Abstract
Background
Climate change is increasing global average temperatures, as well as the frequency of extreme weather events. Both low and high ambient temperatures have been associated with elevated mortality; however, little is known about the cardiovascular impacts of hourly temperature.
Methods
We assessed the association between hourly ambient temperature and risk of myocardial infarction (MI) across adult residents of New York State (NYS). We identified cases across NYS hospitals from 2000 to 2015 in the New York Department of Health Statewide Planning and Research Cooperative System dataset, using ICD codes. Hourly ambient temperature was assessed at each patient’s residential ZIP code, up to 48 hours prior to MI. We employed a time-stratified case-crossover study design matching case to control periods on hour of day, day of week, month and year.
Results
Of the 791,695 primary MI hospital admissions, 45% were female, the mean (standard deviation; SD) age was 70 (15) years, and 49% of cases occurred among New York City residents. The observed temperature range was −29°C to 39°C, with a mean of 10.8°C (10.5°C). Temperature in the 6 hours preceding the MI was positively associated with risk of MI, across the range of observed temperatures, with null or nearly null associations for earlier hours. We estimated a cumulative percent increase in hourly myocardial infarction rate of 7.9% (95% confidence interval [CI]: 5.2%, 10.6%) for an 11°C (median) to 27°C (95th percentile) temperature increase for lag hours 0–5. Men, Medicare-eligible individuals (age <65), and those experiencing their first MI were most sensitive.
Conclusion
Our study provides evidence that increases in hourly ambient temperature can trigger myocardial infarction risk. Health-based definitions of extreme heat events may better capture the deleterious effects of heat by accounting for hourly temperature. Our findings can inform the design of more effective preparedness strategies for the increasingly frequent extreme heat events.
1. Introduction
As climate change warms our planet1 and increases the frequency and intensity of extreme weather events, including heat waves and cold snaps1, there is a critical need to characterize the health impacts of ambient temperature. Low and high ambient temperatures have been consistently associated with mortality2 and epidemiological evidence suggests a similar nonlinear association between daily temperature and cardiovascular disease (CVD)3, including myocardial infarction (MI)4,5. As CVD, and particularly MI, are leading causes of morbidity and mortality in the US6 and worldwide7, it is critical to investigate modifiable triggers of these acute outcomes, such as exposures to ambient temperature, that occur at a population scale.
A number of studies have observed a U-shaped exposure-response relationship between temperature and CVD, with elevated risk at both high and low daily temperatures3,5. The timing of this association can vary by temperature: high temperature days have been associated5 with elevated CVD risk in the 0–4 days following exposure, and low temperature days have showed a delayed association up to a week5,8,9. Previous epidemiological studies have typically focused on mean or max daily temperature exposure or longer exposure windows5. Yet, temperature exposure at ultra short-time scales (i.e., hourly) can also impact the cardiovascular system, e.g., blood pressure10–12. Additionally, a previous study assessed 2-hour average temperature and observed a negative association between temperature for lags 10–24 and risk of stroke13. However, there has been limited research into the role of ultra short-term ambient temperature on acute cardiovascular events.
In this study we investigated the association between hourly ambient temperature and risk of MI in an administrative cohort in New York State (NYS). We hypothesized that both lower and higher temperatures during the 48 hours prior to MI event would be associated with elevated MI risk. We additionally assessed heterogeneity of the association by individual-level factors and time-varying conditions.
2. Methods
2.1. Study Population
Hospital records from 2000–2015 across NYS were obtained from the NYS Department of Health Statewide Planning and Research Cooperative System (SPARCS)14, an administrative dataset of ~98% of all inpatient stays and outpatient visits from all nonmilitary acute care facilities, including hospitals and hospital extension clinics, in NYS14. As of 2015, 222 acute care facilities across the state reported to SPARCS14. For each admission, SPARCS includes up to 25 International Classification of Disease (ICD) diagnosis code, hour and date of admission, patient residential address, age, sex, and self-reported race. Our study population was restricted to adult (≥18 years) NYS residents, excluding encounters with missing or incorrectly transcribed patient residential ZIP code (e.g., an entry with only 4 digits) or date or hour of admission. Columbia University Institutional Review Board approval was obtained to conduct the analysis and informed consent was waived.
2.2. Case Ascertainment
Cases were identified based on ICD, 9th Revision (ICD-9; code 410.x1), pre-2015, or ICD10 (code I21), in 2015, codes, in the first four diagnostic positions. ICD-9 codes for MI in reimbursement-based administrative datasets have high sensitivity, specificity, and positive predictive value15,16. We included inpatient and outpatient admissions and excluded any admissions with “newborn” or “trauma” admission type. Due to the nature of hospitalization records, if a subject died before admission they were not included in SPARCS, but fatal MIs for which the death occurred after admission are included. We included reinfarctions and recurrent MI admissions, except for admissions that took place within two days after a previous MI admission for that patient, to avoid readmission for the same ischemia event. First/reinfarction/recurrent MI status was determined using hospitalization data from 1995–2015; therefore, if an individual experienced an MI in 1995–1999, then they would only experience recurrent MI during the study period (2000–2015). For all analyses, we assumed that MI events took place three hours prior to the recorded time of hospital admissions, based on a nationwidewide assessment that observed a median delay of 2.6 hours among non-ST-elevated MI (non-STEMI)17.
2.3. Hourly Temperature Exposure Assessment
Hourly temperature estimates were obtained from the North American Land Data Assimilation System, NLDAS-2 Forcing18. NLDAS reports hourly parameter values at 0.125 degree grids (~11 km × 14 km in NYS). We aggregated hourly temperature to ZIP codes via area-weighted averaging. Relative humidity (RH) was calculated from specific humidity, pressure, and temperature. We constructed temperature and RH exposure profiles for each admission and control period (Section 2.4) up to 48 hours prior to the estimated time of the event, by matching to residential ZIP code of each patient.
2.4. Study Design and Statistical Analysis
We used a case-crossover design19,20 to assess the hourly temperature–MI association. In this design an individual’s exposure prior to the event is compared to their exposure prior to a comparable time when the disease did not occur (control time). Since exposures are compared within individuals, the case-crossover design eliminates confounding from factors that vary between individuals or over long time scales. We selected control hours based on bidirectional time-stratified matching21,22; control hours were matched on year, month, day of week, and hour of day, therefore also effectively eliminating confounding by long-term, seasonal, and diurnal trends. We used a natural spline with 4 df to non-linearly adjust for 48-hour average RH, which varies diurnally and could induce confounding.
We fit conditional logistic models to estimate the independent temperature associations 48 hours prior to MI while accounting for the matching structure of the case-crossover design. To account for the high temporal autocorrelation of hourly temperature, we modeled temperature exposure using Distributed Lag Nonlinear Models (DLNMs)23,24. These models allow estimation of independent associations of the exposure of interest for different lags, by mutually adjusting for all lags examined, allowing the association to vary smoothly over time. DLNMs also allow for nonlinear exposure-response curves. We used natural splines with 4 degrees of freedom (df) to capture non-linearity of the exposure-response relationship. We used natural splines to constrain the coefficients across lags, and selected df among a range of plausible df (3– 7) based on Akaike Information Criteria (AIC)25. To compare with previous studies, we also assessed the association between the mean temperature of the previous day (midnight-to-midnight) and risk of MI, using the same design, with a natural spline with 4 df for mean daily temperature, adjusting for same-day mean RH.
Analyses were performed using R statistical software version 3.5.2 (R Project for Statistical Computing). Statistical significance was assessed at α=0.05. We used dlnm version 2.3.9 to construct the distributed lag cross-basis, and ggplot2 version 3.2.1 to plot. All code is available on GitHub (https://github.com/s-rowland).
2.5. Effect Modification
We assessed effect modification by age, sex, time of day, season, quartiles of 48-hour mean RH, and first/recurrent status26, using the same DLNM constraints as in the main model. We conducted stratified analyses and assessed whether subgroup effect estimates for low and high temperature were significantly different from each other27. To account for multiple testing (30 total comparisons), we set a Bonferroni-corrected significance level αBc = 0.0016. Age was categorized to reflect distinct risk regimes for MI: Medicare-ineligible (<65) and Medicare-eligible (≥65). Season was dichotomized as cold (October 1–May 31) and warm (June 1–September 31), and times of day were defined by estimated time of the MI: morning (06–11), afternoon (12–17), evening (18– 22), and night (23–05). As a secondary analysis, we additionally stratified by combined sex and age categories to assess whether the modifying role of sex was consistent by age.
2.6. Sensitivity Analyses
We conducted several sensitivity analyses to assess the robustness of our results. Specifically, we assessed (1) sensitivity to choices of the DLNM parameters by (a) changing the maximal lag of hourly temperature to 24 or 58 hours, (b) placing the knots evenly located across the log of the lags23, and (c) using 5 df for the natural spline for the exposure dimension. For the 1a sensitivity analyses, we chose the df to correspond to the spline intervals of the main cross basis (i.e., 3 df for 24 hours and 7 df for 58 hours, with an additional knot at 48 hours). We also assessed (2) sensitivity to case definition by re-defining cases according to the following: (a) 410.x1 in the principal diagnosis, (b) 410.x1 or 410.x0 in the four diagnostic positions, (c) 410.xx in the principal diagnosis, and (d) 410.x1 in the first four diagnostic positions, but excluding recurrent MI within 6 months of a previous MI admission. In addition, we assessed (3) sensitivity to choice of adjustment for hourly RH over the 48 hours, via (a) a second DLNM term with the same constraints as the temperature DLNM and (b) no RH adjustment. Finally, to assess the impact of our assumption of a single delay time across MI severity, we (4) assumed a two-hour delay for STEMI (all MI codes excluding 410.71 and I21.4) and re-estimated hourly temperature exposures accordingly. We retained the same three-hour delay for non-STEMI events. For all sensitivity analyses (except #1b), we used the same knot locations as in the main model.
3. Results
3.1. Study Participants and Temperature Conditions
In the main analysis, a total of 791,695 primary MI admissions in adult NYS residents were observed for the study period, among 621,137 unique individuals. We excluded 1,151 primary MI admissions due to missing time/date or address information (0.1%). The mean (standard deviation; SD) age of MI cases was 70 years (15 years), and 45% were female (Table 1). MI events were more common on Mondays (16% of cases), late morning/afternoon (40% of admissions occurred at 11–17 hours), and in January (9.3%) (eFigure 1). Nearly half (49%) of cases occurred among New York City residents, and 63% of patients were on public insurance.
Table 1:
Descriptive Statistics of Study Population: NYS CVD Hospitalizations (2000 – 2015)
| Variable | N | Percentage |
|---|---|---|
| Total Sample | 791,695 | 100.0 |
| Sex | ||
| Female | 359,295 | 45.4 |
| Male | 432,388 | 54.6 |
| Unidentified | 12 | 0.0 |
| Age | ||
| < 40 | 16,974 | 2.1 |
| 40 ≤ Age <65 | 256,899 | 32.5 |
| ≥ 65 | 517,822 | 65.4 |
| Race/Ethnicity | ||
| Asian American | 1,723 | 0.2 |
| Black | 77,560 | 9.8 |
| Hispanic | 37,235 | 4.7 |
| Other + Multi-Racial | 160,822 | 20.3 |
| White | 514,355 | 65.0 |
| Recurrent Case Status | ||
| First MI Admission | 566,360 | 71.5 |
| Reinfarctiona | 49,911 | 6.3 |
| Recurrent MIa | 175,424 | 22.2 |
If an individual had no previous record of MI admission up to 1995 (when our SPARCS data begin), then their first recorded MI hospitalization was considered to be their first MI. Following the Fourth Universal Definition of Myocardial Infarctions, subsequent admissions for MI within 28 days after an MI admission are considered reinfarctions and admissions more than 28 days later are considered recurrent MI26.
Among case and control hours, the average (SD) temperature was 10.8°C (10.5°C), with a range of −29°C to 39°C (5th percentile: −5°C; 95th percentile: 27°C); the mean temperature at lag 0 among cases was 10.8 °C (10.4°C) and the mean temperature among controls was 10.9°C (10.4°C). Hourly temperatures showed high autocorrelation; the correlation between consecutive hours was 0.99, which remained >0.81 for at least 48 lagged hours. The mean (SD) 48-hour average RH was 0.78 (0.09), with a range of 0.31–0.98 (5th percentile: 0.61, 95th percentile: 0.91). Temperature at the hour of MI was weakly correlated with 48-hour RH (Pearson’s correlation=−0.06).
3.2. Hourly Temperature and Risk of MI
Based on AIC, we selected 6 df across the 48 lags for the lag constraint (eTable 1). Temperature in the 6 hours preceding the MI was positively and nearly-linearly associated with MI risk, across the range of observed temperatures (Figure 1 and 2A). We estimated a percent increase in hourly MI rate of 1.9% (95%CI: 1.2%, 2.5%) for a temperature increase from the median (11°C) to the 95th percentile (27°C) of the temperature distribution in lag hour 1. Associations were null or nearly null for earlier lags, except for a negative association for lags 44–47 (Figure 1). We estimated a cumulative change of 7.9% (95%CI: 5.2%, 10.6%) for an 11°C to 27°C temperature increase for lags 0–5 (Figure 2B), and 2.1% (95%CI: 0.1, 4.2%) for all 48 hours (eFigure 2).
Figure 1: Exposure-Response Relationship Across Lags.
Percent increase in hourly MI rate for a change in hourly temperature for each lag from median temperature (11°C) to (A) the 5th percentile of temperature (−6°C) and (B) the 95th percentile (27°C). Shaded bands represent 95% confidence intervals.
Figure 2: Exposure-Response Curve for Selected Lags.
In panel A, each curve illustrates the exposure-response relationship for selected lags. Only the first 4 even-numbered lags are included for visibility. Panel B illustrates the cumulative association for an increase from median temperature (11°C) to the 95th percentile (27°C) for lags 0–6. Shaded areas represent 95% confidence intervals.
In the analysis of mean daily temperature, we estimated that the lowest risk occurred at a mean daily temperature of 18°C, with significant associations for both lower and higher temperatures (eFigure 3).
3.3. Effect Modification of the Temperature – MI Relationship
High Temperatures
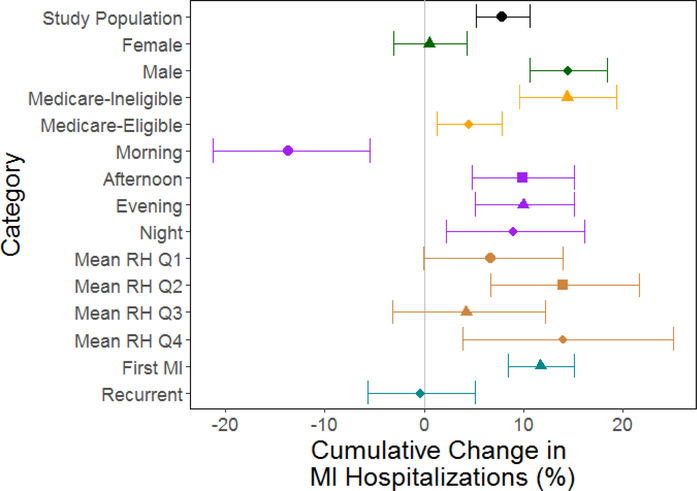
We observed a stronger association among males, a null association among females (pvalue for modification < 0.001) and a larger association among Medicare-ineligible adults than among Medicare-eligible adults (p-value = 0.001; Figure 3, eTable 2, and eFigures 4–5). In the secondary analysis of doubly-stratified models by age and sex, we observed a similar pattern of modification by sex, but attenuated among the Medicare-eligible population, and the same direction of modification by age group among males, but not among females (eFigure 10).
Figure 3: Cumulative Association Between Temperature for Lags 0–5 and MI.
Cumulative associations for an increase in temperature from median temperature (11°C) to the 95th percentile of temperature (27°C) over lags 0–5 are shown for the total study population, and for stratified models. Error bars represent 95% confidence intervals. Q1–Q4 represent the four relative humidity quartiles, with Q1 the lowest.
We observed a protective association with high temperatures for MI events that occurred in the morning and a nonsignificant harmful association with low temperatures. This was in contrast to other times of the day for which we observed harmful association with high temperatures (p-value for difference between morning vs. other times < 0.0016; eFigure 6). We did not observe any evidence for effect modification by warm/cold season or by quartiles of 48-hour mean RH (p-value > αBc for all seasonal and RH comparisons; eFigures 7–8). We observed a stronger association among first MI than among recurrent MI, with a null association among recurrent MI (p-value for modification < 0.001; eFigure 9).
Low Temperatures
We did not observe any evidence of effect modification for the association between low temperatures and MI (p-value for all comparisons > αBc).
3.4. Sensitivity Analyses
Our sensitivity analyses consistently replicated our overall finding of a positive, nearlylinear association between hourly temperature and MI during the few hours preceding the event. However, the exact shape and magnitude of this association varied by choice of DLNM parameters and outcome definition, but not method of RH adjustment. The model with a maximal lag of 24 hours showed associations very similar to the main model; the models slightly diverged at lags earlier than 20 hours (eFigure 11). The 58hour model showed similar, albeit slightly attenuated, associations as the main model for lags 0–10 (eFigure 12). A negative association between temperature and MI was observed for lags 48–55, rather than for lags 44–47, as observed in the main model. When the knots were placed at log intervals, the association for lags 0–5 showed a much sharper peak, with wider CIs, and smaller but similarly-null associations for lags 7–47 (eFigure 13). Increasing the flexibility in the estimated exposure-response relationship yielded consistent results with the main model (eFigure 14). The exposurelag-response relationship was consistent across case definitions, but the associations for lags 0–5 were larger in magnitude when only the principal position was used and attenuated for the most expansive definition (eFigures 15). Similarly, using a DLNM to adjust for RH, making no adjustment, or assuming a 2-hour delay for STEMI cases (40% of the total cases) did not alter the results (eFigure 15).
4. Discussion
We assessed the association between hourly temperature and MI risk in a publiclyavailable, population-wide hospital admissions cohort across NYS. We observed a positive association between increasing hourly temperatures and MI risk, even at low temperatures, for the 6 hours preceding MI, but a null association for lags more than 6 hours before MI. Our overall finding of a positive association for the hours directly preceding the event was consistent across choices of DLNM constraints, outcome definitions, and RH adjustment method. However, the exact magnitude and timing of the association were sensitive to choice of knot location across lags, a sensitivity also noted in other DLNM studies23, and the maximal lag of exposure assessed. Our sensitivity analyses suggest that the negative association observed for lags 45–47 was not robust to choice of maximal lag. Alternatively, it may represent a delayed effect of low temperature identified in previous studies29,30.
This study is one of the first studies to assess hourly ambient temperature, and the first to consider hourly temperature and MI. A previous study assessing sub-daily temperature and MI measured temperature exposure by the number of hours exceeding a comfort threshold, and observed elevated risk only at higher temperatures for same day and lag day 1, but a U-shaped curve for lag day 231. These findings were limited by using daily hospitalization data, which may have induced exposure measurement error and inhibited identifying the relevant exposure lags. By using hourly hospitalization data, we minimized exposure measurement error (by reducing temporal misalignment of outcome and exposure) and specifically assessed the timing of health effects by lag hour.
Our results are consistent with prior studies reporting a positive association between higher temperatures and risk of MI5,29,32. However, our work differs from other studies that detected a negative association for increasing temperature, even during summer33; but this may be due to their use of a linear term for temperature5,34, which would obscure a U-shaped curve. There is evidence that heat exposure impacts cardiovascular performance through the autonomic system. High temperature exposure can activate the sympathetic nervous system35, leading to elevated heart rate and decreased heart rate variability36. Experiments of combined exposure to heat and exercise have observed that heat exposure decreases aerobic capacity in athletes35; a recent experiment found a similar relationship in healthy elderly women performing a typical non-exercise task12. Wu et al.37 and Ren et al.38 observed that higher hourly ambient temperatures were associated with decreased heart rate variability, suggesting that ultra short-term high temperature exposure impacts autonomic function, even during non-exercise activities. It is also important to consider potential indirect pathways, such as elevated nighttime temperature leading to sleep disruption39, that can in turn lead to increased cardiovascular risk40.
We observed a protective association for MI risk following temperatures lower than the median temperature, unlike some previous studies33,34. This protective association is somewhat surprising because exposure to lower ambient temperature has been consistently associated with cardiovascular risk factors such as increased blood pressure41,42 and increased markers of inflammation such as C-reactive protein42. This discrepancy may be because we only examined exposure during the 48-hour period prior to the MI, whereas some previous studies observed a delayed effect of lower temperature, with a delay longer than 48 hours prior to the event2,5,30. For example, one study found that low temperatures more than two days prior to the event (daily lags 3–11) were associated with elevated MI30. Alternatively, ultra short-term temperature exposure may have different cardiovascular effects than daily temperature—e.g., a single hour of low temperature exposure may not have the same impact as a full day of low temperature exposure. This theory is supported by our finding of a harmful association between low mean daily (midnight-to-midnight) temperature and MI risk using the same study population and design.
We observed suggestive evidence of effect modification by a few key factors. The association was stronger among males than among females, and among nonMedicare-eligible individuals than among Medicare-eligible individuals. There is inconclusive evidence on sensitivity by sex for the high temperature–MI relationship, with prior studies observing stronger associations both in females43 and in males29,30. Generally, though not all30, previous studies have observed stronger associations among older populations29,44. To date, no other study has assessed effect modification by time of day. Our protective findings in the morning may be due to (a) within-day harvesting, (b) unique triggers for morning MI, such as waking45, or (c) different physiological response to temperature during sleep. Other studies43,46 have observed modification by season that we did not detect. Further research is needed to identify chronic diseases and other conditions that may increase vulnerability to temperature.
Strengths and Limitations
Our study benefited from a number of strengths. First, since SPARCS records include the hour of admission, we were uniquely able to estimate the time of events and investigate the timing of ultra short-term health effects. Second, our large study population enabled us to investigate the independent associations of hourly temperature, despite temperature’s strong autocorrelation. By sampling across NYS, our effect estimates are more representative of population-wide responses than cityspecific estimates. Third, the case-crossover design eliminated most potential sources of confounding.
Our findings should be interpreted in light of our limitations. First, we used ICD codes to identify MI cases. Although ICD codes for MI admissions have been found highly reliable15,16, some potential outcome misclassification is still likely. In the sensitivity analyses, the stricter case definition yielded slightly stronger in magnitude effect estimates, suggesting some small outcome misclassification in our main analysis. Second, we made assumptions about the timing of the outcome relative to admissions. Severity of the MI symptoms (e.g., STEMI vs. non-STEMI) likely influences this delay time28. However, our estimated effects did not change in our sensitivity analysis taking into account a different delay for STEMI vs. non-STEMI events. While our assumption of a single pre-hospital delay time across the study population could introduce random measurement error, we expect this error to be non-differential with respect to exposure and most likely introduce noise into our estimates and potentially induce a bias towards the null. It is important to note that daily exposure windows also introduce exposure measurement error, as an individual who experienced an event at hour 23 would be assigned the same exposure as an individual who experienced an event at hour 02 of the same day. Third, we employed a DLNM, which is sensitive to the choice of lag constraints. This issue is common across flexible modeling approaches. Nonetheless, to our knowledge, no other methods currently exist to more robustly study the nonlinear effects of highly correlated exposure lags. We performed numerous analyses to assess the sensitivity of our results to modeling assumptions and our findings of a significant positive association for lags 0–5 are robust. Fourth, we only considered an hourly exposure window, up to 48 hours prior to the MI, which likely does not capture the totality of temperature’s impact on MI risk. The exposure window of interest must be chosen based on the research question; the goal of this study was to specifically assess the role of ultra short-exposure to ambient temperature. Fifth, we assessed ambient outdoor temperature using area-weighted ZIP code averages, based on patient residential ZIP code, which may have introduced exposure measurement error, as subjects likely spend time outside their residential ZIP code. Finally, it is important to note that our findings are based on ambient temperature, and not personal or indoor exposure.
Conclusions
This is the first study, to date, providing evidence that even very short-term exposure to increasing ambient temperature can trigger MI. Further research is needed to evaluate this relationship in different populations and geographic regions, and among populations who have adapted to different climate regimes. Our study suggests that ultra short-term temperature increases should be considered when designing health-based definitions of extreme heat events and heat action alerts.
Supplementary Material
Highlights.
Increases in hourly temperature were associated with risk of Myocardial Infarction
The association persisted for 6 hours following exposure
Association was strongest among male, Medicare-eligible individuals, and those experiencing their first MI
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health [grants T32 ES023770, P30 ES023515, P30 ES009089, R00 ES023450].
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None Reported.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional Contributions: Dr. Nicholas B. DeFelice provided valuable suggestions for interpretation of the results.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sebastian T. Rowland, Department of Environmental Health Sciences, Columbia University Mailman School of Public Health, New York, New York.
Amelia K. Boehme, Departments of Neurology, Columbia University Medical School and Epidemiology, Columbia University Mailman School of Public Health, New York, New York.
Johnathan Rush, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, New York.
Allan C. Just, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, New York.
Marianthi-Anna Kioumourtzoglou, Department of Environmental Health Sciences, Columbia University Mailman School of Public Health, New York, New York.
References
- 1.[Core Writing Team, Pachauri RK and Meyer LA (eds.)]. Climate Change 2014: Synthesis Report Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC; 2014:151. [Google Scholar]
- 2.Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. The Lancet. 2015;386(9991):369–375. doi: 10.1016/S0140-6736(14)62114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phung D, Thai PK, Guo Y, Morawska L, Rutherford S, Chu C. Ambient temperature and risk of cardiovascular hospitalization: An updated systematic review and meta-analysis. Sci Total Environ. 2016;550:1084–1102. doi: 10.1016/j.scitotenv.2016.01.154 [DOI] [PubMed] [Google Scholar]
- 4.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Effects of ambient temperature on the incidence of myocardial infarction. Heart. 2009;95(21):1760–1769. doi: 10.1136/hrt.2009.175000 [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Chen C, Xu D, Li T. Effects of ambient temperature on myocardial infarction: A systematic review and meta-analysis. Environ Pollut. 2018;241:1106–1114. doi: 10.1016/j.envpol.2018.06.045 [DOI] [PubMed] [Google Scholar]
- 6.Johnson NB, Hayes LD, Kathryn B, Hoo EC, Ethier KA. CDC National Health Report: Leading Causes of Morbidity and Mortality and Associated Behavioral Risk and Protective Factors - United States, 2005 – 2013.pdf. CDC Morb Mortal Wkly Rep. 2014;63(4):3–32. [PubMed] [Google Scholar]
- 7.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient Temperature and Morbidity: A Review of Epidemiological Evidence. Environ Health Perspect. 2012;120(1):19–28. doi: 10.1289/ehp.1003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavigne E, Gasparrini A, Wang X, et al. Extreme ambient temperatures and cardiorespiratory emergency room visits: assessing risk by comorbid health conditions in a time series study. Environ Health. 2014;13(1). doi: 10.1186/1476-069X-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue Y, Nakao M, Araki T, Ueda H. Thermoregulatory responses of young and older men to cold exposure. Eur J Appl Physiol. 1992;65(6):492–498. doi: 10.1007/BF00602354 [DOI] [PubMed] [Google Scholar]
- 11.Dehghan H, Bastami M, Mahaki B. Evaluating combined effect of noise and heat on blood pressure changes among males in climatic chamber. J Educ Health Promot. 2017;6(1):39. doi: 10.4103/jehp.jehp_107_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stotz A, Rapp K, Oksa J, et al. Effect of a Brief Heat Exposure on Blood Pressure and Physical Performance of Older Women Living in the Community—A PilotStudy. Int J Environ Res Public Health. 2014;11(12):12623–12631. doi: 10.3390/ijerph111212623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostofsky E, Wilker EH, Schwartz J, et al. Short-Term Changes in Ambient Temperature and Risk of Ischemic Stroke. Cerebrovasc Dis Extra. 2014;4(1):9–18. doi: 10.1159/000357352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statewide Planning and Research Cooperative System (SPARCS). https://www.health.ny.gov/statistics/sparcs/.
- 15.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 16.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of Myocardial Infarction Diagnoses in Administrative Databases: A Systematic Review. Guo Y, ed. PLoS ONE. 2014;9(3):e92286. doi: 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ting HH, Chen AY, Roe MT, et al. Delay From Symptom Onset to Hospital Presentation for Patients With Non–ST-Segment Elevation Myocardial Infarction. Arch Intern Med. 2010;170(20). doi: 10.1001/archinternmed.2010.385 [DOI] [PubMed] [Google Scholar]
- 18.Cosgrove BA, Lohmann D, Mitchell KE, et al. Real-time and retrospective forcing in the North American Land Data Assimilation System (NLDAS) project. J Geophys Res Atmospheres. 2003;108(D22). doi: 10.1029/2002JD003118 [DOI] [Google Scholar]
- 19.Maclure M The Case-Crossover Design: a Method for Studying Transient Effects on the Risk of Acute Events.pdf. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853 [DOI] [PubMed] [Google Scholar]
- 20.Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014;43(5):1645–1655. doi: 10.1093/ije/dyu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janes H, Sheppard L, Lumley T. Case-Crossover Analyses of Air Pollution Exposure Data: Referent Selection Strategies and Their Implications for Bias. Epidemiology. 2005;16(6):717–726. doi: 10.1097/01.ede.0000181315.18836.9d [DOI] [PubMed] [Google Scholar]
- 22.Mittleman MA. Optimal Referent Selection Strategies in Case-Crossover Studies: A Settled Issue. Epidemiology. 2005;16(6):715–716. doi: 10.1097/01.ede.0000183170.92955.25 [DOI] [PubMed] [Google Scholar]
- 23.Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29(21):2224–2234. doi: 10.1002/sim.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparrini A Distributed Lag Linear and Non-Linear Models in R : The Package dlnm. J Stat Softw. 2011;43(8). doi: 10.18637/jss.v043.i08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparrini A Modelling Lagged Associations in Environmental Time Series Data: A Simulation Study. Epidemiology. 2016;27(6):835–842. doi: 10.1097/EDE.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138(20). doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 27.Altman DG. Statistics Notes: Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219–219. doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting HH, Bradley EH, Wang Y, et al. Factors Associated With Longer Time From Symptom Onset to Hospital Presentation for Patients With ST-Elevation Myocardial Infarction. Arch Intern Med. 2008;168(9):959. doi: 10.1001/archinte.168.9.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Lee E, Park MS, et al. Short-Term Effect of Temperature on Daily Emergency Visits for Acute Myocardial Infarction with Threshold Temperatures. Sun Q, ed. PLoS ONE. 2014;9(4):e94070. doi: 10.1371/journal.pone.0094070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammadi R, Soori H, Alipour A, Bitaraf E, Khodakarim S. The impact of ambient temperature on acute myocardial infarction admissions in Tehran, Iran. J Therm Biol. 2018;73:24–31. doi: 10.1016/j.jtherbio.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 31.Morabito M, Modesti PA, Cecchi L, et al. Relationships between weather and myocardial infarction: A biometeorological approach. Int J Cardiol. 2005;105(3):288–293. doi: 10.1016/j.ijcard.2004.12.047 [DOI] [PubMed] [Google Scholar]
- 32.Koken PJM, Piver WT, Ye F, Elixhauser A, Olsen LM, Portier CJ. Temperature, air pollution, and hospitalization for cardiovascular diseases among elderly people in Denver. Environ Health Perspect. 2003;111(10):1312–1317. doi: 10.1289/ehp.5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhaskaran K, Armstrong B, Hajat S, Haines A, Wilkinson P, Smeeth L. Heat and risk of myocardial infarction: hourly level case-crossover analysis of MINAP database. BMJ. 2012;345(dec13 2):e8050–e8050. doi: 10.1136/bmj.e8050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danet S, Richard F, Montaye M, et al. Unhealthy Effects of Atmospheric Temperature and Pressure on the Occurrence of Myocardial Infarction and Coronary Deaths: A 10-Year Survey: The Lille-World Health Organization MONICA Project (Monitoring Trends and Determinants in Cardiovascular Disease). Circulation. 1999;100(1). doi: 10.1161/01.CIR.100.1.e1 [DOI] [PubMed] [Google Scholar]
- 35.Casa DJ. Exercise in the Heat. I. Fundamentals of Thermal Physiology, Performance Implications, and Dehydration. J Athl Train. 1999;34(3):246–252. [PMC free article] [PubMed] [Google Scholar]
- 36.Vaseghi M, Shivkumar K. The Role of the Autonomic Nervous System in Sudden Cardiac Death. Prog Cardiovasc Dis. 2008;50(6):404–419. doi: 10.1016/j.pcad.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Deng F, Liu Y, et al. Temperature, traffic-related air pollution, and heart rate variability in a panel of healthy adults. Environ Res. 2013;120:82–89. doi: 10.1016/j.envres.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 38.Ren C, O’Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient Temperature, Air Pollution, and Heart Rate Variability in an Aging Population. Am J Epidemiol. 2011;173(9):1013–1021. doi: 10.1093/aje/kwq477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obradovich N, Migliorini R, Mednick SC, Fowler JH. Nighttime temperature and human sleep loss in a changing climate. Sci Adv. 2017;3(5):e1601555. doi: 10.1126/sciadv.1601555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisinger C, Heier M, Löwel H, Schneider A, Döring A. Sleep Duration and Sleep Complaints and Risk of Myocardial Infarction in Middle-aged Men and Women from the General Population: The MONICA/KORA Augsburg Cohort Study. Sleep. 2007;30(9):1121–1127. doi: 10.1093/sleep/30.9.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alpérovitch A, Lacombe J-M, Hanon O, et al. Relationship Between Blood Pressure and Outdoor Temperature in a Large Sample of Elderly Individuals: The Three-City Study. Arch Intern Med. 2009;169(1):75. doi: 10.1001/archinternmed.2008.512 [DOI] [PubMed] [Google Scholar]
- 42.Hong Y-C, Kim H, Oh S-Y, et al. Association of cold ambient temperature and cardiovascular markers. Sci Total Environ. 2012;435–436:74–79. doi: 10.1016/j.scitotenv.2012.02.070 [DOI] [PubMed] [Google Scholar]
- 43.Lam HCY, Chan JCN, Luk AOY, Chan EYY, Goggins WB. Short-term association between ambient temperature and acute myocardial infarction hospitalizations for diabetes mellitus patients: A time series study. Thomson M, ed. PLOS Med. 2018;15(7):e1002612. doi: 10.1371/journal.pmed.1002612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ. 2010;341(aug10 1):c3823–c3823. doi: 10.1136/bmj.c3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thrall G, Lane D, Carroll D, Lip GYH. A Systematic Review of the Prothrombotic Effects of an Acute Change in Posture. Chest. 2007;132(4):1337–1347. doi: 10.1378/chest.06-2978 [DOI] [PubMed] [Google Scholar]
- 46.Versaci F, Biondi-Zoccai G, Giudici AD, et al. Climate changes and ST-elevation myocardial infarction treated with primary percutaneous coronary angioplasty. Int J Cardiol. 2019;294:1–5. doi: 10.1016/j.ijcard.2019.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.