Abstract
AIM
To investigate changes in macular vessels and thickness in myopic eyes after intraocular collamer lens (ICL) implantation using quantitative optical coherence tomography angiography (OCTA).
METHODS
This retrospective included 73 myopic eyes of 73 patients (average age, 27.53±6.16y) who underwent ICL implantation (28 eyes were Toric ICL). Axial length (AL), uncorrected visual acuity (UCVA), refractive dioptre (RD), intraocular pressure (IOP) and OCTA were measured and compared with before and 1wk, 1, and 3mo after surgery. OCTA was used to image vessel density (VD) and skeleton density (SD) in both the superficial (SCP) and deep capillary plexus (DCP). Central retinal thickness (CRT) and ganglion cell-inner plexiform layer thickness (GCT) were also measured. Changes between pre- and postoperative measurements were analysed by repeated measures analysis of variance.
RESULTS
Compared with preoperative data, postoperative data on UCVA revealed significant improvements in all patients (P<0.05). However, there was no significant difference in IOP. After the operation, CRT and GCT exhibited significant changes (P<0.05). Among these measures, CRT was significantly higher at one and three months postoperative (all P<0.01). GCT was significantly higher at 1wk, 1, and 3mo postoperative (all P<0.01). Changes in VD and SD were nonsignificant in both the SCP and DCP. There was no difference in postoperative changes between the ICL and Toric ICL groups.
CONCLUSION
ICL and Toric ICL implantation both have good efficacy and safety for myopic eyes, but macular area changes that occur after surgery need attention.
Keywords: intraocular collamer lens implantation, vessel density, macular thickness, optical coherence tomography angiography
INTRODUCTION
In 2000, approximately 1.4 billion people had myopia worldwide; by 2050, this number will rise to 4.7 billion[1]. Current mainstream refractive surgery, like laser-assisted in situ keratomileusis (LASIK) and femtosecond laser small incision corneal stromal lens extraction (SMILE), can lead to corneal thinning as well as structural alterations in corneal biomolecules. For patients with high myopia and thin corneas, these procedures are risky[2]–[3]. Intraocular collamer lens (ICL) implantation could correct a wider range of myopia without changing corneal morphology while retaining accommodation. Therefore, ICL implantation could be a good option for patients with the above conditions[4]. A large number of short and long term studies have confirmed the safety, efficacy and predictability of ICL surgery[5]–[6]. However, due to changes in fundus structure and function in myopic patients, the risk of vitreoretinal complications during various operations will be greatly increased. As an internal eye operation, ICL implantation will lead to physiological changes in the eye that may also lead to vitreoretinal complications and visual impairment. However, there are few reports about the effect of ICL implantation on the posterior segment of the eye. Optical coherence tomographic angiography (OCTA) is a new technique for observing the morphology of retinal blood vessels. OCTA can display the three-dimensional morphology of retinal structure and blood vessels clearly with high resolution and quantify the retinal microcirculation accurately[7]–[10]. Recently, OCTA has been used to evaluate the changes induced by a local inflammation reaction after cataract surgery[11]. Here, we used OCTA to observe changes in macular microvasculature and thickness after ICL implantation and to explore the effect of ICL implantation on the posterior segment.
SUBJECTS AND METHODS
Ethical Approval
This retrospective study was approved by the Lixiang Eye Hospital of Soochow University Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from each subject before enrolment.
Study Sample
Patients were enrolled in this study from Lixiang Eye Hospital between January 2018 and July 2019. The inclusion criteria were as follows: myopic patients who underwent ICL implantation in our hospital and were willing to cooperate with follow-up. The eye operated on first was enrolled if both patient's eyes qualified for the study. The exclusion criteria were as follows: myopic retinopathy, choroidal neovascularization, uveitis, macular oedema, vitreous haemorrhage, macular degeneration, glaucoma, intraocular surgery history, hypertension, diabetes and other systemic diseases affecting the retina. Patients who could not complete the follow-up on time and those who had intraoperative complications or serious complications after operation were excluded. During follow-up, the subjects whose OCTA image signal intensity was less than 8 (maximum intensity 10) or whose image was blurred or obviously interrupted were completely excluded, and those who exceeded or were earlier than the prescribed follow-up time by 3d were completely excluded.
All patients underwent complete preoperative examination, including uncorrected distance visual acuity (UDVA) as a logarithm of the minimum angle of resolution (logMAR), best corrected visual acuity (BCVA) and refractive dioptre (RD) measured with a standardized comprehensive optometry and then converted into equivalent spherical dioptres, which is equal to spherical dioptres plus half of astigmatism. Intraocular pressure (IOP) was measured with a noncontact tonometer (Topcon, Inc. Japan), axial length (AL) was measured with partial coherence interferometry (IOLMaster Zeiss 500; Carl Zeiss Meditec, Inc. Germany), and a dilated fundus examination was performed by a 3-mirror contact lens.
Image Acquisition
A commercially available Zeiss Cirrus HD-OCT 5000-2328 (Zeiss Meditec, Inc. Germany) with OMAG AngioPlex (software version 10.0.0.14618) was used for image acquisition. This device had a scanning wavelength of 840 nm, a scanning speed of 68 000 A-scans per second and a resolution of 5 µm. During the examination, the patient was asked to fix their head position and look at the internal visual indicator of the instrument. The Fast Trace mode was turned on to track the eye position in real time. The scanning range was 3×3 mm2 in the macular centre and 2 mm in depth. The whole scan volume was then divided into the superficial and deeper capillary plexus (SCP and DCP) by an automated segmentation algorithm in AngioPlex. Briefly, SCP covered 70% of the superficial of the retina and extends from internal limiting membrane (ILM) to 110 µm above the retinal pigment epithelium (RPE), and the DCP was defined as the remaining 30%[12]. Then the manual “Remove Projections” option was conducted in AngioPlex software for the DCPs.
Ganglion cell-inner plexiform layer thickness (GCT) and central retinal thickness (CRT) within 1 mm of the macular fovea were obtained using tomography by the Machine-Built software CIRRUS. GCT was the sum of the thickness of the inner plexiform layer and ganglion cell layer[13]–[14]. Images with obvious errors in the determination of GCT were excluded.
En Face Imaging Analysis
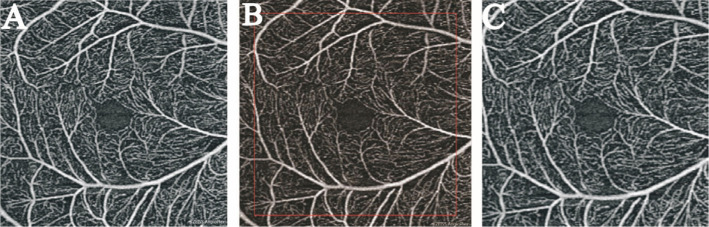
En face OCTA images analysis was performed using Image J (USA). As the AL of the myopic eye lengthens, the fundus image of OCTA would be magnified. Hence, appropriate magnification correction is necessary to evaluate the construction of the retina. Bennett's formula based on the AL was used to adjust the image magnification. The formula was expressed as t=p×q×s and the scaling factor (p×q) =3.3823×0.0130623×(AL-1.82), as described in the previous study[15]–[17]. Since the pixel size of high myopia image changes after adjusting the scale factor according to Bennett formula, the original pixel size (759×759 pixels) is pruned to correct the image (Figure 1).
Figure 1. Magnification correction of OCTA image.
A: The original image was 759×759 pixels. B: The image was magnified 1.15× according to the AL (AL=27.90 mm), the resolution was 873×873 pixels. C: Finally, it was trimmed to the original resolution of 759×759 pixels.
In order to calculate vessel density (VD) and skeleton density (SD), we applied threshold algorithm to en face images of each capillary plexus to calculate a binarized image. VD calculation was the proportion of the total pixel area of white pixel in binarization image in the total pixel area of binarization image. Further from this binary map, skeletonized image was created to represent vessels with a width of 1 pixel. SD was defined as the ratio of the vessel skeleton length to the total area of the image. Similar methods have been described in detail in many previous studies[8],[18] (Figure 2).
Figure 2. Process of quantitative analysis.
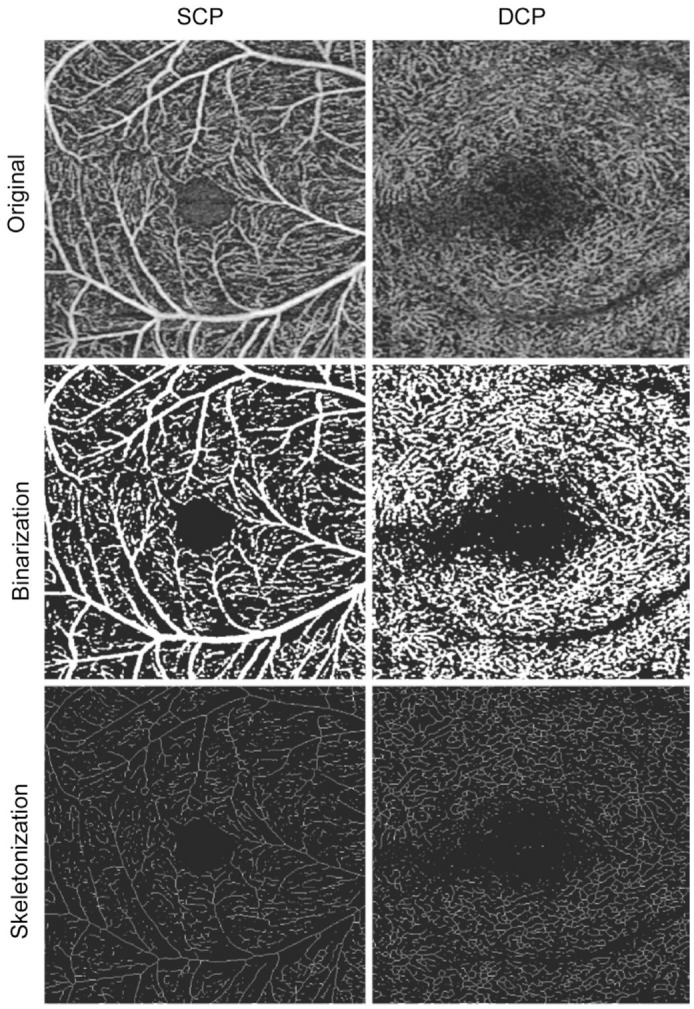
The first column shows the SCP, and the second column shows the DCP; the first row shows the original image, the second row shows the binary graph used to calculate the VD, and the third row shows the skeleton image that was generated from the binary image and used to calculate the SD.
Surgical Procedure
All surgeries were performed by the same experienced surgeon (corresponding author), and the surgical procedures followed the standard procedures. Briefly, a 3-mm clear temporal corneal incision was made after topical anesthesia (proparacaine hydrochloride, Nanjing, China). After injection of hyaluronic acid (Shanghai, China) into the anterior chamber, a STAAR ICL VICMO or STAAR TORIC ICL VTICMO was implanted and then positioned in the posterior chamber. Finally, hyaluronic acid was completely removed from the eye using a manual I/A device. All surgeries were successfully completed without any intraoperative complications. Following surgery, tobramycin 0.3%-dexamethasone 0.1% eye drops (Tobradex) were prescribed for use three times daily for 2wk.
Follow-Up
At 1wk, 1 and 3mo postoperatively, all the parameters were collected except for the preoperative examination of the ocular axis and comprehensive optometry. All examinations were performed by the same examiner between 2 p.m. and 4 p.m.
Statistical Analysis
Statistical analysis was performed using SPSS 18.0. All data are expressed as the mean±standard deviation. Changes between pre- and postoperative measurements were analysed by repeated measures analysis of variance with Bonferroni corrections. Independent sample t-test was used to compare the two groups. The Chi-square test was used for comparisons of enumeration data between the two groups. Correlation analysis was performed using Spearman's correlation coefficient to determine the relationships between the magnitude of the changes in parameters from baseline to each timepoint and identify related factors. A P value less than 0.05 was considered statistically significant.
RESULTS
A total of 73 patients (73 eyes) were followed up successfully. These included 35 right eyes, 38 left eyes, 25 eyes of male patients and 48 eyes of female patients. TORIC ICL VTICMO was implanted in 28 eyes and ICL VICMO in 45 eyes. Patient age ranged from 19 to 46y, with an average age of 27.53±6.16y. The AL of the eye ranged from 23.85 mm to 28.85 mm, with an average of 26.73±1.15 mm. The refractive state ranged from -2.00 D to -13.00 D, with an average of -8.27±2.46 D (Table 1).
Table 1. Subject data.
| Characteristics | VICMO | VTICMO | P |
| Patients (eyes), right/left | 45, 21/24 | 28, 14/14 | 0.782a |
| Age (y) | 27.44±5.79 | 27.68±6.82 | 0.876 |
| Sex, male/female | 13/32 | 12/16 | 0.221a |
| Dioptre (D) | -7.52±2.21 | -8.10±2.34 | 0.298 |
| UCVA | 1.47±0.23 | 1.45±0.33 | 0.826 |
| BCVA | -0.05±0.06 | -0.04±0.04 | 0.701 |
| AL (mm) | 26.61±1.15 | 26.91±1.17 | 0.293 |
| IOP (mm Hg) | 13.77±2.54 | 14.00±3.14 | 0.736 |
| SCP | |||
| VD (%) | 49.84±5.10 | 48.71±4.85 | 0.352 |
| SD (mm−1) | 22.33±2.05 | 21.83±1.95 | 0.305 |
| DCP | |||
| VD (%) | 40.32±6.93 | 39.27±7.82 | 0.553 |
| SD (mm−1) | 18.92±2.84 | 18.37±3.26 | 0.445 |
| CRT | 247.40±17.19 | 253.71±20.71 | 0.163 |
| GCT | 76.62±6.63 | 78.18±6.34 | 0.325 |
Independent sample t-test. aChi-square test. UCVA: Uncorrected visual acuity; BCVA: Best corrected visual acuity; AL: Axial length; IOP: Intraocular pressure; SCP: Superficial capillary plexuses; DCP: Deep capillary plexuses; CRT: Central retinal thickness; GCT: Ganglion cell-inner plexiform thickness.
Visual Acuity and Intraocular Pressure
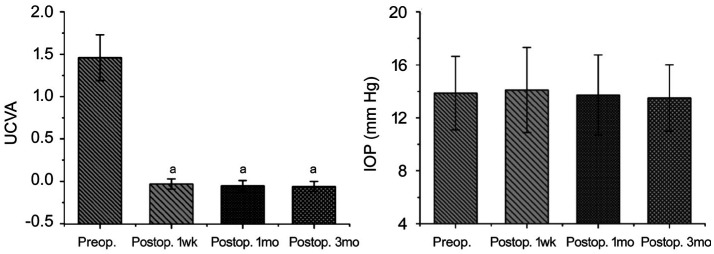
Compared with before surgery, after surgery, there was significant improvement in UCVA at 1wk, 1 and 3mo (P<0.01 for all), and the pre- and postoperative UCVA values were 1.46±0.27, -0.03±0.06, -0.05±0.06, and -0.06±0.06, respectively. In contrast, there was no significant difference in IOP between before and after surgery (P=0.148), and the preoperative and postoperative IOP values were 13.86±2.77, 14.10±3.21, 13.73±3.02, and 13.50±2.51 mm Hg, respectively (Figure 3).
Figure 3. Changes in clinical indicators between before and after surgery.
A: The change in UCVA between before and after surgery; B: The change in IOP between before and after surgery. UCVA: Uncorrected visual acuity; IOP: Intraocular pressure. aP<0.05.
Image Analysis
The results of repeated measures showed there as no significant difference between VD and SD either before or after the operation, whether in SCP (P=0.532, 0.199) or in DCP (P=0.226, 0.258). There was a significant difference between CRT and GCT both before and after the operation (P<0.001, <0.001). CRT was higher at 1 and 3mo postoperatively (from 249.96±18.83 µm at baseline to 252.28±18.34 µm at 1mo and 251.46±18.95 µm at 3mo after surgery, all P<0.001). In contrast, GCT was higher at all time points after the operation (from 77.22±6.52 µm at baseline to 79.86±6.22 µm at 1wk, 78.53±6.47 µm at 1mo and 78.36±6.09 µm at 3mo after surgery, all P<0.001; Table 2).
Table 2. The parameters of OCTA at preoperative and postoperative time points with repeated measures.
| Parameters | Preoperative | 1wk after surgery | 1mo after surgery | 3mo after surgery | Correlation with time (P)a |
| SCP | |||||
| VD (%) | 49.41±5.00 | 48.96±4.05 | 49.64±5.16 | 49.84±4.88 | 0.532 |
| SD (mm−1) | 22.14±2.01 | 21.97±1.74 | 22.35±2.14 | 22.48±2.04 | 0.199 |
| DCP | |||||
| VD (%) | 39.92±7.25 | 39.30±7.10 | 38.18±7.30 | 39.10±7.27 | 0.226 |
| SD (mm−1) | 18.71±3.00 | 18.45±2.96 | 18.03±3.07 | 18.45±3.00 | 0.258 |
| CRT (µm) | 249.96±18.83 | 250.40±19.26 | 252.28±18.34 | 251.46±18.95 | <0.001 |
| Pb | Ref. | 0.460 | <0.001 | <0.001 | |
| Ref. | <0.001 | 0.056 | |||
| Ref. | 0.177 | ||||
| GCT (µm) | 77.22±6.52 | 79.86±6.22 | 78.53±6.47 | 78.36±6.09 | <0.001 |
| Pb | Ref. | <0.001 | <0.001 | <0.001 | |
| Ref. | <0.001 | <0.001 | |||
| Ref. | 0.430 |
aRepeated measures, significance P values of repeated factor ‘time’; bSignificance P values of the comparisons between mean values with respect to reference time, post hoc Bonferroni test. SCP: Superficial capillary plexuses; DCP: Deep capillary plexuses; CRT: Central retinal thickness; GCT: Ganglion cell-inner plexiform thickness; VD: Vessel density; SD: Skeleton density; Ref.: Reference mean value.
Repeated measures analysis of variance showed no difference in the changes of the VD or SD in SCP or DCP after surgery in either the VICMO or VTICMO group (all P>0.05; Table 3). Changes in the CRT and the GCT at each time point were also not significantly different between the two groups (Table 3).
Table 3. Changes in OCTA parameters at different time points.
| Parameters | Central retinal thickness change |
Ganglion cell-inner plexiform thickness change |
||||
| 1wk after surgery | 1mo after surgery | 3mo after surgery | 1wk after surgery | 1mo after surgery | 3mo after surgery | |
| VICMO | -0.07±4.62 | 1.62±4.74 | 0.89±3.28 | 2.76±2.11 | 1.54±2.41 | 1.04±1.68 |
| VTICMO | 1.25±5.70 | 3.11±5.49 | 2.14±3.66 | 2.46±1.95 | 1.18±2.15 | 1.29±1.49 |
| t | 1.076 | 1.225 | 1.518 | -0.589 | -0.661 | 0.623 |
| P | 0.285 | 0.225 | 0.133 | 0.557 | 0.511 | 0.535 |
Independent sample t-test.
µm
Correlation Analysis
The changes in GCT at 1wk and 3mo were positively correlated with AL (r=0.280; P=0.016), but the changes at other time points were not correlated with AL. There was no significant correlation between changes of CRT and AL (Table 4).
Table 4. Spearman correlation analysis between changes in CRT and GCT at each timepoint after surgery and ocular variables.
| Parameters | Axial length |
Refractive dioptre |
||
| r | P | r | P | |
| Changes in CRT | ||||
| 1wk after surgery | 0.098 | 0.409 | -0.072 | 0.547 |
| 1mo after surgery | 0.018 | 0.877 | -0.195 | 0.097 |
| 3mo after surgery | 0.011 | 0.924 | -0.090 | 0.448 |
| Changes in GCT | ||||
| 1wk after surgery | 0.280 | 0.016a | 0.186 | 0.115 |
| 1mo after surgery | 0.137 | 0.247 | 0.172 | 0.145 |
| 3mo after surgery | 0.258 | 0.028a | 0.248 | 0.034a |
aP<0.05.
DISCUSSION
The results of our study show that UCVA improved rapidly and steadily, and no significant change in IOP was observed after ICL implantation, supporting the safety and efficacy of ICL implantation for myopia correction, in accordance with the previous literature[19]–[21].
In this study, we investigated microvascular structural changes in both superficial and deep capillary plexuses. However, there were no significant changes in VD and SD before and after the operation in either the SCP or the DCP, indicating that the effect of ICL implantation on the macular microvasculature was limited. Zhao et al[22] and Pilotto et al[11] found that retinal vessel density increased after uncomplicated phacoemulsification surgery. Zhao et al[22] suggested that this may be related to decreased postoperative IOP, increased inflammation, and increased light exposure. In our study, no reduction in IOP was found, and light exposure was unchanged after ICL implantation; these findings may explain why we did not observe changes in the microvasculature.
We found that CRT was significantly higher at 1 and 3mo postoperatively. In contrast, GCT significantly increased at all time points after the operation; GCT peaked one week, followed by a slow decrease. Changes in macular thickness after internal eye surgery are often associated with macular oedema, which is the most common cause of vision loss after cataract surgery. Macular oedema is also an important cause of vision loss after other operations, such as vitrectomy, penetrating keratoplasty and YAG posterior capsulotomy[23].
The mechanism of macular oedema after surgery has not been clarified. Some scholars believe that it is caused by mechanical vitreous traction and the stimulation of inflammatory mediators after surgery. Following intraocular surgery, inflammatory mediators disrupt the blood-retinal barrier, increasing the permeability of the perifoveal capillaries, resulting in perifoveal intraretinal fluid accumulation, especially in the outer plexiform layer[7],[24]–[25]. Therefore, changes in the thickness of the outer plexiform layer may be easier to detect. We speculate that similar changes may occur after ICL implantation, which may partly explain CRT thickening. However, in this study, the changes in GCT were more obvious and earlier than those in CRT. Because the GCT contains only the ganglion cell layer and inner plexiform layer, the above theory cannot be fully explained.
Recently, Pilotto et al[11] found that changes in the inner retinal layer and outer retinal layer were inconsistent after uncomplicated cataract surgery. One week after cataract surgery, the inner retinal volume increased while the outer retinal volume decreased. One month after cataract surgery, both volumes increased. Since we did not measure the outer retinal layer separately, the thickening of the GCT may have concealed the thinning of the outer retinal layer in CRT, and this is generally consistent with our conclusion. Moreover, Pilotto et al[11] found that hyper-reflective intraretinal spots (HRS) first appeared in the inner retinal layer, gradually developed in the outer retinal layer, and continued to increase until one month at which point they began to slow down. HRS represent activated, aggregated microglial cells and play an important role in the inflammatory response in the retina[26]–[27]. The changes in HRS coincide with those in CRT and GCT in our study. Therefore, we speculate that similar to cataract surgery, inflammation begins in the inner retina after ICL implantation, leading to an increase in GCT. Then, inflammation gradually develops throughout the entire retina, leading to increased CRT. However, after one month, as the inflammation subsides, CRT and GCT decrease. In this process, the role of Müller cells deserves further study. Müller cells extend from the photoreceptor inner segment to the inner limiting membrane through the entire retina. These neuronal structural support cells were connecting elements between neuronal and vascular cells involved in regulating neuronal nutrition, development, and metabolism. In addition, Müller cells participate in the local inflammatory response and increase proinflammatory factor production, which in turn increases microglial activation in a positive feedback loop, upregulates chemokine and adhesion protein expression, allows Müller cells to attract and adhere to microglia, and directs intraretinal mobilization of migrating microglia in the outer retina[26],[28]. The cell bodies of Müller cells were mainly located in the inner retina, which may partly explain why inflammation first occurred in the inner retina and then spread to the outer retina[29]–[30]. In addition, since changes in CRT and GCT were most likely caused by inflammation, anti-inflammatory treatment after ICL implantation is recommended for at least one month.
The commonly used criterion for OCT in judging macular oedema is that macular thickness increases by more than 30%[31]. In this study, we found no macular oedema after surgery in any patient. Postoperative CRT and GCT changed by much less than 30%. ICL implantation had an impact on the posterior segment of the eye, but these changes were within the safe range. Canan et al[32] have reported a case of macular oedema after astigmatic Toric-ICL implantation. The authors believe that this oedema may be caused by constant friction between the IOL and posterior iris surface or anterior suspension ligament. Vitreous traction during implantation may also be an important factor. Additionally, continuous repositioning following Toric-ICL implantation may increase the risk of CME[32]. However, in our study, there was no significant difference in the postoperative changes in retinal thickness between the ICL and Toric-ICL groups, suggesting that Toric-ICL may not increase the degree of postoperative inflammation or the incidence of postoperative macular oedema.
In addition, we found that the changes in GCT at 1wk and 3mo after surgery were positively correlated with AL. Inflammation was also more obvious in the inner retinal layer one week after surgery, suggesting that inflammation tended to be more serious with the extension of the ocular axis in the early stage after ICL implantation. Based on previous studies, high myopia is an inflammation-related disease[33]–[34], which is more likely to occur in inflammation-related diseases such as choroidal neovascularization, multifocal choroiditis[35]–[37], and capsular contraction syndrome after cataract surgery[38]. However, we have not found any research reports on the correlation between macular thickness changes after ICL implantation and the ocular axis. There is no difference between the macular thickness changes after cataract surgery in patients with high myopia and those with emmetropia[38]–[39]. This lack of differences is probably because previous reports focused only on measurements of retinal thickness in the whole macular area, which would cover changes in the inner retina. We suggest that anti-inflammatory therapy should be strengthened in patients with high myopia after ICL implantation.
This study also has certain limitations. First, it remains unclear when CRT and GCT return to the baseline, and we will further investigate this issue in follow-up studies. Second, although artefact removal is currently the best solution for DCP, part of the blood flow signal is lost when an artefact is removed. We expect more advanced technology and equipment algorithms to be developed in the future. Third, although we found that retinal thickness changed after ICL implantation, the magnitude of the change was very limited, and its effect on ocular function is still unclear and deserves further exploration.
In conclusion, we confirmed the safety and efficacy of ICL implantation and observed thickening of the central retina and the ganglion cell-inner plexiform layer after ICL implantation. Although macular oedema and microvascular changes were not detected, they still need attention.
Acknowledgments
We thank professor Song (Department of Ophthalmology, Lixiang Eye Hospital of Soochow University) for the kind contribution of the instrument used in this study.
Conflicts of Interest: Zhu QJ, None; Wang MY, None; Yu P, None; Liang XS, None; Ma L, None; Xiao HX, None; Yuan Y, None.
REFERENCES
- 1.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Brenner LF, Alió JL, Vega-Estrada A, Baviera J, Beltrán J, Cobo-Soriano R. Clinical grading of post-LASIK ectasia related to visual limitation and predictive factors for vision loss. J Cataract Refract Surg. 2012;38(10):1817–1826. doi: 10.1016/j.jcrs.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Masket S, Fram NR, Shen TT, Talamo JH, Manche EE. Refractive surgical problem: April consultation. J Cataract Refract Surg. 2013;39(4):652–655. doi: 10.1016/j.jcrs.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Miao HM, Chen X, Tian M, Chen YJ, Wang XY, Zhou XT. Refractive outcomes and optical quality after implantation of posterior chamber phakic implantable collamer lens with a central hole (ICL V4c) BMC Ophthalmol. 2018;18(1):141. doi: 10.1186/s12886-018-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igarashi A, Shimizu K, Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular lens implantation for moderate to high myopia. Am J Ophthalmol. 2014;157(3):532–539.e1. doi: 10.1016/j.ajo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Moya T, Javaloy J, Montés-Micó R, Beltrán J, Muñoz G, Montalbán R. Implantable collamer lens for myopia: assessment 12y after implantation. J Refract Surg. 2015;31(8):548–556. doi: 10.3928/1081597X-20150727-05. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology. 2007;114(5):881–889. doi: 10.1016/j.ophtha.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, Shi YG, Wang RK. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100. doi: 10.1016/j.preteyeres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nesper PL, Fawzi AA. Human parafoveal capillary vascular anatomy and connectivity revealed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2018;59(10):3858–3867. doi: 10.1167/iovs.18-24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan ACS, Tan GS, Denniston AK, Keane PA, Ang M, Milea D, Chakravarthy U, Cheung CMG. An overview of the clinical applications of optical coherence tomography angiography. Eye (Lond) 2018;32(2):262–286. doi: 10.1038/eye.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilotto E, Leonardi F, Stefanon G, Longhin E, Torresin T, Deganello D, Cavarzeran F, Miglionico G, Parrozzani R, Midena E. Early retinal and choroidal OCT and OCT angiography signs of inflammation after uncomplicated cataract surgery. Br J Ophthalmol. 2019;103(7):1001–1007. doi: 10.1136/bjophthalmol-2018-312461. [DOI] [PubMed] [Google Scholar]
- 12.Durbin MK, An L, Shemonski ND, Soares M, Santos T, Lopes M, Neves C, Cunha-Vaz J. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):370–376. doi: 10.1001/jamaophthalmol.2017.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WJ, Kim YK, Park KH, Jeoung JW. Trend-based analysis of ganglion cell-inner plexiform layer thickness changes on optical coherence tomography in glaucoma progression. Ophthalmology. 2017;124(9):1383–1391. doi: 10.1016/j.ophtha.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Shin JW, Sung KR, Lee GC, Durbin MK, Cheng D. Ganglion cell-inner plexiform layer change detected by optical coherence tomography indicates progression in advanced glaucoma. Ophthalmology. 2017;124(10):1466–1474. doi: 10.1016/j.ophtha.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232(6):361–367. doi: 10.1007/BF00175988. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Wang JH, Jiang H, Yang XL, Feng LM, Hu L, Wang L, Lu F, Shen MX. Retinal microvasculature alteration in high myopia. Invest Ophthalmol Vis Sci. 2016;57(14):6020–6030. doi: 10.1167/iovs.16-19542. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Yang Y, Jiang H, Gregori G, Roisman L, Zheng F, Ke BL, Qu DY, Wang JH. Retinal microvascular network and microcirculation assessments in high myopia. Am J Ophthalmol. 2017;174:56–67. doi: 10.1016/j.ajo.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim AY, Rodger DC, Shahidzadeh A, Chu ZD, Koulisis N, Burkemper B, Jiang XJ, Pepple KL, Wang RK, Puliafito CA, Rao NA, Kashani AH. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;171:101–112. doi: 10.1016/j.ajo.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi M, Kong J, Li X, Yan Q, Zhang J. Observing implantable collamer lens dislocation by panoramic ultrasound biomicroscopy. Eye (Lond) 2015;29(4):499–504. doi: 10.1038/eye.2014.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Luo HH, Zhuang J, Yu KM. Comparison of anterior section parameters using anterior segment optical coherence tomography and ultrasound biomicroscopy in myopic patients after ICL implantation. Int J Ophthalmol. 2016;9(1):58–62. doi: 10.18240/ijo.2016.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen P, Cai XX, Xu LJ, Zhang J, Yang Y, Gao QY, Ge J, Yu KM, Zhuang J. Assessing oxygen saturation in retinal vessels in high myopia patients pre- and post-implantable collamer lens implantation surgery. Acta Ophthalmol. 2017;95(6):576–582. doi: 10.1111/aos.13368. [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZN, Wen W, Jiang CH, Lu Y. Changes in macular vasculature after uncomplicated phacoemulsification surgery: Optical coherence tomography angiography study. J Cataract Refract Surg. 2018;44(4):453–458. doi: 10.1016/j.jcrs.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Zur D, Fischer N, Tufail A, Monés J, Loewenstein A. Postsurgical cystoid macular edema. Eur J Ophthalmol. 2011;21(Suppl 6):S62–S68. doi: 10.5301/EJO.2010.6058. [DOI] [PubMed] [Google Scholar]
- 24.Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002;47(Suppl 1):S203–S218. doi: 10.1016/s0039-6257(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 25.Zur D, Loewenstein A. Postsurgical cystoid macular edema. Dev Ophthalmol. 2017;58:178–190. doi: 10.1159/000455280. [DOI] [PubMed] [Google Scholar]
- 26.Wang MH, Ma WX, Zhao L, Fariss RN, Wong WT. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation. 2011;8:173. doi: 10.1186/1742-2094-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vujosevic S, Bini S, Midena G, Berton M, Pilotto E, Midena E. Hyperreflective intraretinal spots in diabetics without and with nonproliferative diabetic retinopathy: an in vivo study using spectral domain OCT. J Diabetes Res. 2013;2013:491835. doi: 10.1155/2013/491835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JM, van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269(4 Pt 1):G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 29.Distler C, Dreher Z. Glia cells of the monkey retina—II. Müller cells. Vis Res. 1996;36(16):2381–2394. doi: 10.1016/0042-6989(96)00005-3. [DOI] [PubMed] [Google Scholar]
- 30.Powner MB, Gillies MC, Tretiach M, Scott A, Guymer RH, Hageman GS, Fruttiger M. Perifoveal Müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117(12):2407–2416. doi: 10.1016/j.ophtha.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessel L, Tendal B, Jørgensen KJ, Erngaard D, Flesner P, Andresen JL, Hjortdal J. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology. 2014;121(10):1915–1924. doi: 10.1016/j.ophtha.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Canan J, Akkan U, Tuncer K, Elbay A. Postsurgical cystoid macular edema following posterior chamber toric phakic intraocular lens implantation surgery: a case report. Case Rep Ophthalmol. 2015;6(2):223–227. doi: 10.1159/000437013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbort CP, Papadia M, Neri P. Myopia and inflammation. J Ophthalmic Vis Res. 2011;6(4):270–283. [PMC free article] [PubMed] [Google Scholar]
- 34.Kung YJ, Wei CC, Chen LH, Chen JY, Chang CY, Lin CJ, Lim YP, Tien PT, Chen HJ, Huang YS, Lin HJ, Wan L. Kawasaki disease increases the incidence of myopia. Biomed Res Int. 2017;2017:2657913. doi: 10.1155/2017/2657913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neelam K, Cheung CMG, Ohno-Matsui K, Lai TYY, Wong TY. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res. 2012;31(5):495–525. doi: 10.1016/j.preteyeres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Ziemssen F, Lagrèze W, Voykov B. Secondary diseases in high myopia. Ophthalmologe. 2017;114(1):30–43. doi: 10.1007/s00347-016-0390-x. [DOI] [PubMed] [Google Scholar]
- 37.Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res. 2018;63:92–106. doi: 10.1016/j.preteyeres.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Wang DD, Yu XY, Li ZL, Ding XX, Lian HL, Mao JY, Zhao YY, Zhao YE. The effect of anterior capsule polishing on capsular contraction and lens stability in cataract patients with high myopia. J Ophthalmol. 2018;2018:8676451. doi: 10.1155/2018/8676451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Du J, Yang M, Xu Y, Guan HJ, Wu J. Distinct macular thickness changes after femtosecond laser-assisted cataract surgery of age-related cataract and myopia with cataract. Sci Rep. 2018;8(1):3279. doi: 10.1038/s41598-018-21698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]