Abstract
Sepsis associated acute kidney injury (SA-AKI) is a common clinical syndrome that occurs among hospitalized patients and significantly impacts mortality. Furthermore, survival after sepsis is intricately dependent on recovery of kidney function. In this review, we discuss the role of iron imbalance in mediating the pathogenic events during sepsis. Intracellular ferritin serves as a repository for iron and prevents iron-mediated injury and may limit the availability of iron to pathogens. Circulating levels of ferritin also increase during sepsis and often correlate with severity of sepsis. Herein, we examine pre-clinical and clinical data and discuss recent findings that suggest immunomodulatory roles for ferritin. We also discuss the possible mechanistic roles for ferritin in mitigating the pathogenic sequelae of sepsis and highlight current gaps in knowledge.
Keywords: ferritin, iron, sepsis-associated kidney injury, cytokine storm
Introduction
Sepsis is defined as a “life-threatening dysfunction caused by dysregulated host response to infection.” Sepsis is the leading cause of acute kidney injury (AKI) with 4570% of all AKI considered to be sepsis associated-AKI (SA-AKI). SA-AKI significantly impacts mortality and recovery from AKI and is intricately linked to survival. [1] Increase in mortality during the early acute phase may be attributed to the inflammatory cytokine storm, oxidative stress, mitochondrial dysfunction and disrupted iron homeostasis. This review will examine and discuss the potential effects of iron imbalance and ferritin, an intracellular iron storage protein, in the pathogenesis of sepsis.
Impact of regulating iron levels during sepsis
Iron plays a major role in many metabolic reactions and therefore both the host and pathogen compete for iron. In an effort to starve the pathogens of iron, the liver synthesizes and releases hepcidin. Hepcidin prevents egress of iron from cells by inducing degradation of ferroportin, an iron exporter. This causes hypoferremia, a rapid decline in levels of iron in circulation, which may prevent bacterial proliferation and dampen oxidative stress by preventing iron-mediated free radical generation. Based on this physiological response, several studies have investigated the effects of iron chelation or supplementation during sepsis and have produced conflicting results (Table 1). Iron supplementation was associated with a decrease in all-cause mortality and a reduced sepsis related mortality rate in a cohort of dialysis patients.[2] Conversely, intravenous iron supplementation of patients with SA-AKI had no signficant effect on recovery of AKI or mortality.[3] However, in contrast, another study showed that parenteral iron supplementation given at the time of sepsis resulted in worse outcomes due to an increase in oxidative stress and Tumor Necrosis Factor alpha (TNF- α).[4] High levels of serum iron were independently associated with an increase in 90-day mortality rates in patients with sepsis.[5] These data indicate that iron supplementation could exacerbate SA-AKI and align with previous findings that iron starvation may augment host defense mechanisms.[6] In a pre-clinical study, iron chelation via desferoxamine (DFX), an iron chelator, resulted in increased survival of rats and was associated with an increase in renal expression of the pro-apoptotic protein, Bax during sepsis.[7] Interestingly, pre-treatment of mice with hepcidin also protects against SA-AKI and mortality. Hepcidin mediated macrophage iron retention and consequently increased FtH expression.[8] Several studies have demonstrated that iron chelation prior to induction of AKI is protective (Table 1); however, the effect of iron chelators following established renal injury is not well-studied. Also, iron restriction using agents such as DFO or hepcidin may be most effective against siderophilic bacteria and extracellular pathogens but may not be protective against nonsiderophilic or intracellular pathogens, including Mycobacterium tuberculosis. It should also be recognized that while acute iron chelation may be beneficial against bacteremia and oxidative stress, prolonged iron chelation may negatively impact the host by inducing anemia and subsequently organ dysfunction. Therefore, the potential of iron chelation during SA-AKI requires further investigation.
Table 1.
Effects of iron modulation during sepsis-AKI
| Iron Supplementation | |||||
| Species | Treatment | Time of Treatment | Outcome | Results | PMID |
| Humans | 12.5 mg − 62.5 mg of Intravenous iron (ferric gluconate) | Weekly | Reduced mortality | Positive | 25462819 |
| Humans | 125 mg/day of Sodium Ferric Gluconate | Post SA-AKI for 2–8 days | No adverse effect in recovery of AKI or mortality | No Change | 26801821 |
| Mice | 2 mg of intravenous iron Fe3+ in the form of Fe sucrose | Time of sepsis | Increase in ROS, TNF-a and mortality | Negative | 15149323 |
| Iron Chelation | |||||
| Species | Treatment | Time of Treatment | Outcome | Results | PMID |
| Mice | 50–100 ug of hepcidin | Pre- or post-LPS or CLP | Pre-Tx reduced SA-AKI and mortality. Post-Tx reduced SA-AKI and bacteremia. | Positive | 31244655 |
| Mice | 40 mg/kg DIBI | Post Colon ascendens stent peritonitis (CASP) 3h or 13 h | Reduced bacteremia | Positive | 26235905 |
| Rats | 20 mg/kg of N-acetylcysteine (NAC) and DFO | NAC: 3–24 h after CLP, subcutaneously DFO: 3–24 h after CLP, subcutaneously | Reduced septic shock by decreasing ROS, neutrophil infiltration, mitochondrial dysfunction and mortality. | Positive | 14758146 |
| Rats | 40mg/kg of DFX | 20mg/kg subcutaneously Immediately post-CLP, 20mg/kg subcutaneously again 6h post-CLP | Reduced mortality | Positive | 15037222 |
Elevation of circulating ferritin during sepsis
Ferritin is a ubiquitously expressed spherical protein that comprises 24 subunits of heavy (FtH) and light (FtL) chains. FtH is a ferroxidase which converts ferrous iron into ferric iron, which is subsequently stored within the ferritin shell. FtL does not have ferroxidase activity and its presumed function was limited to iron nucleation. Intracellular tissue ferritin can store up to 4500 atoms of iron in a safe and bioavailable form. However, circulating (serum) ferritin is predominantly composed of FtL and is low in iron content.[9] While the anti-oxidant and iron repository role of tissue ferritin is extensively studied, the function of circulating ferritin is still unclear. In the clinic, serum ferritin is routinely used to evaluate body iron stores and inflammation; a decrease in circulating ferritin levels is indicative of low iron whereas an increase is indicative of iron overload. However, ferritin levels also increase during inflammation and confound the clinical utility of ferritin in iron management.
Ferritin is elevated during sepsis and hyperferritinemia often correlates with severity of sepsis.[10] Additionally, treatment of hyperferritinemic patients with an interleukin-1 receptor antagonist reduced sepsis-induced mortality from 66% to 35%, suggesting that elevation of ferritin was associated with inflammation.[11] While the preponderance of studies establish a correlation between elevated ferritin and worse outcomes, few studies demonstrate that a failure to increase ferritin was also associated with mortality.[12] Additionally, serum ferritin levels predicted renal function recovery in patients with acute kidney injury.[13] It should be noted that these clinical studies were associative and do not necessarily establish a causal relationship with severity of AKI or mortality. Of note, FtL hyperferritinemia, an autosomal dominant syndrome is associated with increased intracellular FtL and elevated serum ferritin levels. Also, serum ferritin levels are significantly higher in transgenic mice heterozygous for FtH gene. Interestingly, such elevation in FtL (in both humans and rodents) is benign and is not associated with increased inflammation, suggesting a contradiction in the presumed association of serum ferritin with inflammation. Therefore, the significance of elevated ferritin during infection and inflammatory conditions warrants further investigation.
Ferritin in pre-clinical models of sepsis-AKI
As described in the previous section, circulating ferritin levels are often elevated during sepsis. However, the contributory role of ferritin in mediating the pathogenic events during sepsis could not be established in these clinical studies. In this connection, Lipinski et al. demonstrated that administration of tissue ferritin (derived from horse spleen, murine liver or bovine spleen) significantly protected mice against a lethal Escherichia coli infection.[14] Interestingly, iron saturation of these tissue ferritins varied from 11% to 46% but did not influence mortality, suggesting that the protective effects of ferritin were independent of iron loading. They also found a dose- and time-dependent effect of ferritin, with maximal protection derived from administration of ferritin 24h prior to infection. Similarly, Weis et al. recapitulated these findings in a mouse model of polymicrobial sepsis using apoferritin that is completely devoid of iron.[15] These intriguing findings suggest that the protective effects of ferritin may not be limited to iron sequestration.
Host survival is dependent on resistance to infections; however data shows that tolerance is equally important to preserving host metabolism and promoting survival. Ramos et al. showed that FtH expression in renal proximal tubules is essential to establish tolerance against Plasmodium spp.-induced AKI.[16] FtH also sustained gluconeogenesis in hepatocytes during sepsis and promoted tolerance.[15] These studies emphasize the role of FtH in mediating tolerance against infections.
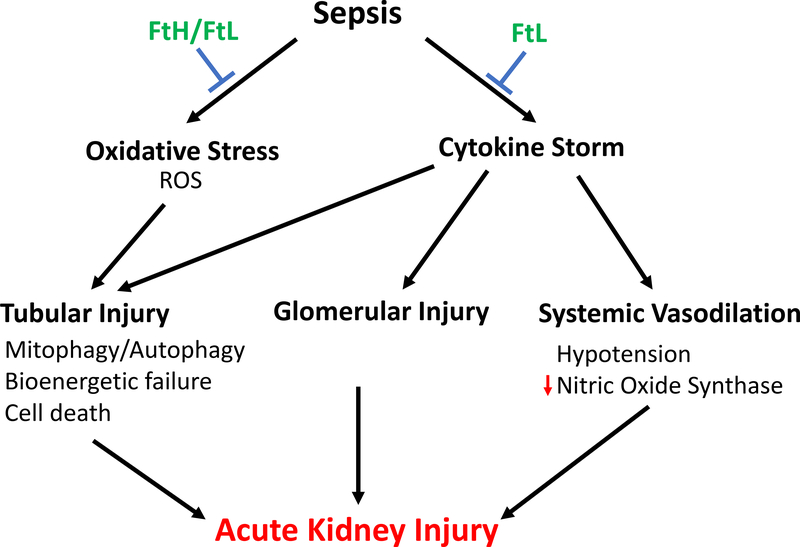
To better understand the role of iron metabolism and ferritin on sepsis-induced inflammation and acute kidney injury, we generated transgenic mice with targeted deletion of FtH in myeloid cells (macrophages and neutrophils). Macrophages are central mediators of iron recycling and systemic iron homeostasis. Also, iron storage requires FtH and therefore, deletion of FtH in macrophages prevents intracellular iron sequestration. Additionally, previous reports suggested that circulating ferritin was predominantly derived from macrophages. Intriguingly, we found that myeloid FtH deletion conferred significant protection against sepsis-AKI and mortality in two models of sepsis, cecal ligation and puncture (CLP) or lipopolysaccharide (LPS) endotoxemia.[17] Corroborating previous reports, wild-type mice had significantly high levels of pro- and anti-inflammatory cytokines (IL-6,TNF- α, IL-1β, IL-4, IL-10) following sepsis induction. In contrast, loss of FtH from the myeloid compartment abrogated the cytokine storm. Sepsis caused leukopenia in both the animal groups and loss of FtH did not influence the number or proportion of immune cells in circulation or kidneys. Interestingly, while CLP increased hepcidin levels in both groups of mice, FtH deficient mice had significantly lower levels of hepcidin compared to wild-type mice, suggesting that hepcidin induced hypoferremia is not the dominant protective mechanism in the absence of FtH. As expected, sepsis led to an increase in circulating ferritin levels. However, loss of FtH was associated with significantly elevated circulating ferritin levels even in animals that underwent sham surgery. Importantly, the increase in serum ferritin did not lower serum iron levels or affect transferrin saturation, suggesting that circulating ferritin does not influence extracellular iron metabolism during sepsis. Myeloid FtH deficient mice expressed significantly higher levels of FtL in macrophages and subsequently in circulation. To determine whether the elevated ferritin levels conferred resistance to myeloid FtH deficient mice, recombinant FtL was administered to wildtype mice prior to sepsis induction. Intriguingly, FtL administration significantly dampened the hyperinflammatory response, reduced organ dysfunction and promoted survival (Fig. 1).
Figure 1. Role of ferritin in sepsis associated – AKI.
Ferritin is an essential iron sequestering, anti-oxidant protein that protects against sepsis-associated kidney injury. Serum ferritin, specifically ferritin light chain, prevents the cytokine storm and consequently SA-AKI.
In order to delineate the mechanism of FtL-mediated immunomodulatory effects, we examined the transcriptomic profile of blood leukocytes following sepsis induction. As expected, the inflammatory response and associated pathways were significantly upregulated in wild-type leukocytes with a dominant NFkB footprint. These findings were also re-capitulated in vitro using bone-marrow derived macrophages. Additionally, activation of Mitogen-activated protein kinase pathways, including c-Jun N-terminal kinase (JNK) and extracellular signal-related kinase (ERK) were heightened in wild-type macrophages.[17] Both loss of FtH and overexpression of FtL were associated with a reduction in NFkB activation and subsequent inflammatory response. These data underscore an important role for ferritin light chain and serum ferritin in mediating a regulated and measured inflammatory response to sepsis to prevent hyperinflammation and kidney injury.
Conclusion
Iron is a prerequisite for many cellular processes that enable survival, oxidative stress and inflammation. Therefore, acute chelation of iron to limit availability to pathogens is tempting, but prolonged iron chelation may lead to detrimental consequences, including metabolic dysfunction, anemia and organ injury. Levels of circulating ferritin, predominantly FtL, are often elevated during sepsis and correlate with severity of disease. Our recent findings shed light on this evolutionarily conserved protein and identify FtL as a potent immunomodulatory effector. Collectively, we speculate that FtH is an essential iron sequestering, anti-oxidant that promotes tolerance. On the other hand, FtL may mediate immunoregulation and prevent sepsis-AKI (Fig. 1). These findings also underscore the under-appreciated role of ferritin in preventing AKI during sepsis. Further investigation into the functions of ferritins during sepsis may enable development of new treatments for SA-AKI that could alleviate the significant burden of sepsis induced morbidity, mortality and substantial health care expenditures.
Acknowledgments
Funding Sources
This work was supported in part by a NIH grant (DK103931 to SB) and an ASN grant (Carl W. Gottschalk award to SB).
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019. November;96(5):1083–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitt E, Sturm G, Kronenberg F, Neyer U, Knoll F, Lhotta K, et al. Iron supplementation and mortality in incident dialysis patients: an observational study. PLoS One. 2014;9(12):e114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark BA, Osadchuk L, John J, Culver T, Marcus R. Effect of intravenous iron on outcomes of acute kidney injury. Transfusion. 2016. April;56(4):933–7. [DOI] [PubMed] [Google Scholar]
- 4.Zager RA, Johnson AC, Hanson SY. Parenteral iron therapy exacerbates experimental sepsis. Kidney Int. 2004. June;65(6):2108–12. [DOI] [PubMed] [Google Scholar]
- 5.Lan P, Pan KH, Wang SJ, Shi QC, Yu YX, Fu Y, et al. High Serum Iron level is Associated with Increased Mortality in Patients with Sepsis. Sci Rep 2018. July 23;8(1):11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz T Iron in innate immunity: starve the invaders. Curr Opin Immunol. 2009. February;21(1):63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messaris E, Antonakis PT, Memos N, Chatzigianni E, Leandros E, Konstadoulakis MM. Deferoxamine administration in septic animals: improved survival and altered apoptotic gene expression. Int Immunopharmacol. 2004. March;4(3):455–9. [DOI] [PubMed] [Google Scholar]
- 8.Scindia Y, Wlazlo E, Leeds J, Loi V, Ledesma J, Cechova S, et al. Protective Role of Hepcidin in Polymicrobial Sepsis and Acute Kidney Injury. Front Pharmacol 2019;10:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010. August;1800(8):760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017. November 1;29(9):401–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, Dimopoulos G, Pantazi A, Orfanos SE, et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med 2017. September 18;15(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carcillo JA, Simon DW, Podd BS. How We Manage Hyperferritinemic Sepsis-Related Multiple Organ Dysfunction Syndrome/Macrophage Activation Syndrome/Secondary Hemophagocytic Lymphohistiocytosis Histiocytosis. Pediatr Crit Care Med 2015. July;16(6):598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrijevic ZM, Salinger-Martinovic SS, Jankovic RJ, Mitic BP. Elevated Serum Ferritin Levels Are Predictive of Renal Function Recovery among Patients with Acute Kidney Injury. Tohoku J Exp Med. 2019. June;248(2):63–71. [DOI] [PubMed] [Google Scholar]
- 14.Lipinski P, Jarzabek Z, Broniek S, Zagulski T. Protective effect of tissue ferritins in experimental Escherichia coli infection of mice in vivo. Int J Exp Pathol. 1991. December;72(6):623–30. [PMC free article] [PubMed] [Google Scholar]
- 15.Weis S, Carlos AR, Moita MR, Singh S, Blankenhaus B, Cardoso S, et al. Metabolic Adaptation Establishes Disease Tolerance to Sepsis. Cell. 2017. June 15;169(7):1263–75 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos S, Carlos AR, Sundaram B, Jeney V, Ribeiro A, Gozzelino R, et al. Renal control of disease tolerance to malaria. Proc Natl Acad Sci U S A. 2019. March 19;116(12):5681–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarjou A, Black LM, McCullough KR, Hull TD, Esman SK, Boddu R, et al. Ferritin Light Chain Confers Protection Against Sepsis-Induced Inflammation and Organ Injury. Front Immunol. 2019;10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]