Summary
Background
In rural Burkina Faso, a package of six low-technology, post-partum contraceptive interventions (ie, refresher training for providers, a counselling tool, supportive supervision, daily availability of contraceptive services, client appointment cards, and invitation letters to attend appointments for partners), aimed at strengthening existing primary health-care services and enhancing demand for them, doubled the use of modern contraceptives at 12 months post partum (ie, 55% uptake in intervention recipients vs 29% in routine-care users). This study assessed the effect of a similar package but in urban settings of Kinshasa province, Democratic Republic of the Congo, in an effort to reduce the unmet need for post-partum family planning.
Methods
Yam Daabo was a multi-intervention, single-blinded, cluster-randomised controlled trial done in six primary health-care centres (clusters) in Kinshasa. Centres were randomly allocated to receive the six-component intervention or standard antenatal and postnatal care in matched pairs (1:1) on the basis of number of monthly births, the ratio of health workers per population in the health zone, and the urban and suburban settings. Only data analysts could be masked to cluster allocation. Health-care facilities were eligible if they provided a continuum of antenatal, delivery, and postnatal care, were well stocked with contraceptives, and were situated close to the main study centre. All pregnant women presenting to the six centres were eligible if they were in their third pregnancy trimester and had no counterindications to deliver in the facility. The main outcome was prevalence of use of modern contraceptives at 12 months after delivery. Analysis was by modified intention-to-treat using generalised linear mixed models or Fisher's exact test for small groups. Prevalence ratios were adjusted for cluster effects and baseline characteristics. This study was registered with the Pan-African Clinical Trials Registry (PACTR201609001784334).
Findings
From July 1, 2016, to Feb 2, 2017, eight of 52 clinics assessed for eligibility met the criteria and were randomised. Of 690 women approached, 576 (83%) women were enrolled: 286 in the four intervention clusters and 290 in the four control clusters. Of them, 519 (90%) completed the 12-month study exit interview (252 in the intervention group and 267 in the control group) and were included in the intention-to-treat analysis. At 12 months, 115 (46%) of 252 women in the intervention group and 94 (35%) of 267 in the control group were using modern contraceptives (adjusted prevalence ratio [PR] 1·58, 95% CI 0·74–3·38), with significant differences in the use of contraceptive implants (22% vs 6%; adjusted PR 4·36, 95% CI 1·96–9·70), but without difference in the use of short-acting contraceptives (23% vs 28%; 0·92, 0·29–2·98) and non-modern or inappropriate methods (7% vs 18%; 0·45, 0·13–1·54). There were no serious adverse events or maternal deaths related to the study.
Interpretation
The Yam Daabo intervention package did not have a significant effect on the overall use of effective modern contraceptives but significantly increased implant use in women post partum who live in urban settings in Kinshasa up to a year after childbirth. However, interferences from external family planning initiatives in the control group might have diminished differences between the services received. Such an intervention could be potentially relevant in similar contexts in DR Congo and other countries.
Funding
Government of France; UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction.
Research in context.
Evidence before this study
We searched PubMed for papers published since inception to April 1, 2019, using the term “randomized controlled trial” combined with “post partum” and “contraception” or “family planning” for publications in English focusing on trials in low-income and middle-income countries. We identified six trials since the most recent systematic review at the time, which was published in 2016. All six trials were in Africa and investigated a specific intervention: men's involvement in maternity care in Burkina Faso; advance counselling and provision of emergency contraceptive pills in Egypt; integration of post-partum family planning in immunisation clinics in Rwanda; implant services after childbirth in Uganda; and two Kenyan studies investigating weekly mobile phone text message reminders, and text message reminders combined with vouchers for modern contraceptives. Effectiveness was shown in all studies except for the Kenyan study testing text messaging alone. The urban-based study in Burkina Faso, which is particularly relevant to our trial, involved male partners of pregnant women in maternity care through men-only group sessions and provided couple counselling sessions before and after delivery. Results showed that the use of any contraceptives at 8 months post partum was marginally different between the intervention group (71%) and control group (64%), with a risk ratio of 1·10 (95% CI 1·02–1·20).
Added value of this study
By contrast, the Yam Daabo trial, implemented in predominantly rural areas in Burkina Faso and in urban settings in the Democratic Republic of the Congo, did not investigate a specific programmatic innovation or the promotion of a particular post-partum family planning method. Instead, fertility and contraceptive choices were made by the women and couples. Results of this cluster-randomised controlled trial in Kinshasa province showed that a package strategy consisting of six low-technology interventions designed in a participatory manner and aimed at strengthening routine antenatal and postnatal care services in primary health clinics could decrease the unmet need for post-partum family planning and contribute to reducing maternal and newborn mortality and morbidity through healthy timing and spacing of pregnancies.
Implications of all the available evidence
On the basis of the growing body of evidence on post-partum family planning, decision makers can opt for different strategies to strengthen contraceptive use depending on health service needs, gaps, and opportunities of specific contexts. This includes the Yam Daabo strategy, which was tested and found to be effective in both rural and urban settings in sub-Saharan Africa. Although cost-effectiveness of the package warrants further study, the simplicity of the interventions and focus on strengthening existing services at the primary health-care level make them suitable for large scale implementation in similar settings in DR Congo, Burkina Faso, and other countries.
Introduction
Post-partum family planning is defined by WHO as the prevention of closely-spaced pregnancies and unwanted pregnancies up to 12 months after delivery, when a new pregnancy presents high risk for mothers and babies. Post-partum family planning could prevent more than 30% of maternal and 10% of infant deaths by effectively spacing birth-to-pregnancy intervals by at least 2 years and birth-to-birth intervals by at least 3 years.1 Therefore, post-partum family planning services aim to assist women and couples to decide on their preferred method of contraception, initiate that method, and continue use for ideally 2 years or longer, depending on their reproductive goals.2 Birth-to-pregnancy intervals in 50% or more of pregnancies in low-income and middle-income countries are too short (<23 months).3 Considering the definition that assumes that the risk of becoming pregnant restarts soon after birth and before the sixth week post partum, and that women should use contraception even if abstinent or before the menstrual cycle resumes, unmet need for family planning reaches 65% in east and southern Africa and 75% in west and central Africa.4
Post-partum family planning is usually designed as an integral part of reproductive, maternal, neonatal, and child health services. Despite some progress in accessing these services in sub-Saharan African countries, improvement in reducing the unmet need for effective post-partum contraceptives is slow.5 According to various literature reviews, the evidence is often weak or incomplete in terms of research design and quality, details about the interventions, or women's perspectives.6, 7, 8 There is also a paucity of studies looking at operationally feasible ways to integrate post-partum family planning into existing antenatal and postnatal care, for example through meaningful involvement of community actors.2 In response, the Yam Daabo trial (meaning “your choice” in Mooré, one of the local languages in Burkina Faso) was designed to test the effectiveness of a low-technology, post-partum family planning intervention package, established using participatory action research, on contraceptive uptake.9 In predominantly rural settings in Burkina Faso, results showed that the use of modern contraceptives almost doubled at 12 months post partum, reaching 55% of women in the intervention group versus 29% of women who received routine care in the control group (adjusted prevalence ratio 1·79, 95% CI 1·30–2·47).9 Significant differences were also found in the proportion of women in the two groups using long-acting contraceptives, mostly implants (29% vs 17%), and short-acting methods (26% vs 12%), with injectables prevailing.
The objective of our twin study was to assess the effect of a similar package on modern contraceptive prevalence in urban settings of Kinshasa province, Democratic Republic of the Congo, where poverty, hunger, and underdevelopment are important challenges.10 Demographic and Health Survey data from DR Congo (2013–14) show a country-wide median breastfeeding duration of 22 months (2 months of exclusive breastfeeding), a median post-partum sexual abstinence of 8 months, and median amenorrhoea duration of 13 months.11 The median duration of birth intervals is 31 months, but 27% of babies are born less than 24 months after their previous sibling. Most women (88%) attend antenatal care and deliver in a health facility (80%). Around half (44%) of women who have given birth receive postnatal care within 48 h of delivery, and 45% of children aged 12–23 months are adequately immunised. Despite the use of routine reproductive, maternal, neonatal, and child health services that should allow regular opportunities to address the contraceptive needs of women and couples post partum, the unmet need for family planning among these women in the whole country is high at 66% immediately after birth, 48% after 6 months of amenorrhoea, and 36% at the end of amenorrhoea.12 In Kinshasa province, 45% of married women of reproductive age use any type of contraceptive method (vs 20% of women country-wide), and 19% use a modern method (vs 8%). The unmet need for family planning is high in Kinshasa (23% of women who are married or in a union and want to stop or delay childbearing have no access to family planning services) and nationwide (28%). UN data show that maternal mortality is very high (693 of 100 000 livebirths in 2015), as is total fertility (61 children per woman in 2016).13
Methods
Study design and participants
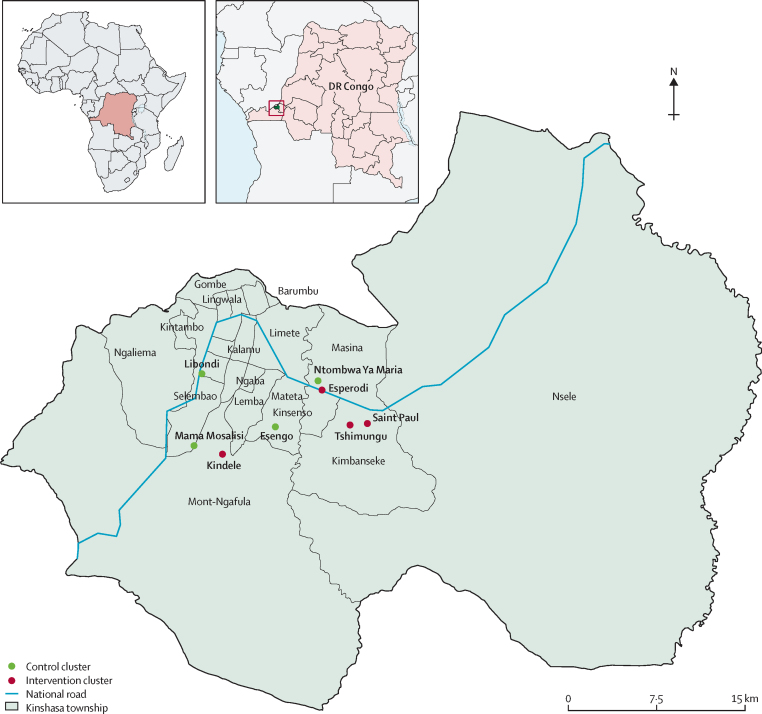
The Yam Daabo study was a pragmatic, cluster-randomised, multi-interventional trial done in eight primary health centres offering reproductive, maternal, neonatal, and child health services in Kinshasa province. The centres were Esengo, Esperodi, Kindele, Libondi, Mama Mosalisi, Ntombwa Ya Maria, Saint Paul, and Tshimungu (figure 1, appendix p 2).14 The central research centre in DR Congo was based at the School of Public Health of the University of Kinshasa, and the overall research coordinating centre was at WHO headquarters (Geneva, Switzerland). Our post-partum family planning intervention package, identified during the formative study phase, comprised three facility-based interventions (ie, refresher training of service providers, regularly scheduled and strengthened supportive supervision of providers, and enhanced availability of contraceptive services 7 days a week), and three individual-based interventions (ie, a post-partum family planning counselling tool, appointment cards for women, and invitation letters for partners to attend appointments). The implementation of three of the six components favoured a cluster design for the trial, with randomisation at the level of the study centre. Furthermore, potential contamination between intervention and control groups for the other three individual-based interventions could be minimised by the cluster design. The intervention package was offered to women allocated to the intervention group and routine care (information about the benefits for mothers and children of spacing births and provision of basic contraceptives) to those allocated to the control group (appendix pp 4–6).
Figure 1.
Kinshasa province and study area with intervention clusters (red) and control clusters (green)
Health facilities were eligible if they provided a continuum of antenatal, delivery, and postnatal care; had at least three modern contraceptive methods available, including a barrier method such as condoms, a short-term method such as pills, a long-term method such as an intrauterine device, and referrals for permanent methods; experienced no depletion of stock of contraceptives during the 6 months preceding the study; cumulated an average of at least 30 deliveries per month; were located within a 4 h drive from the research centre; and were willing to participate. All pregnant women attending the clinic for antenatal care were invited to participate in the study. They were eligible if they were in their third pregnancy trimester; their health and pregnancy situation allowed for a birth at the health centre; they had the intention to attend antenatal, delivery, and postnatal care at the health centre; they were not participating in another study; and they provided written informed consent (verbal informed assent for minors aged <18 years and written assent from their parents or guardians).
Study approval was obtained by the School of Public Health (University of Kinshasa) ethics committee in DR Congo (reference number ESP/CE/039b/2016) and WHO Research Ethics Review Committee in Geneva, Switzerland (protocol ID RPC757). This study is registered with the Pan African Clinical Trials Registry (PACTR201609001784334).
Randomisation and masking
The eight sites were matched in pairs according to the number of monthly births, the ratio of health workers per population in the health zone, and the urban and suburban settings, without specific thresholds for these criteria (appendix p 3). Within each of the four study centre pairs, a site was randomly assigned to the intervention group or to the control group (1:1 ratio). This computer-based randomisation was done four times, once for each pair. No restriction in the randomisation process was required. All eligible participants consecutively presenting at the health centres were included in the clusters. The nature of the interventions did not allow masking to cluster assignment of participants, health staff, research assistants assigned to each centre, and the rest of the research team members. Only data analysts were masked to cluster assignment (ie, they received no information about the cluster allocation and did not interact with the field team). Masking of data was achieved by replacing study site names with numbers and group names with letters.
Procedures
The rationale for our trial approach is based on WHO's seminal 2013 publication of programmatic strategies for post-partum family planning2 and three other systematic reviews (without meta-analysis) on post-partum family planning interventions in low-income and middle-income countries published between 2014 and 2016.6, 7, 8 These publications suggest that the following interventions could have a positive effect on post-partum contraceptive uptake: counselling activities during antenatal care; provision of post-partum family planning information, education, and counselling materials before women are discharged from health facilities after birth, including provision of emergency contraception for women using the lactational amenorrhoea method; promotion by community-based counsellors of exclusive breastfeeding practices before 5−6 months post partum; access to contraceptive methods immediately after birth, including intrauterine devices; provider competencies in quality counselling and the provision of quality services with several readily available products; and long programmes with several contact points between providers and clients across the continuum of care versus short antenatal interventions.
The intervention package was designed through participatory action research and the process and contents were detailed elsewhere (appendix pp 4–6).15, 16 The working hypotheses for the selection of the post-partum family planning interventions to be included in the package were as follows. Interventions should strengthen existing antenatal and postnatal care services by means of low-dose, high-frequency interventions. We assumed that they would be more effective than high-dose, low-frequency strategies that promote a specific method over another and restrict services to a narrow timeframe, such as before home discharge after delivery, or during the 6-week postnatal care visits dedicated to post-partum family planning. Drawing from participatory action research principles,17 key actors, including clients and providers, should be meaningfully engaged in the package design, implementation, and research. Such participation would ensure that the package reflects field reality, including restricted clinical capacity and human and financial resources, and is feasible, sustainable, and scalable while also aligned with national health policies.
We followed WHO recommendations for medical eligibility of contraceptive use to define modern contraceptives appropriate up to 12 months post partum.18 We categorised modern contraceptives as: long-acting and reversible, including implants and intrauterine devices; short-acting, including injectables, pills, emergency contraception, male and female condoms, and other less commonly used methods; permanent methods (male and female sterilisation); and lactational amenorrhoea. Contraceptives were further defined as modern and appropriate and non-modern or inappropriate. Non-modern contraceptives used traditional methods such as withdrawal and abstinence. Inappropriate contraceptives were lactational amenorrhoea if used after 6 months and calendar-based methods if used during the first 12 months post partum. We assumed that as most women breastfeed up to 2 years, they would not fulfil the initiation requirement of calendar-based methods of having at least three regular menstrual cycles before 12 months.
Participants received individual-based interventions during third-trimester antenatal care visits and postnatal care follow-up visits according to national practice (typically on clinic discharge at 24–48 h after delivery, then at 1 week, 6 weeks, then at months 6 and 9 post partum) before trial exit at month 12. At each visit, providers were instructed to use the counseling tool to offer information and services and give women an appointment card for the following visit. Providers discussed the invitation letter for the partner during the first study visit and let the participants choose whether to take it. In all study sites, research assistants held a journal where they recorded events that could have an influence on the implementation or outcomes of the trial for its interpretation, but they were not included in the analysis and did not modify the trial (eg, staff turnover, depletion of stocks of contraceptives, local civil unrest).
Outcomes
The primary outcome was prevalence of use of modern contraceptives at month 12 post partum. We amended our original protocol14 to extend the follow-up period from month 9 to month 12 post partum to allow for better comparability with other published research, after the initial funder's research grant deadline was chosen. This extension was approved by the ethics committees at WHO headquarters and country level. We also reported key secondary outcomes after delivery: prevalence of modern contraceptive adoption before discharge from the health facility (assessed within 48 h after delivery); at 1 week (assessed on day 7); at 6 weeks (assessed at 45 days), which coincided with the visit that women are encouraged to attend to specifically discuss post-partum family planning options as per national recommendations; and at 6 months (assessed on the first day of month 6), which corresponded to the latest point when transition from the lactational amenorrhoea method to another modern method should occur. Contraceptive method mix was also reported for each measurement point. Serious adverse events had to be announced to the principal investigator who assessed whether they were related to the study.
Statistical analysis
We used the following assumptions when calculating the target population size. Women in the intervention group wanting to restrict or space their pregnancies would already use a modern contraceptive method at 6 months after delivery when the lactational amenorrhoea method would no longer be suitable. Therefore, although the main study outcome was prevalence of modern contraceptive use at 12 months post partum, we used country data at 6 months to determine our population size. The population size was estimated using the 2013–14 Demographic and Health Survey data:11 we assumed a 5% uptake of modern contraceptives in the control group (on the basis of reported prevalence of modern contraceptive use of 5% in women at 6 months post partum),12 and an increase to 20% in the intervention group (on the basis of reported prevalence of modern contraceptive use of 15% in women in the general population, to which we added 5 percentage points given the high unmet need for post-partum family planning).19 Assuming an intracluster correlation coefficient of 0·02 (no specific evidence existed for its value from the literature),20 the experimental group and the control group each had four study sites with at least 60 participants per site.21 This number allowed for a statistical power of 93% to detect a difference of 15% to a level of significance of 5% at the individual level (and not at the facility level). Assuming a 10% participant loss to follow-up, each facility recruited at least 70 pregnant women for a cohort of at least 280 pregnant women in each study group, and in total at least 560 participants.
The research coordination team at WHO in Geneva developed the paper-based study case report forms with inputs from the country researchers who tested advanced drafts with an appropriate sample of mock clients from sites included in the formative study phase. Data for the trial were collected by trained research assistants when women attended clinic visits or by phone or a home visit when they did not. Data entry was done by the research team of the School of Public Health in Kinshasa and checked in Geneva by use of OpenClinica (version 3.11).
Data from all eligible participants were analysed with IBM SPSS Statistics (version 21.0), R (version 3.4.3), and WINPEPI (PEPI-for-Windows, version 11.50). Generalised linear mixed models (log binomial and log Poisson) were used to assess the effect of the package on prevalence ratios (PRs) of main outcomes with 95% CIs, comparing intervention and control groups, while accounting for clustering and adjusting for potential confounders (women's baseline characteristics that were imbalanced between groups). Fisher's exact test was used when samples were small and models did not converge. WINPEPI was used to obtain a post-hoc global estimation of the intracluster correlation coefficient. We did an intention-to-treat analysis that included all women irrespective of whether they continued to visit the clinic after enrolment. Because women had to see a provider to receive the intervention package, we planned to do a per-protocol analysis focused on participants who attended all recommended follow-up visits, but this was not done because of low trial completion by participants in both groups.
Role of the funding source
The study funder had no role in study design, data collection, data analysis, data interpretation, or writing the article. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
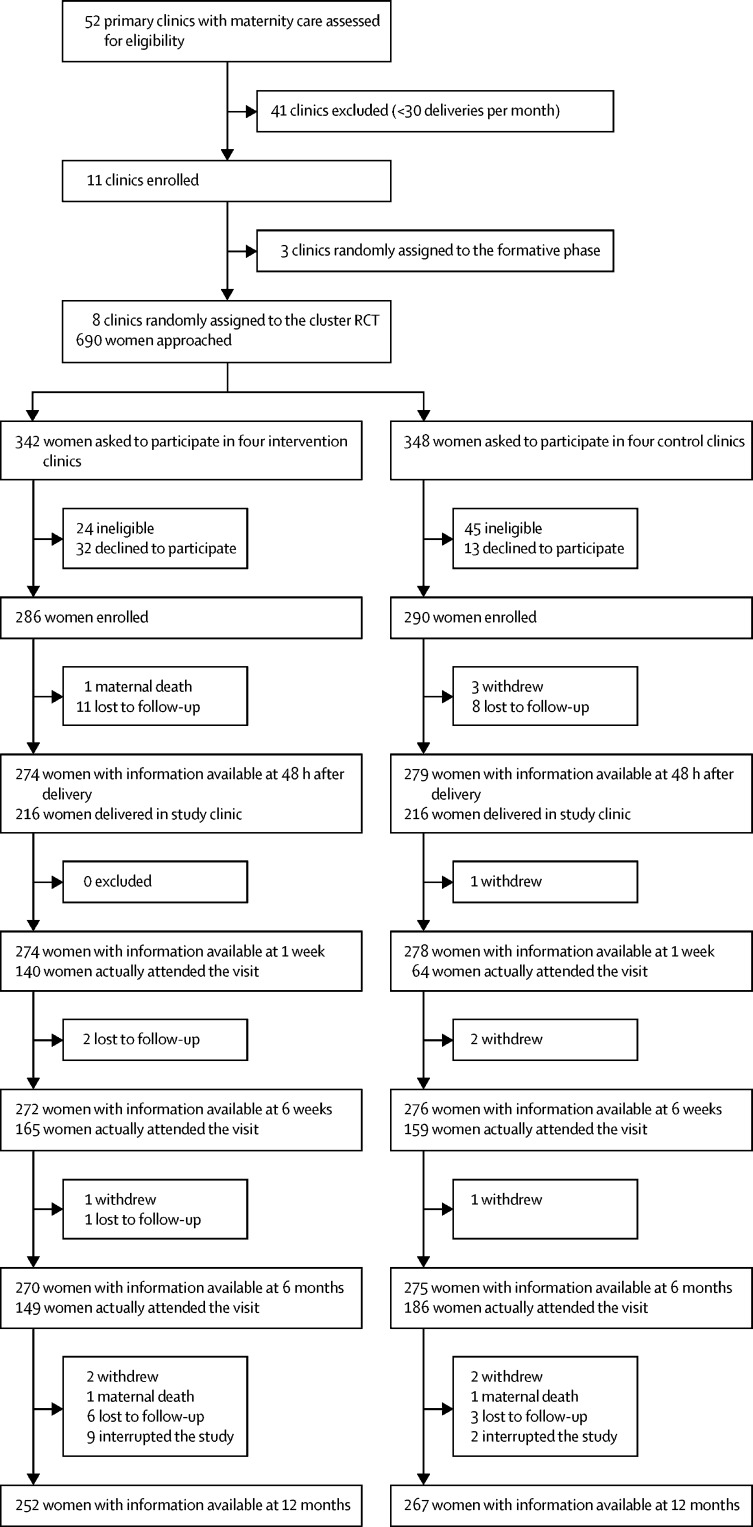
Of 52 primary clinics assessed for eligibility, eight met the criteria and were randomised to the two groups in four pairs (four clinics in each group). From July 1, 2016, to Feb 2, 2017, of 690 women asked to participate, 576 (83%) were enrolled in the study (286 in the intervention group and 290 in the control group), of whom 519 (90%; 252 in the intervention group and 267 in the control group) completed the study exit interview at 12 months and were included in the intention-to-treat analysis (full details on patient exclusion and loss to follow-up are in figure 2). Exit interviews concluded on Feb 1, 2018. More than three-quarters of women in both groups gave birth in their assigned study clinics (216 [79%] of 274 women in the intervention group evaluable at the time of delivery and 216 [77%] of 279 in the control group). Between the intervention and control arms, the proportion of timely visits differed significantly at 1 week (140 [51%] of 274 women in the intervention group vs 64 [23%] of 278 in the control group, p<0·0001) and 6 months (149 [55%] of 270 vs 186 [67%] of 275, p=0·0028), but not at 6 weeks (165 [61%] of 272 and 159 [58%] of 276, p=0·47). The proportion of participants who attended all the recommended follow-up visits from clinic discharge to 12 months post partum differed significantly between the two groups (p<0·0001) with 39 (15%) of 252 in the intervention group versus six (2%) of 267 in the control group. As only six participants in the control group and 39 in the intervention group completed all recommended visits, per-protocol analysis was not done. Between the intervention and control groups, some baseline differences were noted (table 1), including education level, living setting, usual travel time to health centre, and employment.
Figure 2.
Trial profile
RCT=randomised controlled trial.
Table 1.
Baseline characteristics of study population at enrolment
| Intervention (n=286) | Control (n=290) | ||
|---|---|---|---|
| General characteristics of women | |||
| Median maternal age, years | 28 (15–46) | 28 (15–42) | |
| Marital status | |||
| Married or in a relationship | 264 (92%) | 262 (90%) | |
| Single, widow, separated, or divorced | 22 (8%) | 28 (10%) | |
| Education level | |||
| Never attended school | 7 (2%) | 5 (2%) | |
| Primary school | 38 (13%) | 67 (23%) | |
| Secondary school | 187 (65%) | 184 (63%) | |
| Higher education | 54 (19%) | 34 (12%) | |
| Able to read an official language | 249 (87%) | 259 (89%) | |
| Living setting | |||
| Suburban | 282 (99%) | 117 (40%) | |
| Urban | 4 (1%) | 173 (60%) | |
| Employment | |||
| Housewife or farmer | 196 (69%) | 110 (38%) | |
| Student | 11 (4%) | 13 (5%) | |
| Business | 20 (7%) | 18 (6%) | |
| Other | 59 (21%) | 149 (51%) | |
| Usual travel time to health centre | |||
| <30 min | 137/219 (63%) | 68/282 (24%) | |
| 30–60 min | 61/219 (28%) | 164/282 (59%) | |
| >1 h | 21/219 (10%) | 50/282 (18%) | |
| Maternity and contraception | |||
| Previous pregnancies | |||
| Yes | 233 (81%) | 229 (79%) | |
| Median number | 2 (0–11) | 2 (0–9) | |
| Livebirths | 210/233 (90%) | 214/229 (93%) | |
| Living biological children | |||
| Yes | 210/233 (90%) | 212/229 (93%) | |
| Median number | 2 (0–9) | 2 (0–8) | |
| Age of last child | |||
| ≥2 years | 175/207 (84%) | 184/212 (87%) | |
| Breastfeeding of last child | 216/233 (93%) | 212/229 (93%) | |
| Length of exclusive breastfeeding, months | 210/233 (90%) | 212/229 (93%) | |
| <1 | 17/210 (8%) | 7/212 (3%) | |
| 1–5 | 127/210 (61%) | 128/212 (60%) | |
| ≥6 | 66/210 (31%) | 77/212 (37%) | |
| Interpregnancy interval, years | |||
| ≥2 | 121/230 (53%) | 133/226 (59%) | |
| <2 | 109/230 (47%) | 93/226 (41%) | |
| Current pregnancy | |||
| Planned | 92 (32%) | 84/281 (30%) | |
| Any place of delivery | 274 (96%) | 274/281 (98%) | |
| Delivered in study health centre | 241/274 (88%) | 235/274 (84%) | |
| Main reason of non-use of contraception | 146 (51%) | 139 (48%) | |
| Did not know about methods | 84/146 (58%) | 60/139 (43%) | |
| Wanted pregnancy | 24/146 (16%) | 33/139 (24%) | |
| Partner or family opposition | 21/146 (14%) | 6/139 (4%) | |
| Fear of side-effects | 10/146 (7%) | 17/139 (12%) | |
| Other | 7/146 (5%) | 23/139 (17%) | |
| Contraception during year before pregnancy | 140 (49%) | 151 (52%) | |
| Injectable | 7/140 (5%) | 11/151 (7%) | |
| Pill | 17/140 (12%) | 15/151 (10%) | |
| Implant | 6/140 (4%) | 8/151 (5%) | |
| Male condom | 34/140 (24%) | 14/151 (10%) | |
| Standard-day method | 31/140 (22%) | 41/151 (27%) | |
| Intrauterine device | 0 | 2/151 (1%) | |
| Traditional methods | 45/140 (32%) | 60/151 (43%) | |
| General characteristics of male partners* | |||
| Age reported | 233 (81%) | 222 (77%) | |
| Median age, years | 35 (17–57) | 35 (20–69) | |
| Education level, | 264 | 262 | |
| Never attended school | 3 (1%) | 4 (1%) | |
| Primary school | 7 (3%) | 19 (7%) | |
| Secondary school | 154 (59%) | 143 (55%) | |
| Higher education | 100 (38%) | 96 (37%) | |
| Able to read an official language | 258 | 251 | |
| Yes | 251 (97%) | 246 (98%) | |
| Employment | 264 | 261 | |
| None | 27 (10%) | 14 (5%) | |
| Student | 2 (1%) | 1 (1%) | |
| Farmer | 10 (4%) | 2 (1%) | |
| With regular salary | 113 (43%) | 96 (37%) | |
| Business | 21 (8%) | 30 (11%) | |
| Crafts | 2 (1%) | 18 (7%) | |
| Other | 89 (34%) | 100 (38%) | |
Data are N, n/N (%), or median (range).
Information collected from the enrolled women about their partners; male partners were not interviewed directly.
At 12 months post partum, the prevalence of use of modern contraceptives in the intervention group was not significantly different from that in the control group (115 [46%] of 252 women in the intervention group vs 94 [35%] of 267 in the control group; adjusted PR 1·58, 95% CI 0·74−3·38; table 2). The prevalence ratio of long-acting contraceptive use in the intervention group was more than four times higher than that in the control group, mostly because of implants (table 2). In both groups, fewer than 1% of women used intrauterine devices and no one opted for sterilisation. The use of short-acting contraceptives was comparable between both study groups, with a preference for male condoms, injectables, and pills. The proportions of users of short-acting and long-acting contraceptives were similar in the intervention group, whereas women in the control group were four times more likely to use short-acting than long-acting contraceptives. Women in the intervention group tended to resort less to non-modern or inappropriate methods than their counterparts in the control group, with the standard-day method being the most common, followed by withdrawal.
Table 2.
Prevalence of use of contraceptive methods at 12 months post partum
| Intervention (n=252) | Control (n=267) | Adjusted PR (95% CI)* | |||
|---|---|---|---|---|---|
| Modern and appropriate methods | 115 (46%) | 94 (35%) | 1·58 (0·74–3·38) | ||
| Long-acting or permanent methods | 56 (22%) | 18 (7%) | 4·47 (2·05–9·74) | ||
| Implant | 55 (22%) | 16 (6%) | 4·36 (1·96–9·70) | ||
| Intrauterine device | 1 (<1%) | 2 (1%) | 0·53 (0·49–2·05)† | ||
| Female sterilisation | 0 | 0 | 0 | ||
| Vasectomy | 0 | 0 | 0 | ||
| Short-acting methods | 59 (23%) | 76 (28%) | 0·92 (0·29–2·98) | ||
| Injectable | 20 (8%) | 23 (9%) | 1·20 (0·31–4·60) | ||
| Pill | 12 (5%) | 22 (9%) | 0·75 (0·29–1·95) | ||
| Male condom | 27 (11%) | 27 (10%) | 1·02 (0·31–3·40) | ||
| Female condom | 0 | 0 | 0 | ||
| Emergency contraception | 0 | 4 (2%) | 0‡ | ||
| Spermicide | 0 | 0 | 0 | ||
| Non-modern or inappropriate methods | 18 (7%) | 47 (18%) | 0·45 (0·13–1·54) | ||
| Lactational amenorrhoea method | 0 | 0 | 0 | ||
| Withdrawal | 2 (1%) | 9 (3%) | 0·09 (0·02–0·49) | ||
| Abstinence | 2 (1%) | 3 (1%) | 1·81 (0·15–22·44) | ||
| Standard-day method | 11 (4%) | 32 (12%) | 0·38 (0·08–0·31) | ||
| Others | 3 (1%) | 3 (1%) | 0·99 (0·03–34·68) | ||
| No method | 119 (47%) | 126 (47%) | 0·84 (0·31–2·24) | ||
Data are n (%). PR=prevalence ratio.
Accounting for clustering effect and adjusted for living setting, education level, employment, and usual travel time to health centre.
Adjusting for clustering effect not possible.
Two-sided p value Fisher's exact test >0·05.
Findings at 12 months reflected earlier similar trends in both groups: implant uptake was already reported at 48 h in the intervention group and increased at each of the follow-up timepoints (table 3). Conversely, women in the control group began using implants only at 6 months (only one woman opted for an implant at 6 weeks; table 3). By comparison, uptake of short-acting contraceptives was reported earlier in the control group than in the intervention group and also increased at each of the follow-up timepoints, with male condoms being the most prevalent, followed by pills and injectables (table 3). At 6 weeks, a significantly higher proportion of women in the control group already reported the use of non-modern or inappropriate methods (mainly withdrawal), compared with women in the intervention group (table 3). However, at 6 months, the difference between women who used non-modern or inappropriate methods was already not significant (table 3). In both groups, few women used the lactational amenorrhoea method or intrauterine devices and none chose sterilisation (table 3). The post-hoc global estimation of the intracluster correlation coefficient was 0·153 (0·121 for the intervention group and 0·184 for the control group).
Table 3.
Prevalence of use of contraceptive methods at 48 hours, 1 week, 6 weeks, and 6 months post partum
| Intervention (n=274) | Control (n=279) | Adjusted PR (95% CI)* | ||
|---|---|---|---|---|
| 48 hours | ||||
| Modern and appropriate methods | 9 (3%) | 3 (1%) | 1·83 (0·43–7·74) | |
| Long-acting or permanent methods | 6 (2%) | 0 | 0† | |
| Implant | 5 (2%) | 0 | 0† | |
| Intrauterine device | 1 (<1%) | 0 | 0 | |
| Female sterilisation | 0 | 0 | 0 | |
| Vasectomy | 0 | 0 | 0 | |
| Short-acting methods | 0 | 2 (1%) | 0 | |
| Pill | 0 | 0 | 0 | |
| Male condom | 0 | 2 (1%) | 0 | |
| Female condom | 0 | 0 | 0 | |
| Spermicide | 0 | 0 | 0 | |
| Lactational amenorrhoea method | 3 (1%) | 1 (<1%) | 2·60 (0·20–33·60) | |
| Non-modern or inappropriate methods | 1 (<1%) | 0 | 0 | |
| Abstinence | 1 (<1%) | 0 | 0 | |
| No method | 264 (96%) | 276 (99%) | 0·98 (0·77–1·24) | |
| 1 week | ||||
| Modern and appropriate methods | 15 (5%) | 3 (1%) | 2·09 (0·78–10·75) | |
| Long-acting or permanent methods | 12 (4%) | 0 | 0 | |
| Implant | 11 (4%) | 0 | 0 | |
| Intrauterine device | 1 (<1%) | 0 | 0 | |
| Female sterilisation | 0 | 0 | 0 | |
| Vasectomy | 0 | 0 | 0 | |
| Short-acting methods | 0 | 2 (1%) | 0 | |
| Pill | 0 | 0 | 0 | |
| Male condom | 0 | 2 (1%) | 0 | |
| Female condom | 0 | 0 | 0 | |
| Spermicide | 0 | 0 | 0 | |
| Lactational amenorrhoea method | 3 (1%) | 1 (<1%) | 2·51 (0·20–31·11) | |
| Non-modern or inappropriate methods | 1 (<1%) | 1 (<1%) | 1·01 (0·06–16·14)‡ | |
| Abstinence | 1 (<1%) | 1 (<1%) | 1·01 (0·06–16·14)‡ | |
| No method | 258 (94%) | 274 (99%) | 0·96 (0·75–1·22) | |
| 6 weeks | ||||
| Modern and appropriate methods | 32/272 (12%) | 16/276 (6%) | 6·79 (0·86–53·79) | |
| Long-acting or permanent methods | 21/272 (8%) | 1/276 (<1%) | 72·37 (4·75–1103·82) | |
| Implant | 20/272 (7%) | 1/276 (<1%) | 66·10 (4·33–1009·06) | |
| Intrauterine device | 1/272 (<1%) | 0 | 0 | |
| Female sterilisation | 0 | 0 | 0 | |
| Vasectomy | 0 | 0 | 0 | |
| Short-acting methods | 9/272 (3%) | 15/276 (5%) | 3·21 (0·02–651·22) | |
| Injectable | 1/272 (<1%) | 0 | 0 | |
| Pill | 0 | 0 | 0 | |
| Male condom | 8/272 (3%) | 15/276 (5%) | 2·07 (0·00–4238·70) | |
| Female condom | 0 | 0 | 0 | |
| Emergency contraception | 0 | 0 | 0 | |
| Spermicide | 0 | 0 | 0 | |
| Lactational amenorrhoea method | 2/272 (<1%) | 0 | 0 | |
| Non-modern or inappropriate methods | 3/272 (1%) | 19/276 (7%) | 0·10 (0·01–0·90) | |
| Withdrawal | 1/272 (<1%) | 9/276 (3%) | 0·06 (0·00–0·91) | |
| Abstinence | 2/272 (1%) | 8/276 (3%) | 0·23 (0·04–1·24) | |
| Standard-day method | 0 | 1/276 (<1%) | 0 | |
| Others | 0 | 1/276 (<1%) | 0 | |
| No method | 237/272 (87%) | 241/276 (87%) | 1·17 (0·89–1·52) | |
| 6 months | ||||
| Modern and appropriate methods | 82/269 (30%) | 58/275 (21%) | 1·58 (0·60–4·15) | |
| Long-acting or permanent methods | 41/269 (15%) | 12/275 (4%) | 3·43 (1·39–8·48) | |
| Implant | 40/269 (14%) | 10/275 (4%) | 4·06 (1·58–10·40) | |
| Intrauterine device | 1/269 (<1%) | 2/275 (1%) | 0·92 (0·04–23·32) | |
| Female sterilisation | 0 | 0 | 0 | |
| Vasectomy | 0 | 0 | 0 | |
| Short-acting methods | 40/269 (15%) | 46/275 (17%) | 1·04 (0·26–4·14) | |
| Injectable | 13/269 (5%) | 5/275 (2%) | 5·24 (1·02–26·85) | |
| Pill | 4/269 (1%) | 8/275 (3%) | 1·09 (0·20–5·89) | |
| Male condom | 23/269 (9%) | 29/275 (11%) | 0·58 (0·07–4·83) | |
| Female condom | 0 | 0 | 0 | |
| Emergency contraception | 0 | 4/275 (1%) | 0 | |
| Spermicide | 0 | 0 | 0 | |
| Lactational amenorrhoea method | 1/269 (<1%) | 0 | 0 | |
| Non-modern or inappropriate methods | 16/269 (6%) | 30/275 (11%) | 0·64 (0·11–3·78) | |
| Withdrawal | 5/269 (2%) | 9/275 (3%) | 0·28 (0·08–1·01) | |
| Abstinence | 2/269 (1%) | 1/275 (<1%) | 2·04 (0·19–22·42)† | |
| Standard-day method | 9/269 (3%) | 15/275 (6%) | 0·65 (0·15–2·72) | |
| Others | 0 | 5/275 (2%) | 0 | |
| No method | 171/269 (64%) | 187/275 (68%) | 0·88 (0·43–1·79) | |
Data are n (%) or n/N (%), unless otherwise indicated. PR=prevalence ratio.
Accounting for clustering effect and adjusted for living setting, education level, employment, and usual travel time to health centre.
2-sided p value Fisher's exact test >0·05.
Adjusting for clustering effect not possible.
Three of the four control health centres (Esengo, Libondi, and Mama Mosalisi) benefited from family planning activities that were initiated by other non-governmental organisations after study enrolment started. These activities, albeit not focused on post-partum family planning, were linked to HIV or family planning programming and were also offered to women post partum, including to Yam Daabo participants. The Esengo facility received support from the US Agency for International Development to deliver free family planning services, including long-acting and reversible contraceptives. The Libondi centre was supported by the Global Fund, Pathfinder, and the International Center for AIDS Care and Treatment Programs for free contraceptive methods and services (except for implant, for which the method was free but insertion services were not). The Elisabeth Glaser Pediatric Aids Foundation backed the Mama Mosalisi site with free condom distribution and other family planning methods and services (except for implant, for which the method was free but insertion services were not). None of the other study sites reported support from external partners with potential impact on modern contraceptive uptake.
Discussion
Although not significant for the primary outcome, the results of the Yam Daabo trial in DR Congo were overall consistent with those from rural Burkina Faso. They showed that a package strategy combining six low-technology interventions focusing on post-partum family planning was effective in increasing the use of modern contraceptives, particularly implants, up to 12 months after childbirth in women from urban settings in Kinshasa. Along with its twin study in rural Burkina Faso, our study was, to our knowledge, pioneering research using a post-partum family planning package approach in Africa. It adds to the relatively scarce literature on the effectiveness of different intervention packages on post-partum contraceptive use. The Yam Daabo package showed several differences and similarities in its implementation in Burkina Faso and DR Congo.
First, the dynamic of overall prevalence of modern contraceptive use over 12 months and the resulting PRs and level of significance differed between countries. An explanation might be the fact that the integrity of routine care in control facilities in DR Congo was compromised by the unforeseen family planning activities initiated by other non-governmental organisations. In terms of the prevalence dynamic, the use of modern contraceptives in the intervention groups in both countries increased from the time of clinic discharge up to 12 months post partum. This dynamic differed in the control groups, as the prevalence in Burkina Faso did not change from month 6 to month 12, when it reached 29%, a proportion that did not overtake the 30% in national prevalence projected for 2018.22 In DR Congo, however, the prevalence in the control group kept increasing until 12 months, when it reached 35% and surpassed by 8 percentage points the 27% in projected prevalence for 2017 for married women in Kinshasa.23 It was unlikely that women from control study sites went to intervention sites to receive the package, or that components of the package reached contraceptive services in control sites, as neither situations were reported by service providers or research assistants assigned to each of the centres. Instead, externally funded family planning activities documented by the research team in three of the four control sites probably affected the uptake of modern contraceptives. With few resources and investments from the Ministry of Health, most of the health programmes and facilities in DR Congo have been relying on external sources of support.24 Although it was a prerequisite for health centres to be free from other family-planning-related support at the time of study site enrolment, we could not prevent centres from receiving other programmatic assistance for family planning during the study period. As this was a pragmatic trial, which reflected real-life settings and events, we decided to pursue the study course regardless of the confounding effect of external programmatic assistance. In the end, the Yam Daabo intervention package was compared with other interventions that had relevant family planning components and were offered to women in the control group.
Second, although the 12-month PR in Kinshasa was not significant probably because of these interferences, it is important to emphasise the clinically significant differences in the types of contraceptive methods between the intervention and control groups: long-acting and reversible methods are well known to fail less often than short-acting methods.25 The mix of modern contraceptive methods in the intervention group reflected, to some extent, the one reported in Kinshasa in 2017 (38% implants, 2% intrauterine devices, 20% injectables, 17% pills, 17% male condoms, and 6% other methods).23 As in Burkina Faso, women in the intervention group in Kinshasa used more implants, which have a failure rate of 0·05% within the first year of typical use and are therefore more cost-effective than short-acting methods for pregnancy prevention (male condoms have a failure rate of 18%, pills 9%, and injectables 6%).25 In Burkina Faso, there was no difference between groups regarding the use of non-modern inappropriate contraceptives, unlike in Kinshasa where more women in the control group tended to use such methods, including withdrawal or methods based on fertility awareness, which have a typical failure rate of use of 22% and 24%.25
When compared with general contraceptive services that are not offered post partum, providers' specialised knowledge of the appropriate timing of different contraceptive methods after childbirth, breastfeeding status, or menstrual return is crucial for effective post-partum counselling of family planning and contraceptive method provision. For instance, the 2015 revision of WHO's medical eligibility criteria for contraceptive use allowed implants for all women post partum without restriction. This is a key change in clinical practice, especially for auxiliary midwives or nurses who typically staff primary health-care centres and are allowed to provide implants but not intrauterine devices. This revision was disseminated nationwide by the Ministry of Health with key changes related to post-partum contraceptives integrated into the Yam Daabo counselling tool. As such, although our approach did not focus on a specific method to the detriment of another, results showed that women in the post-partum family planning intervention group were more likely to adopt contraceptives with higher effectiveness and were less likely to use non-modern or inappropriate methods, such as withdrawal or standard-day method than women in the control group.
Third, Yam Daabo's low-dose, high-frequency approach encouraged women to attend routinely recommended follow-up visits to ensure time-appropriate contraceptive uptake. In Burkina Faso, client counselling supported by the post-partum family planning tool, in conjunction with appointment card distribution, might have contributed to significant differences in timely visits, with more women in the intervention group attending scheduled visits at 6 weeks (64%) than women in the control group (34%) and 6 months (16% vs 6%). However, results in Kinshasa showed no difference at 6 weeks and a marked difference in visits at 6 months in favour of the control group. This higher attendance in the control group could further indicate the interference in routine care that other family planning-related activities (eg, free family planning services and methods, including for long-acting contraceptives) potentially exerted on control sites.
Fourth, as for the very infrequent use of lactational amenorrhoea as a contraceptive method on facility discharge, at 1 week, 6 weeks, and 6 months post partum, results in Kinshasa did not contrast with those from Burkina Faso and reflected the situation in other low-income countries, where early supplementation of breastfeeding with other fluids anchored in customary practices are common.26
Fifth, our study contributed to a global post-hoc estimation of the intracluster coefficient, which was about 0·15, and larger than the initially assumed value of 0·02 and the 0·03 estimated post hoc in the Burkina Faso trial. Under this assumption of 0·15, the power to detect the expected difference is low at less than 50%, which is consistent with the non-significant results for the primary outcome. The post-hoc estimation of the intracluster coefficient was not as large for other relevant outcomes, such as the prevalence of long-acting or permanent methods at 12 months, which was 0·01.
There were several limitations to our study. First, the integrity of routine care in three of the four control sites could not be preserved during the study period. In DR Congo, the Yam Daabo intervention package was not compared with typical routine care, but with other family-planning-related activities. Additionally, incorporation of elements of the package into other sites could not be fully excluded. However, our post-partum family-planning-focused intervention, compared with other family-planning-related activities in control sites, proved more effective in enabling women to find a combination of contraceptive methods that reduced the use of non-modern or inappropriate methods and offered increased pregnancy protection. Second, the trial included eight clusters that were enough according to our statistical hypotheses but were still relatively few, potentially increasing the risk of groups not being fully similar. Third, this pragmatic trial based in health centres did not permit masking of participants, providers, and research assistants, and we could not rule out assessment or observer bias. Fourth, we could not exclude an enhanced effect size due to bias towards socially desirable answers, especially in this facility-based research, although independent research assistants were employed. However, information bias and assessment bias could not alone contribute to the increased use of effective contraceptives reported at different measurement points. Fifth, policy makers and programme managers might prefer a single most effective intervention over a package approach. Our research design was not meant to discriminate which intervention in the package had the greatest effect on contraceptive use, for which additional research would be needed. However, interventions were selected on the basis of the perspectives of multiple stakeholders and integrated into the package because they were perceived to be simple, easily accessible, and adapted to resource-constrained settings, while strengthening existing services, making them easy to implement and replicate. Sixth, women using modern contraceptives at 12 months should be followed up until 2 years and beyond to study their patterns of contraceptive use and evaluate whether they continue to be more successful in limiting or spacing pregnancies according to their reproductive choices. We acknowledge the limitations of using prevalence of modern contraceptive use as our primary outcome because there is potential for contraceptive uptake to be encouraged to achieve targets, without accounting for women's or couples' fertility intentions and their right to determine the timing of their pregnancies.
Reflecting the effectiveness of a similar intervention package in rural Burkina Faso, the implementation in urban settings of six low-technology, post-partum family planning interventions established through multi-stakeholder participation increased the proportion of women choosing modern contraceptives post partum, and in particular implants, while avoiding the use of less effective methods up to 12 months after childbirth. Other primary health centres in Kinshasa or in similar urban settings in DR Congo or other countries could benefit from introducing a similar package to reduce the unmet need for family planning in women and couples post partum.
Data sharing
Requests for the anonymised, coded trial data can be made to the Department of Reproductive Health and Research, WHO (reproductivehealth@who.int). Data sharing is subject to WHO data-sharing policies and data-use agreements with the participating research centres.
Acknowledgments
Acknowledgments
We thank all participants, health-centre staff, and research assistants in the study sites. The Government of France provided funding for this research grant in the context of the Muskoka Initiative on Maternal and Child Health. The UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction funded the grant extension to allow participant follow-up until 12 months post partum.
Contributors
NTT, AS, SL, MEG, and DMK, designed the study protocol and instruments. BT, MM, BK, BMN, JNK, FL, RY, and DMK contributed to the final study protocol, study instruments, and led the field implementation. NTT, SL, BK, BMN, ACK, JK, and MEG monitored the study implementation. AS, SL, MM, BT, and NTT contributed to data management and analysis. ACK coordinated the project between DR Congo and WHO. NTT wrote the first draft of the manuscript with the contributions of MEG, ACK, JK, AS, SL, DMK, RY, BT, JNK, FL, MM, BMN and BK. All authors contributed toward data analysis, drafting, and revising the paper and agreed to be accountable for all aspects of the work.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Cleland J, Bernstein S, Ezeh A, Faundes A, Glasier A, Innis J. Family planning: the unfinished agenda. Lancet. 2006;368:1810–1827. doi: 10.1016/S0140-6736(06)69480-4. [DOI] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; Geneva, Switzerland: 2013. Programming strategies for postpartum family planning. [Google Scholar]
- 3.Moore Z, Pfitzer A, Gubin R, Charurat E, Elliott L, Croft T. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and middle-income countries. Contraception. 2015;92:31–39. doi: 10.1016/j.contraception.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Rossier C, Bradley SEK, Ross J, Winfrey W. Reassessing unmet need for family planning in the postpartum period. Stud Fam Plann. 2015;46:355–367. doi: 10.1111/j.1728-4465.2015.00037.x. [DOI] [PubMed] [Google Scholar]
- 5.Ross JA, Winfrey WL. Contraceptive use, intention to use and unmet need during the extended postpartum period. Int Fam Plann Perspect. 2001;27:20–27. [Google Scholar]
- 6.Sonalkar S, Mody S, Gaffield ME. Outreach and integration programs to promote family planning in the extended postpartum period. Int J Gynaecol Obstet. 2014;124:193–197. doi: 10.1016/j.ijgo.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleland J, Shah IH, Daniele M. Interventions to improve postpartum family planning in low- and middle-income countries: program implications and research priorities. Stud Fam Plann. 2015;46:423–441. doi: 10.1111/j.1728-4465.2015.00041.x. [DOI] [PubMed] [Google Scholar]
- 8.Blazer C, Prata N. Postpartum family planning: current evidence on successful interventions. Open Access J Contracept. 2016;7:53–67. doi: 10.2147/OAJC.S98817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran NT, Seuc A, Coulibaly A. Post-partum family planning in Burkina Faso (Yam Daabo): a two group, multi-intervention, single-blinded, cluster-randomised controlled trial. Lancet Glob Health. 2019;7:e1109–e1117. doi: 10.1016/S2214-109X(19)30202-5. [DOI] [PubMed] [Google Scholar]
- 10.Longondjo C. Urbanization and poverty in Kinshasa: thinking beyond 2015 Millennium Development Goals. In: Andrews N, Khalema NE, Assié-Lumumba NT, editors. Millennium Development Goals (MDGs) in retrospect. Springer; Switzerland: 2015. pp. 31–44. [Google Scholar]
- 11.Ministère du Plan et Suivi de la Mise en œuvre de la Révolution de la Modernité. Ministère de la Santé Publique. ICF International . MPSMRM, MSP, ICF International; Rockville, MD, USA: 2014. République Démocratique du Congo enquête démographique et de santé 2013–2014. [Google Scholar]
- 12.Borda M, Winfrey W. Jhpiego; Baltimore, MD, USA: 2010. Postpartum fertility and contraception: an analysis of findings from 17 countries. [Google Scholar]
- 13.WHO . World Health Organization; Geneva, Switzerland: 2015. Trends in maternal mortality: 1990–2015: estimates from WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division: executive summary. [Google Scholar]
- 14.Tran NT, Gaffield M, Seuc A. Effectiveness of a package of postpartum family planning interventions on the uptake of contraceptive methods until twelve months postpartum in Burkina Faso and the Democratic Republic of Congo: the YAM DAABO study protocol. BMC Health Serv Res. 2018;18:439. doi: 10.1186/s12913-018-3199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran NT, Yameogo WME, Langwana F. Participatory action research to identify a package of interventions to promote postpartum family planning in Burkina Faso and the Democratic Republic of Congo. BMC Womens Health. 2018;18:122. doi: 10.1186/s12905-018-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran NT, Langwana F, Yameogo WME. Birth spacing and informed family planning choices after childbirth in Burkina Faso and the Democratic Republic of Congo: participatory action research to design and evaluate a decision-making tool for providers and their clients. Patient Educ Couns. 2018;101:1871–1875. doi: 10.1016/j.pec.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Bergold J, Thomas S. Participatory research methods: a methodological approach in motion. Hist Soc Res. 2012;37:191–222. [Google Scholar]
- 18.WHO . 5th edn. World Health Organization; Geneva, Switzerland: 2015. Medical eligibility criteria for contraceptive use. [PubMed] [Google Scholar]
- 19.Institut National de la Statistique et de la Démographie. ICF International . INSD, ICF International; Calverton, MD, USA: 2012. Burkina Faso enquête démographique et de santé et à indicateurs multiples 2010. [Google Scholar]
- 20.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: a review of recent methodological developments. Am J Pub Health. 2004;94:423–432. doi: 10.2105/ajph.94.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. 2000. https://catalyst.harvard.edu/docs/biostatsseminar/Donner%20slides.pdf [DOI] [PubMed]
- 22.Cahill N, Sonneveldt E, Stover J. Modern contraceptive use, unmet need, and demand satisfied among women of reproductive age who are married or in a union in the focus countries of the Family Planning 2020 initiative: a systematic analysis using the Family Planning Estimation Tool. Lancet. 2018;391:870–882. doi: 10.1016/S0140-6736(17)33104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PMA2020. Tulane University. Johns Hopkins Bloomberg School of Public Health Performance Monitoring and Accountability 2020 (PMA2020) Project: PMA2017/Kinshasa Round 6. 2017. https://www.pma2020.org/sites/default/files/EN-DRC-Kinshasa-R6-FP-Brief_0.pdf
- 24.Kalambay Ntembwa H, Van Lerberghe W. World Health Organization; Geneva, Switzerland: 2015. Democratic Republic of the Congo: improving aid coordination in the health sector. [Google Scholar]
- 25.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabic M, Choi Y. Assessing the quality of data regarding use of the lactational amenorrhea method. Stud Fam Plan. 2013;44:205–221. doi: 10.1111/j.1728-4465.2013.00353.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for the anonymised, coded trial data can be made to the Department of Reproductive Health and Research, WHO (reproductivehealth@who.int). Data sharing is subject to WHO data-sharing policies and data-use agreements with the participating research centres.