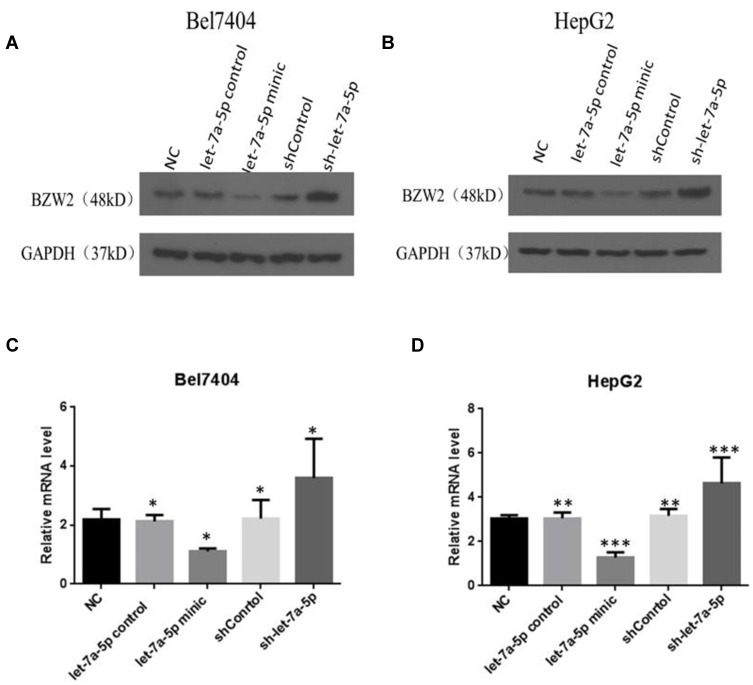
Western blotting results suggested that (Figure 1A and B) BZW2 protein expression decreased following treatment with high miR-let-7a-5p level. Moreover, BZW2 protein expression was increased following the inhibition of miR-let-7a-5p. According to the results of qPCR, BZW2 expression in the let-7a-5p mimic group was lower than that in the control group. However, in the sh-let-7a-5p group, BZW2 expression was higher than that in the control group (Figure 1C and D). These results indicate that BZW2 mRNA expression was significantly downregulated at increased levels of miR-let-7a-5p. Meanwhile, BZW2 mRNA expression was significantly increased upon the inhibition of miR-let-7a-5p.
Figure 1.
BZW2 protein expression level in liver cancer cells detected by Western blotting. In the let-7a-5p mimic group, BZW2 expression decreased; in the sh-let-7a-5p group, BZW2 expression increased (A and B). BZW2 gene expression level in liver cancer cells detected by qPCR. Relative to the control group, in the let-7a-5p mimic group, BZW2 gene expression decreased; in the sh-let-7a-5p group, BZW2 gen expression increased (C and D). *Indicates that the difference between the treated and control cells was statistically significant (P < 0.05). **Indicates that the difference between the treated and control cells was statistically significant (P < 0.01). ***Indicates that the difference between the treated and control cells was statistically significant (P < 0.001).
miR-let-7a-5p Targets 3ʹ-UTR Region of BZW2 Gene
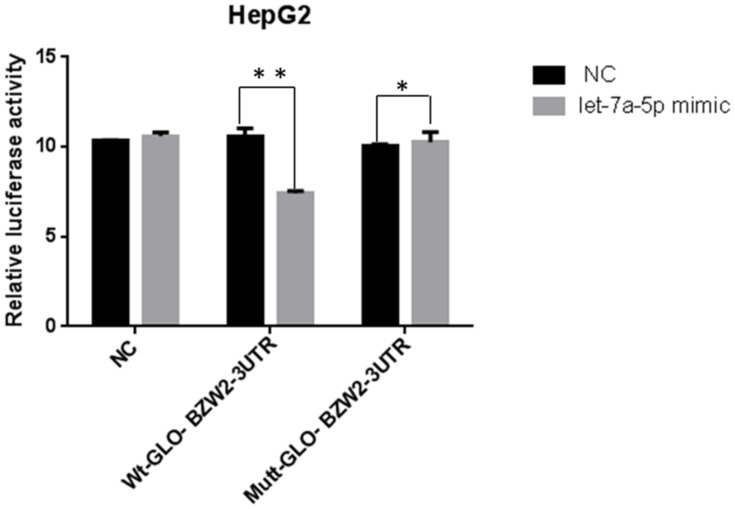
The 3nsxpression was BZW2 was labeled with luciferase to construct Rluc-BZW2 3′-UTR WT/MUT and co-transfected with miR-let-7a-5p into hepatoma cells. Cells were also co-transfected with Wt-GLO-BZW2-3UTR and miR-let-7a-5p mimic. The results showed that miR-let-7a-5p decreased BZW2 expression in the mutant group to a higher extent than that in the control group (Figure 2). Therefore, BZW2 is targeted by miR-let-7a-5p.
Figure 2.
Detection results of luciferase after transfection of hepatoma cells with Wt-GLO-BZW2-3UTR. miRNA let-7a-5p down-regulated BZW2 compared to the control group. *Indicates that the difference between the treated and control cells was statistically significant (P < 0.05). **Indicates that the difference between the treated and control cells was statistically significant (P < 0.01).
miR-let-7a-5p Inhibits Invasion of Human Hepatoma Cells
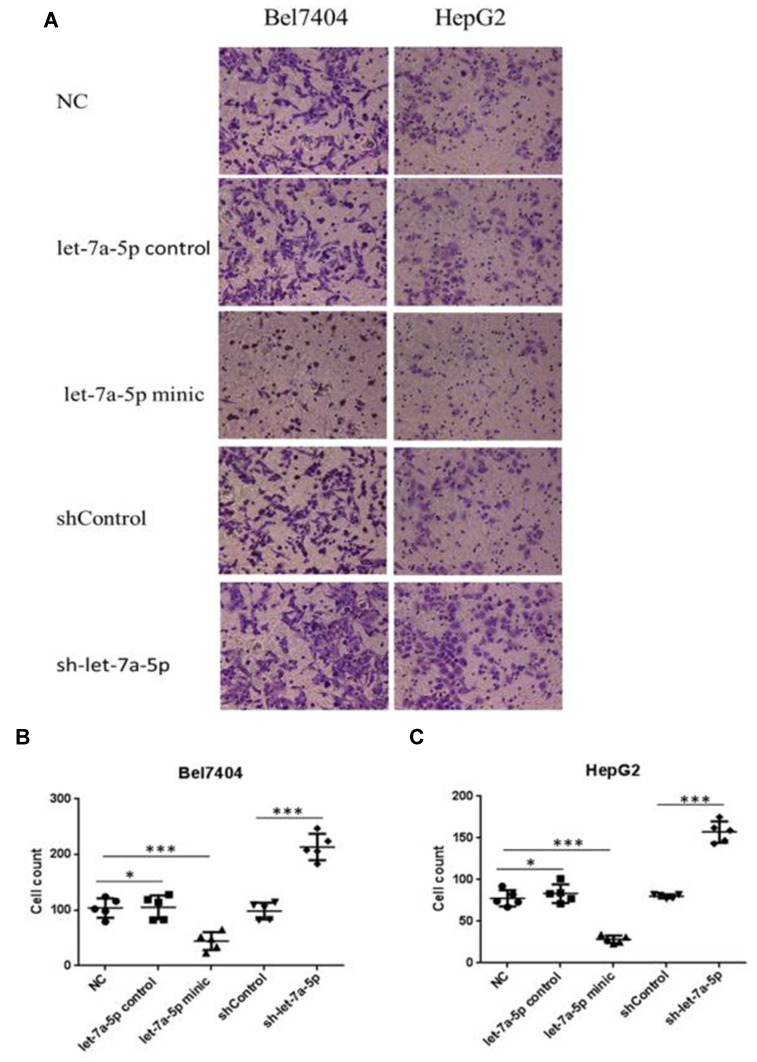
The ability of cells to penetrate the Matrigel can indicate cell invasion capacity. The degree of cell invasion in the miR-let-7a-5p mimic group was lower than that in the miR-let-7a-5p control group. Moreover, the degree of cell invasion in the sh-miR-let-7a-5p group was higher than that in the shControl group (p<0.01; Figure 3A–C). These results suggest that high expression of miR-let-7a-5p inhibits the invasion of human hepatoma cells.
Figure 3.
The invasion rate of Bel7404 and HepG2 cells in the let-7a-5p mimic group was lower than that in the let-7a-5p control group; the invasion rate of Bel7404 and HepG2 cells in the sh-let-7a-5p group was higher than that in the shControl group (A). Quantitative analysis of migration ability in Bel7404 cells (B). Quantitative analysis of migration ability in HepG2 cells (C). *Indicates that the difference between the treated and control cells was statistically significant (P < 0.05). ***Indicates that the difference between the treated and control cells was statistically significant (P < 0.001). The ability of human hepatoma cells to penetrate the Matrigel can indicate cell invasion capacity.
miR-let-7a-5p Reduces Migration of Human Hepatoma Cells
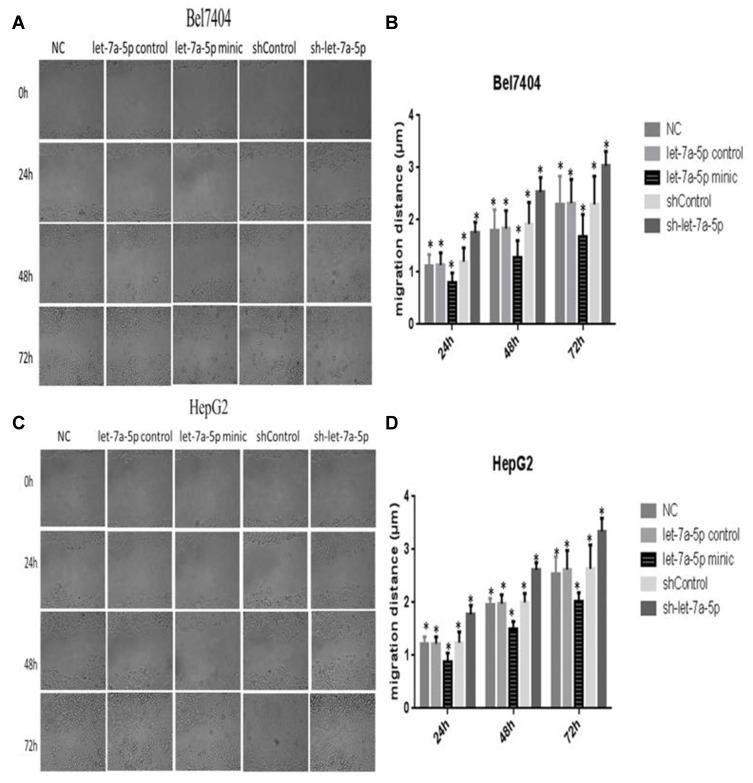
The cell scratch width at three arbitrary sites in each group was measured at 0, 24, 48, and 72 h under a microscope, and the migration distance was calculated as [D(t =24, 48, or 72 h)–D(t=0 h)]/D(t=0 h). The results of the scratch test revealed that the migration distances in the miR-let-7a-5p mimic group at 24, 48, and 72 h were notably shorter than those in the miR-let-7a-5p control group, whereas the migration distances in the sh-miR-let-7a-5p group were longer than those in the shControl group (Figure 4A–D). These results indicate that high expression of miR-let-7a-5p can reduce the migration of human hepatoma cells.
Figure 4.
The migration distance of Bel7404 cells in the let-7a-5p mimic group was lower than that in the let-7a-5p control group, and the migration distance of cells in the sh-let-7a-5p group was higher than that in the shControl group (A). Quantitative analysis of migration ability in Bel7404 cells (B). The migration distance of HepG2 cells in the let-7a-5p mimic group was lower than that in the let-7a-5p control group, and the migration distance of cells in the sh-let-7a-5p group was higher than that in the shControl group (C). Quantitative analysis of migration ability in HepG2 cells (D). *Indicates that the difference between the treated and control cells was statistically significant (P < 0.05).
miR-let-7a-5p Reduces Proliferation of Human Hepatoma Cells
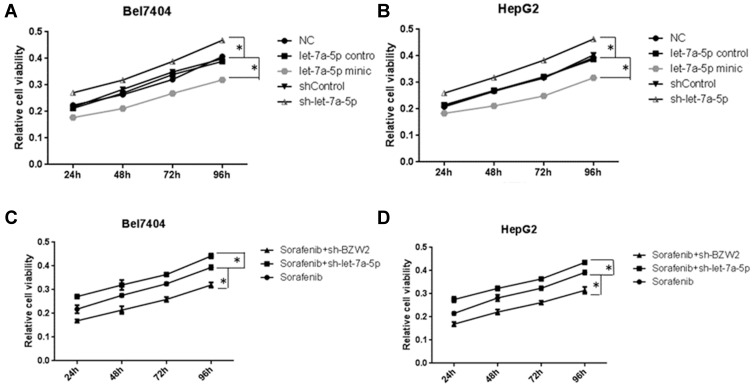
Because miR-let-7a-5p targets BZW2 in HCC, we investigated whether miR-let-7a-5p can affect the proliferation of hepatoma cells. The cellular absorbance at 450 nm in the miR-let-7a-5p mimic and sh-miR-let-7a-5p groups was significantly lower and higher, respectively, than that in the NC group (Figure 5A and B) (P<0. 01). These results suggested that high expression of miR-let-7a-5p can inhibit the proliferation of human hepatoma cells. After treatment with 6 μM sorafenib for 24, 48, 72, and 96 h, CCK-8 was used to assess the effect of silencing miR-let-7a-5p and BZW2 on the sensitivity of hepatoma cells to sorafenib. The cell viability of the sorafenib+sh-miR-let-7a-5p group was higher than that of the sorafenib group, whereas the cell viability of the sorafenib+shBZW2 group was lower than that of the sorafenib group (Figure 5C and D). These results suggest that BZW2 promotes proliferation and miR-let-7a-5p inhibits the proliferation of hepatoma cells. Moreover, miR-let-7a-5p can promote the drug sensitivity of hepatoma cells.
Figure 5.
The relative viability of cells in the let-7a-5p mimic group was lower than that in the NC group, and the relative viability of the cells in the sh-let-7a-5p group was higher than that in the NC group (A and B). Effect of interfering with miRNA let-7a-5p and BZW2 expression on the proliferation of liver cancer cells under sorafenib treatment. Compared with the sorafenib group, the cell viability was increased in the sorafenib+shlet-7a-5p group and decreased in the sorafenib+shBZW2 group (C and D). *Indicates that the difference between the treated and control cells was statistically significant (P < 0.05).
miR-let-7a-5p Promotes Apoptosis of Human Hepatoma Cells
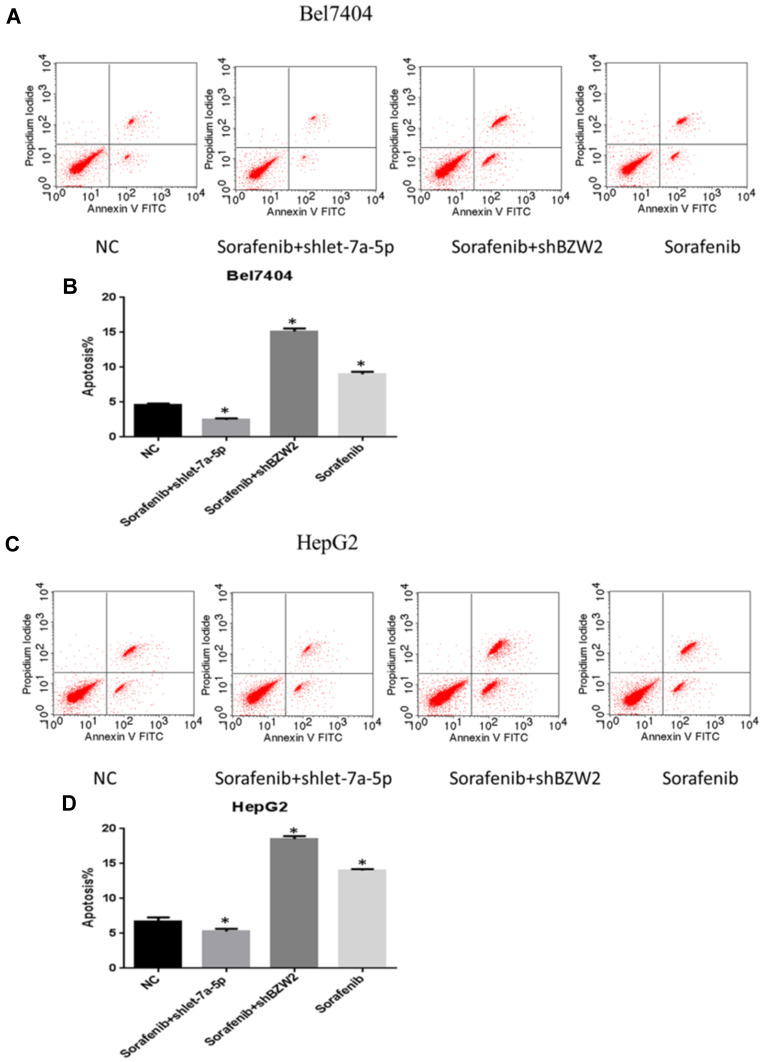
Sorafenib, a multi-kinase inhibitor with antiproliferative, anti-angiogenic, and pro-apoptotic properties, is the only effective first-line drug approved for the treatment of advanced HCC. Despite its efficacy in increasing the survival of patients with HCC, its long-term success is limited owing to the development of resistant cells through several mechanisms.20 The effect of miR-let-7a-5p and BZW2 on apoptosis of hepatoma cells was measured using flow cytometry. miR-let-7a-5p and BZW2 promoted the apoptosis of sorafenib-treated Bel-7404 and HepG2 cells. The number of apoptotic cells in the sorafenib and sorafenib+shBZW2 groups was higher than that in the control group (Figure 6A–D). However, shRNA-mediated knockdown of miR-let-7a-5p reduced apoptosis of hepatoma cells and decreased sensitivity to sorafenib.
Figure 6.
Effect of interfering with the expression of miRNA let-7a-5p and BZW2 on apoptosis of liver cancer cells under drug treatment. Let-7a-5p and BZW2 affects apoptosis in Bel7404 and HepG2 cells treated with sorafenib. Compared with the control group, the cells that were treated with sorafenib alone and the sorafenib+shBZW2 group had increased apoptosis. After knocking let-7a-5p down, hepatocellular carcinoma cells reduced apoptosis and decreased sensitivity to sorafenib (A and C). Quantitative analysis of migration ability in Bel7404 cells (B). Quantitative analysis of migration ability in HepG2 cells (D). *Indicates that thedifference between the treated and control cells was statistically significant (P < 0.05).
Discussion
miRs are endogenous, highly conserved non-coding single-stranded RNAs that are extensively distributed in plant and metazoan genomes. Upon processing by Drosha and Dicer enzymes, mature miRs can bind to the 3′-UTR of target genes to suppress their translation or mark for degradation. miR-et-7a-5p is located between the HOXD4 and HOXD8 genes on chromosome 2p311. Once pre-miR-let-7a-5p enters the cytoplasm, it is processed by the Dicer into the mature miR-let-7a-5p. miR-10b can bind to the RNA-induced silencing complex (RISC) to form the asymmetrical RISC, which can suppress mRNA translation or degrade mRNA and regulate cell metabolism.21,22
Numerous studies have shown that miR-let-7a-5p expression is correlated with the invasive capacity of various malignant tumors. Liu et al23 used a PCR-based assay to demonstrate that miR-let-7a-5p expression was markedly high in esophageal carcinoma and para-carcinoma tissues. Kim et al24 also showed that miR-let-7a-5p expression in metastatic breast cancer cells MDA-MB-231 and SUM1315 is higher than that in non-metastatic breast cancer cell MCF-7. Subsequently, SUM149 and SUM159 were selected for ectopic implantation in nude mice. Consequently, tumor metastases were observed in SUM149 and SUM159 models, but not in the control group, suggesting that miR-let-7a-5p promotes breast cancer metastasis.
Tumor metastasis refers to the condition in which the attachment of the tumor to the vascular endothelium results in vascular endothelial injury, cancer embolus attachment, and invasion into the sub-endothelium.25 BZW2 is found in the vascular subendothelial matrix of the tumor tissue, and can promote platelet-vascular endothelial cell adhesion, platelet-tumor cell adhesion, as well as cancer embolus formation. Therefore, BZW2 plays an important role in tumor metastasis.
Another study showed that BZW2 protein and gene expression increases with a concomitant increase in the tumor grade, suggesting that BZW2 plays an important role in liver cancer progression.8 Herein, human hepatoma cells Bel-7404 and HepG2 were transfected with an miR-let-7a-5p mimic to upregulate miR-let-7a-5p expression. The results of this study demonstrated that BZW2 expression is markedly downregulated at high levels of miR-let-7a-5p. Simultaneously, the proliferation, invasion, and migration of Bel-7404 and HepG2 cells were also inhibited.
Conclusion
The findings of this study indicate that miR-let-7a-5p can suppress hepatoma cell proliferation, invasion, and migration by downregulating BZW2 expression. Therefore, miR-let-7a-5p can be potentially used as a marker to predict liver cancer progression and evaluate therapeutic efficacy. However, further studies are required to understand the precise mechanism of action.
Acknowledgments
The present study was supported by the Natural Science Foundation of Hunan Province (grant no.2018JJ3830) and the Graduate Independent Innovation Project of Central South University (grant no. 2019zzts1044).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Choi KJ, Baik IH, Ye SK, Lee YH. Molecular targeted therapy for hepatocellular carcinoma: present status and future directions. Biol Pharm Bull. 2015;38(7):986–991. doi: 10.1248/bpb.b15-00231 [DOI] [PubMed] [Google Scholar]
- 2.Shaaban S, Negm A, Ibrahim EE, Elrazak AA. Chemotherapeutic agents for the treatment of hepatocellular carcinoma: efficacy and mode of action. Oncol Rev. 2014;8(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrero JA. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33(Suppl S 01):S3–10. doi: 10.1055/s-0033-1333631 [DOI] [PubMed] [Google Scholar]
- 4.Sartorius K, Sartorius B, Aldous C, Govender PS, Madiba TE. Global and country underestimation of hepatocellular carcinoma (HCC) in 2012 and its implications. Cancer Epidemiol. 2015;39(3):284–290. doi: 10.1016/j.canep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 5.Seldeen KL, McDonald CB, Deegan BJ, Bhat V, Farooq A. DNA plasticity is a key determinant of the energetics of binding of Jun-Fos heterodimeric transcription factor to genetic variants of TGACGTCA motif. Biochemistry. 2009;48(51):12213–12222. doi: 10.1021/bi901392k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye J, Koumenis C. ATF4, an ER stress and hypoxia-inducible transcription factor and its potential role in hypoxia tolerance and tumorigenesis. Curr Mol Med. 2009;9(4):411–416. doi: 10.2174/156652409788167096 [DOI] [PubMed] [Google Scholar]
- 7.Loughran G, Firth AE, Atkins JF, Ivanov IP. Translational autoregulation of BZW1 and BZW2 expression by modulating the stringency of start codon selection. PLoS One. 2018;13(2):e0192648. doi: 10.1371/journal.pone.0192648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin X, Liao M, Zhang L, Yang M, Zhao J. Role of the novel gene BZW2 in the development of hepatocellular carcinoma. J Cell Physiol. 2019;234(9):16592–16600. doi: 10.1002/jcp.28331 [DOI] [PubMed] [Google Scholar]
- 9.Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6(3):235–246. doi: 10.1158/2159-8290.CD-15-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17(12):719–732. [DOI] [PubMed] [Google Scholar]
- 11.Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer - an emerging concept. EBioMedicine. 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SN, Chang R, Lin LT, et al. MicroRNA in ovarian cancer: biology, pathogenesis, and therapeutic opportunities. Int J Environ Res Public Health. 2019;16(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G, Yin L, Ouyang X, Zeng K, Xiao Y, Li Y. M2 macrophages promote HCC cells invasion and migration via miR-149-5p/MMP9 signaling. J Cancer. 2020;11(5):1277–1287. doi: 10.7150/jca.35444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Tan X, Huang Z, Chen Z, Lin P, Fu SW. microRNA biomarkers in colorectal cancer liver metastasis. J Cancer. 2018;9(21):3867–3873. doi: 10.7150/jca.28588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamza AA, Heeba GH, Elwy HM, Murali C, El-Awady R, Amin A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: in vivo and in vitro analyses. Sci Rep. 2018;8(1):1270. doi: 10.1038/s41598-018-19492-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Dabbagh B, Elhaty IA, Al Hrout A, et al. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement Altern Med. 2018;18(1):240. doi: 10.1186/s12906-018-2285-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balzeau J, Menezes MR, Cao S, Hagan JP. The LIN28/let-7 pathway in cancer. Front Genet. 2017;8:31. doi: 10.3389/fgene.2017.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elghoroury EA, ElDine HG, Kamel SA, et al. Evaluation of miRNA-21 and miRNA let-7 as prognostic markers in patients with breast cancer. Clin Breast Cancer. 2018;18(4):e721–e726. doi: 10.1016/j.clbc.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 19.Wu WY, Tao SQ, Wang XN, Lobie PE, Wu ZS. XIAP 3ʹ-untranslated region serves as a competitor for HMGA2 by arresting endogenous let-7a-5p in human hepatocellular carcinoma. Tumour Biol. 2017;39(7):1010428317719578. doi: 10.1177/1010428317719578 [DOI] [PubMed] [Google Scholar]
- 20.Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50(10):1–9. doi: 10.1038/s12276-018-0159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifer BJ, Su D, Taylor HS. Circulating miRNAs in murine experimental endometriosis. Reprod Sci. 2017;24(3):376–381. doi: 10.1177/1933719116667228 [DOI] [PubMed] [Google Scholar]
- 22.Fu Y, Sun LQ, Huang Y, et al. miR-142-3p inhibits the metastasis of hepatocellular carcinoma cells by regulating hmgb1 gene expression. Curr Mol Med. 2018;18(3):135–141. doi: 10.2174/1566524018666180907161124 [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Lv GD, Qin X, et al. Role of microRNA let-7 and effect to HMGA2 in esophageal squamous cell carcinoma. Mol Biol Rep. 2012;39(2):1239–1246. doi: 10.1007/s11033-011-0854-7 [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Shin JY, Lee KD, et al. MicroRNA let-7a suppresses breast cancer cell migration and invasion through downregulation of C-C chemokine receptor type 7. Breast Cancer Res. 2012;14(1):R14. doi: 10.1186/bcr3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W, Tang H, Tang L. MicroRNA-34a inhibits metastasis in liver cancer cells. Oncol Lett. 2018;16(6):6960–6965. [DOI] [PMC free article] [PubMed] [Google Scholar]