Abstract
AIM
To study of corneal biomechanical properties and intraocular pressure (IOP) measured with Corvis Scheimpflug Technology (ST) in patients with childhood glaucoma (CG).
METHODS
Cross-sectional study in which 89 eyes were included 56 of them with CG. Only one eye per patient was included. The following variables were obtained from the clinical history and the ophthalmological examination: age, sex, IOP, number of surgeries, and the cup/disc ratio (CDR). The following parameters were recorded using Corvis ST: corrected by biomechanics IOP (bIOP), not corrected IOP (nctIOP), central corneal thickness (CCT), maximum concavity [radius, peak distance (PD) and deformation amplitude], applanation 1 and 2 (length and velocity). The mean age was 23±14.55 and 33±19.5 years old for the control group and CG group, respectively. Totally 36 were males and 53 were females. In the CG group, 7 patients were controlled only with medical treatment. Sixteen had at least one previous goniotomy, 19 had at least one trabeculectomy, and 11 had an Ahmed implant.
RESULTS
A significant and positive intraclass correlation coefficient was found between Goldman IOP and the IOP measured by Corvis in both groups. No differences were found between the IOP measured with Corvis and Goldman using a student t-test. Regarding biomechanical parameters, there were differences in the applanation length 2 (A-L2), in the applanation velocity 2 (A-V2) and in the PD. By sex, only the applanation length 1 (A-L1) was found to be different in control group. A positive and significant Pearson correlation was found between CDR and the A-L1.
CONCLUSION
Corneal biomechanical properties have shown differences between CG and healthy subjects and also between men and women.
Keywords: childhood glaucoma, biomechanical properties, Corvis Scheimpflug Technology
INTRODUCTION
Childhood glaucoma (CG) is the main cause of blindness in children[1]. There are several studies that show how the biomechanical properties of the cornea change in patients with primary open angle glaucoma (POAG). This has been proved using different devices such as Ocular Response Analyzer (ORA; Reichert Ophthalmic Instrument) and Corvis Scheimpflug Technology (ST)[2]. With ORA, it has been reported that corneal hysteresis (CH) and corneal resistance factor (CRF) are significantly reduced in patients with POAG compared to normal subjects[3]–[4]. But there has been little emphasis in the literature on the influence of corneal biomechanical properties in CG patients.
Patients with CG used to have corneas with bigger diameters, and irregular shapes, and major changes in the anterior segment. Moreover, CG patients might require, in some cases, surgical interventions such as corneal transplant.
In these circumstances it has been proved that applanation tonometry induces errors[5]. In addition, an error frequently attributable to applanation tonometry is the influence of central corneal thickness (CCT) and the absence of any reliable formula to correct this error. However, it is well known that other corneal properties apart from CCT influence the intraocular pressure (IOP) measurement. That is the reason why there is no agreement on establishing a validated and useful correction for Goldman tonometry and CCT as it would be an incomplete method. Recent studies show how intrastromal corneal ring surgery modified the IOP measurement. This measurement varies depending on the method used[6]–[7]. Therefore, there is a need to look for another method that can overcome these difficulties. This could be the Corvis ST.
Corvis ST records the reaction of the cornea to an air pulse using a Scheimpflug camera with 4330 frames per second and provides coverage over a horizontal range of 8.5 mm, at the same time, measures the IOP value independent from the biomechanical properties as well as the corneal thickness.
In previous studies the Corvis ST showed good precision (repeatability and reproducibility) for both IOP measurements and for dynamic corneal response parameters in healthy eyes[8]–[9]. In glaucomatous eyes, it is possible that the Corvis ST may reflect the corneal biomechanical changes. Many studies have reported increased stiffness of ocular structures in glaucoma patients[10]–[11].
The aim of this study was to compare the corneal biomechanical properties between healthy subjects and CG patients. Moreover, the measured of IOP with Goldmann tonometer and Corvis ST was compared. Finally, the differences in the corneal biomechanical between genders were also analyzed.
SUBJECTS AND METHODS
Ethical Approval
The study protocol was approved by the institution review board of our Hospital following the guidelines of the Declaration of Helsinki. The number of the certificate of approval given by our Hospital was 18/467-E. Informed consent was obtained from each participant older than 18 years old and from the legal guardian for subjects younger than 18 years old, with assent by children older than 12y before inclusion in the study.
We performed a case–control study in which consecutive patients with CG were screened at the Glaucoma Service of Clínico San Carlos University Hospital in Madrid for a period of 2mo, between October 2018 and November 2018. Only patients diagnosed with CG, following the diagnostic criteria established at the 5th International Congress of Glaucoma were included[1]. The inclusion criteria consisted on the presence of at least two of the following clinical features: increased corneal diameter (>12 mm) along with elevated IOP (>21 mm Hg or >16 mm Hg under general anaesthesia) and/or Haab striae, corneal edema, glaucomatous optic disc head appearance and glaucomatous visual fields (when available).
Exclusion criteria were the presence of any other ocular pathology different from CG, any corneal alteration that makes the test impossible to perform, intellectual disability or any other limitation for obtaining informed consent and participation in any other research study in the 6mo before starting the study. The age of the patients and the treatment given were not the exclusion criteria.
Controls were recruited from the staff of the same institutions and healthy relatives of the patients. Before including relatives, an exhaustive exploration was carried out, and it was verified that they were healthy. Only one eye per subject was analyzed. In the case of controls, we have only included one of the two eyes, fixing the right eye for this. In the case of CG, we have included both because the disease is very asymmetric.
All participants underwent a full eye examination. The visual acuity was tested using decimal visual acuity charts. The anterior chamber was examined with slit-lamp biomicroscopy using a 90 diopter lens for the examination of the posterior pole. The cup/disc ratio (CDR) was measured at the slit-lamp using a handheld high power convex lens of 90 diopters. The slit beam was coaxial with the observation axis, a narrow beam was used to measure the vertical disc diameter using the inner margin of the white Eisching's ring as reference. And after a correction factor of 1.36 was used. The CDR value was measured following the criteria of the European Guidelines of Glaucoma fourth edition.
Perkins tonometry (Haag-Streit, Koniz, Switzerland) was used after instillation of one drop of Fluotest (topical fluorescein 0.25% and oxybuprocain 0.4%) to measure the IOP. The number of surgeries was taken from the medical records of the patient.
The Corvis ST, was used to measure the corneal biomechanical properties during the air puff. Tow specific moments should be recorded: the corneal applanation and the highest concavity. Because of the viscoelastic properties of the cornea, there are two applanation moments. One is when the length and the velocity are measure (A-V1, A-V2), (A-L1, A-L2) and the other when the moment of highest concavity of the cornea is reached. The following measures were obtained (highest concavity time); central curvature radius at the highest concavity (highest concavity curvature); distance between the two surrounding peaks of the cornea at the highest concavity [peak distance (PD)], and maximum deformation amplitude (DA; measured from the start of deformation to the highest concavity) of the corneal apex[1].
We first take the IOP with Corvis ST and then we take it with Goldman/Perkins.
RESULTS
The age range was 5-73y in the control group and 5-54y in the group of children with glaucoma.
The mean age was 23±14.55 and 33±19.5 years old for the control group and CG group, respectively. The 36 were males and 53 were females. In the case of controls, we have only included one of the two eyes, fixing the right eye for this. In the case of CG, we have included both because the disease is very asymmetric. Primary congenital glaucoma (PCG) was the most frequent diagnosis reaching the 47 patients.
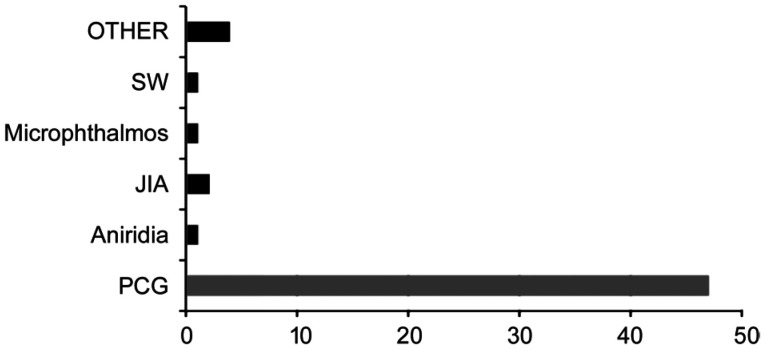
In addition, 9 patients were classified as secondary CG, being 2 patients of the patients associated with congenital microphthalmos; 1 patient with glaucoma secondary to juvenile idiopathic arthritis (JIA); 2 patients glaucoma patients associated with Sturge Weber Syndrome (SW); 1 patient with aniridia; and 4 patients with other type of secondary CG (Figure 1).
Figure 1. Varieties of childhood glaucoma.
SW: Sturge Weber Syndrome; JIA: Juvenile idiopathic arthritis; PCG: Primary congenital glaucoma.
Regarding the medical treatment used, 15 patients were using prostaglandins and 28 patients had used them at some time in their treatment. In relation to the surgical treatment, all subjects except 7 eyes with CG had undergone some type of surgery. Among those, 16 had at least one previous goniotomy, 19 had at least one trabeculectomy, and 11 had an Ahmed implant.
Related to the corneal biomechanical parameters those were measured by the Corvis ST for the study population (Table 1). Three out of 7 biomechanical parameters were significantly different between eyes with CG and control eyes: A-L2, A-V2 and in PD.
Table 1. Corneal biomechanics comparison between healthy subjects and CG.
| Measurements |
t-test for equality of means |
|||||||
| Sig. | t | Df | Sig.(2-tailed) | Mean difference | Std. error difference | 95%CI of the difference |
||
| lower | upper | |||||||
| A-L1 | 0.199 | -1.51 | 78 | 0.13 | -0.10 | 0.07 | -0.25 | 0.03 |
| A-L2 | 0.001 | -1.5 | 65.85 | 0.13 | -0.15 | 0.10 | -0.36 | 0.05 |
| A-V1 | 0.159 | -0.44 | 78 | 0.65 | -0.03 | 0.06 | -0.16 | 0.10 |
| A-V2 | 0.000 | -3.97 | 60.09 | 0.00 | -0.04 | 0.01 | -0.06 | -0.02 |
| P. dist | 0.002 | 0.81 | 77 | 0.42 | 0.09 | 0.12 | -0.14 | 0.33 |
| Radius | 0.220 | -0.57 | 78 | 0.56 | -0.46 | 0.80 | -2.06 | 1.13 |
| Def. amp. | 0.152 | -0.39 | 78 | 0.69 | -0.08 | 0.22 | -0.52 | 0.35 |
CG: Childhood glaucoma; A-L1: Applanation length 1; A-L2: Applanation length 2; A-V1: Applanation velocity 1; A-V2: Applanation velocity 2; P. dist: Peak distance; Def. amp: Deformation amplitude; Df: Degrees of freedom; Sig.: Significance; Std.: Standard. Independent samples t test.
The A-L2 being 1.88±0.25 mm and 2.04±0.64 mm in controls and GC respectively (P=0.00). The A-V2 being -0.27±0.02 in control group and -0.22±0.07 in CG group (P=0.00). And in the PD 5.02±0.04 in control group and 4.92±0.09 in CG group (P=0.00). Comparison of IOP measurements recorded with Corvis ST [corrected by biomechanics IOP (bIOP)] and Perkins tonometer revealed a mean difference in the control group of 0.63±0.6 (P=0.3) and in the GC group 0.34±0.91 (P=0.71). However, this did not reach statistical significance, although a tendency towards higher IOP measurement by Corvis ST was noted.
The intraclass correlation coefficient (ICC) between the IOP measured with the Goldman tonometer (reference) and the Corvis ST was significant and positive in both groups 0.51, P=0.00 in the glaucoma group and 0.32, P=0.04 in the control group.
Small differences have been found related to the sex. Only the A-L1 was found to be different in control group. It was higher in males. The A-L1 was 2.45±0.06 and 2.23±0.09 in males and women respectively (P=0.00; Table 2).
Table 2. Corneal biomechanics comparison by sex.
| Groups |
t-test for equality of means |
|||||||
| Sig. | t | Df | Sig.(2-tailed) | Mean difference | Std. error difference | 95%CI of the difference |
||
| Lower | Upper | |||||||
| Control group | ||||||||
| A-L1 | 0.001 | 1.91 | 30 | 0.06 | 0.21 | 0.11 | -0.01 | 0.45 |
| A-L2 | 0.287 | -0.502 | 27.66 | 0.62 | -0.04 | 0.09 | -0.23 | 0.14 |
| A-V1 | 0.946 | -0.73 | 29.88 | 0.47 | 0 | 0 | -0.01 | 0 |
| A-V2 | 0.967 | -0.14 | 29.14 | 0.88 | 0 | 0 | -0.01 | 0.01 |
| P. dist | 0.226 | 1.13 | 29.18 | 0.26 | 0.09 | 0.08 | -0.07 | 0.27 |
| Radius | 0.815 | 0.55 | 27.60 | 0.58 | 0.18 | 0.32 | -0.49 | 0.85 |
| Def. amp. | 0.473 | -0.62 | 29.78 | 0.53 | -0.02 | 0.03 | -0.09 | 0.04 |
| CG | ||||||||
| A-L1 | 0.279 | -0.46 | 35.92 | 0.64 | -0.04 | 0.09 | -0.23 | 0.14 |
| A-L2 | 0.949 | 0.26 | 42.72 | 0.79 | 0.05 | 0.18 | -0.33 | 0.43 |
| A-V1 | 0.126 | -0.88 | 27.17 | 0.38 | -0.08 | 0.09 | -0.27 | 0.11 |
| A-V2 | 0.057 | -3.37 | 45.74 | 0 | -0.06 | 0.01 | -0.10 | -0.02 |
| P. dist | 0.167 | 1.99 | 43.46 | 0.05 | 0.36 | 0.18 | -0.00 | 0.73 |
| Radius | 0.195 | -0.80 | 28.91 | 0.42 | -0.90 | 1.12 | -3.19 | 1.38 |
| Def. amp. | 0.130 | -0.79 | 27.44 | 0.43 | -0.24 | 0.30 | -0.88 | 0.38 |
CG: Childhood glaucoma; A-L1: Applanation length 1; A-L2: Applanation length 2; A-V1: Applanation velocity 1; A-V2: Applanation velocity 2; P. dist: Peak distance; Def. amp: Deformation amplitude; Df: Degrees of freedom; Sig.: Significance; Std.: Standard.
The Pearson analysis was used in order to find any correlation between the corneal biomechanical parameters offered by Corvis ST and the clinical examination of the CG patients. We found correlation between the CDR and the A-L1 was positive and significant (0.38, P=0.00).
Pearson correlation was used between the number of surgeries and the biomechanical properties and it has not been significant with any of the corneal biomechanical parameters, measured with Corvis ST (Table 3). Strong tendency towards statistical significance was found between the number of surgeries and the A-L1 (P=0.059). It was found a positive correlation between them r=0.249.
Table 3. Surgical procedures and corneal biomechanical properties.
| Surgeries | A-L1 | A-L2 | P. dist | Radius | A-V1 | A-V2 | Def. amp. |
| Sig. | 0.059 | 0.561 | 0.38 | 0.807 | 0.859 | 0.18 | 0.40 |
A-L1: Applanation length 1; A-L2: Applanation length 2; A-V1: Applanation velocity 1; A-V2: Applanation velocity 2; P. dist: Peak distance; Def. amp: Deformation amplitude; Df: Degrees of freedom; Sig.: Significance.
DISCUSSION
Studying the corneal biomechanical properties in patients with CG may help to understand the physiopathology and the evolution of this disease.
Recent studies carried out with ORA have shown that age can cause a reduction in CH and CRF[12]–[13], proving that with age the cornea would be less resistant to deformation, that is, “softer”. However, another study, also carried out with ORA, has not found a relationship between corneal biomechanical properties and age[14]. In contrast, the study carried out by Valbon et al[15], where corneal biomechanical properties were measured with Corvis ST, as in our study, showed that only the value of the highest concavity time (HC-time) was correlated with age, proving that the corneas of older patients are more resistant, that is, stiffer.
Therefore, analyzing the literature, the relationship between age and biomechanical properties is a controversial aspect. Because of this we have not stablished the age as an exclusion criterion.
The clinical application of the data generated with Corvis ST, has not been well studied because the instrument is not yet widely used. However, as we established in the study, the corneas of patients with CG are more deformable (higher A-L2, lower A-V2 and PD), and this quantifiable data could be useful in complementing the diagnosis of patients with CG. And perhaps further studies could become useful for monitoring follow-up.
In addition, Corvis ST provides an IOP measurement that does not require direct contact with the cornea, so collaboration by children could be easier. Another advantage with respect to the classic Goldman tonometry is that it not only provides measurements of IOP but also measurements of other corneal parameters in the same measurement. Its measurements have been proven to be reproducible[16]–[17].
There is a previous study that uses the Corvis ST to compare the corneal biomechanical parameters of patients with open angle glaucoma (OAG) with those of IOP- and CCT-matched normal control subjects. However, to our knowledge, this is the first study that does it with CG patients.
We found that eyes with CG exhibit a longer A-L2, the change in the shape of the cornea once the air pulse has stopped, measured in the 0.5 mm surrounding the apex. This could mean that subjects with CG would have more deformable corneas.
However, the value of the A-V2 or the outward applanation velocity—the instantaneous speed of the cornea as it returns to its original, undisturbed state—was slower in the CG eye. These results could be associated with a lower capacity of the cornea to recover its previous condition. Moreover, the PD—distant between the two points when the cornea reaches its maximum concavity—resulted also shorter in the CG eyes.
Eyes with glaucoma showed decreased CH, indicating a reduced viscoelastic response compared with that in normal controls[18]–[19]. Moreover, there are several works, which have studied the corneal biomechanical properties in patients with CG using the ORA. These works conclude that corneas with lower hysteresis values present a higher risk of glaucoma progression. It is established that this lower HC values are due to lower CCT values, along with higher corneal diameters, which would make these corneas have less resistance to deformation, in other words, they have “softer” corneas. Softer corneas are more likely to progress[20].
We also find previously published works that find a good correlation between the CH parameters provided by ORA, and the data obtained with Corvis ST[21]. In our study we analyzed the correlation between CDR value and the biomechanical parameters obtained with Corvis ST, finding a positive and significant correlation (r=0.388, P=0.004) with the parameter that analyzes the A-L1. Therefore, it seems that more deformable corneas have greater optic nerve damage.
In our study we found that the value of bIOP, corrected according to the biomechanical data and the CCT, provided by Corvis ST, was not statistically significant different to IOP provided by the Goldmann applanation tonometer.
Previous studies[16],[21], did not find significant differences of IOP provided by Corvis ST and the IOP values obtained with Goldmann applanation tonometry (GAT) in healthy subjects.
In relation to the correlation found between the IOP measured with both methods, it was positive and significant (r=0.545, P=0.00). This high correlation was also shown in the work published in 2013 by Hong et al[16].
Women and men are anatomically and physiologically different. Therefore, corneal biomechanical properties may be also different. In our study, we found statistically significant differences in the A-L1 in control group. A-L1 was greater in the group of males. The greater A-L1 found in men with respect to women could be due to the fact that they have greater corneal diameters[22].
In conclusion, patients with CG have corneas with lower CH, and with greater A-L2, and lower A-V2 and PD which would make these corneas deformable. GAT continues to be the most used and reference method for measuring IOP. Significant differences between the IOP measurements with Corvis ST compared to the IOP provided by GAT has not been found in our study. And moreover, there was a good correlation between both measurements of IOP. Significant differences have been found between men and women in one of the corneal biomechanical parameters analyzed with Corvis ST, although these findings require further studies to clarify the clinical significance they may have.
Acknowledgments
Conflicts of Interest: Caride SG, None; González LP, None; Francés FS, None; Feijoo JG, None.
REFERENCES
- 1.Weinreb RN, Grajewski AL, Papadopoulos M, Grigg J, Freedman S. Childhood glaucoma: the 9th consensus report of the World Glaucoma Association. Amsterdam, The Netherlands: Kugler Publications; 2013. [Google Scholar]
- 2.Tian L, Ko MWL, Wang LK, Zhang JY, Li TJ, Huang YF, Zheng YP. Assessment of ocular biomechanics using dynamic ultra high-speed scheimpflug imaging in keratoconic and normal eyes. J Refract Surg. 2014;30(11):785–791. doi: 10.3928/1081597X-20140930-01. [DOI] [PubMed] [Google Scholar]
- 3.Anand A, de Moraes CGV, Teng CC, Tello C, Liebmann JM, Ritch R. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Invest Ophthalmol Vis Sci. 2010;51(12):6514. doi: 10.1167/iovs.10-5580. [DOI] [PubMed] [Google Scholar]
- 4.Grise-Dulac A, Saad A, Abitbol O, Febbraro JL, Azan E, Moulin-Tyrode C, Gatinel D. Assessment of corneal biomechanical properties in normal tension glaucoma and comparison with open-angle glaucoma, ocular hypertension, and normal eyes. J Glaucoma. 2012;21(7):486–489. doi: 10.1097/IJG.0b013e318220daf0. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Tocino H, Bringas-Calvo R, Iglesias-Cortiñas D. Correlación entre presión intraocular, paquimetría y queratometría en una población normal. Arch Soc Esp Oftalmol. 2007;82(5):267–272. doi: 10.4321/s0365-66912007000500004. [DOI] [PubMed] [Google Scholar]
- 6.Arribas-Pardo P, Mendez-Hernandez C, Cuiña-Sardiña R, Benitez-Del-castillo JM, Garcia-Feijoo J. Tonometry after intrastromal corneal ring segments for keratoconus. Optom Vis Sci. 2017;94(10):986–992. doi: 10.1097/OPX.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 7.Arribas-Pardo P, Mendez-Hernandez C, Cuiña-Sardiña R, Fernandez-Perez C, Diaz-Valle D, Garcia-Feijoo J. Measuring intraocular pressure after intrastromal corneal ring segment implantation with rebound tonometry and Goldmann applanation tonometry. Cornea. 2015;34(5):516–520. doi: 10.1097/ICO.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 8.Lopes BT, Roberts CJ, Elsheikh A, Vinciguerra R, Vinciguerra P, Reisdorf S, Berger S, Koprowski R, Ambrósio R. Repeatability and reproducibility of intraocular pressure and dynamic corneal response parameters assessed by the corvis ST. J Ophthalmol. 2017;2017:1–4. doi: 10.1155/2017/8515742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen IB, Bak-Nielsen S, Vestergaard AH, Ivarsen A, Hjortdal J. Corneal biomechanical properties after LASIK, ReLEx flex, and ReLEx smile by Scheimpflug-based dynamic tonometry. Graefes Arch Clin Exp Ophthalmol. 2014;252(8):1329–1335. doi: 10.1007/s00417-014-2667-6. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez MR, Andrzejewska WM, Neufeld AH. Changes in the extracellular matrix of the human optic nerve head in primary open-angle glaucoma. Am J Ophthalmol. 1990;109(2):180–188. doi: 10.1016/s0002-9394(14)75984-7. [DOI] [PubMed] [Google Scholar]
- 11.Girard MJA, Suh JKF, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52(8):5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celebi ARC, Kilavuzoglu AE, Altiparmak UE, Cosar Yurteri CB. Age-related change in corneal biomechanical parameters in a healthy Caucasian population. Ophthalmic Epidemiol. 2018;25(1):55–62. doi: 10.1080/09286586.2017.1351997. [DOI] [PubMed] [Google Scholar]
- 13.El Massry AAK, Said AA, Osman IM, Bessa AS, Elmasry MA, Elsayed EN, Bayoumi NHL. Corneal biomechanics in different age groups. Int Ophthalmol. 2020;40(4):967–974. doi: 10.1007/s10792-019-01273-8. [DOI] [PubMed] [Google Scholar]
- 14.Kirwan C, O'Keefe M, Lanigan B. Corneal hysteresis and intraocular pressure measurement in children using the reichert ocular response analyzer. Am J Ophthalmol. 2006;142(6):990–992. doi: 10.1016/j.ajo.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 15.Valbon BF, Ambrósio-Jr R, Fontes BM, Alves MR. Effects of age on corneal deformation by non-contact tonometry integrated with an ultra-high-speed (UHS) Scheimpflug camera. Arq Bras Oftalmol. 2013;76:229–232. doi: 10.1590/s0004-27492013000400008. [DOI] [PubMed] [Google Scholar]
- 16.Hong JX, Xu JJ, Wei AJ, Deng SX, Cui XH, Yu XB, Sun XH. A new tonometer—the corvis ST tonometer: clinical comparison with noncontact and goldmann applanation tonometers. Invest Ophthalmol Vis Sci. 2013;54(1):659. doi: 10.1167/iovs.12-10984. [DOI] [PubMed] [Google Scholar]
- 17.Nemeth G, Hassan Z, Csutak A, Szalai E, Berta A, Modis L. Repeatability of ocular biomechanical data measurements with a Scheimpflug-based noncontact device on normal corneas. J Refract Surg Thorofare N J. 2013;29(8):558–563. doi: 10.3928/1081597X-20130719-06. [DOI] [PubMed] [Google Scholar]
- 18.Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141(5):868–875. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Mangouritsas G, Morphis G, Mourtzoukos S, Feretis E. Association between corneal hysteresis and central corneal thickness in glaucomatous and non-glaucomatous eyes. Acta Ophthalmol. 2009;87(8):901–905. doi: 10.1111/j.1755-3768.2008.01370.x. [DOI] [PubMed] [Google Scholar]
- 20.Perucho-González L, Martínez de la Casa JM, Morales-Fernández L, Bañeros-Rojas P, Saenz-Francés F, García-Feijoó J. Intraocular pressure and biomechanical corneal properties measure by ocular response analyser in patients with primary congenital glaucoma. Acta Ophthalmol. 2016;94(5):e293–e297. doi: 10.1111/aos.12912. [DOI] [PubMed] [Google Scholar]
- 21.Bañeros-Rojas P, Martinez de la Casa JM, Arribas-Pardo P, Berrozpe-Villabona C, Toro-Utrera P, García-Feijoó J. Comparison between goldmann, icare pro and corvis ST tonometry. Arch Soc Esp Oftalmol. 2014;89(7):260–264. doi: 10.1016/j.oftal.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Gharaee H, Abrishami M, Abrishami M, Mirhosseini SM, Mehrabi Bahar MR, Eghbali P. Anterior and posterior corneal curvature: normal values in healthy Iranian population obtained with the Orbscan II. Int Ophthalmol. 2014;34(6):1213–1219. doi: 10.1007/s10792-014-0005-y. [DOI] [PubMed] [Google Scholar]