Abstract
Background:
Childhood exposure to air pollution has been linked with maladaptive cognitive development; however, less is known about the association between prenatal fine particulate matter (PM2.5) exposure and childhood behavior.
Objectives:
Our aim was to assess the association between prenatal PM2.5 exposure and behavioral development in 4–6 year old children residing in Mexico City.
Methods:
We used data from 539 mother-child pairs enrolled in a prospective birth cohort in Mexico City. We estimated daily PM2.5 using a 1km2 satellite-based exposure model and averaged over each trimester of pregnancy. We assessed childhood behavior at 4–6 years of age using the parent-completed Behavioral Assessment Scale for Children (BASC-2) composite scores and subscales. We used linear regression models to estimate change in BASC-2 T-scores with trimester specific 5-μg/m3 increases in PM2.5. All models were mutually adjusted for PM2.5 exposures during the other trimesters, maternal factors including age, education, socioeconomic status, depression, and IQ, child’s age at study visit, and season. We additionally assessed sex-specific effects by including an interaction term between PM2.5 and sex.
Results:
Higher first trimester PM2.5 exposure was associated with reduced Adaptive Skills scores (β: −1.45, 95% CI: −2.60, −0.30). Lower scores on the Adaptive Skills composite score and subscales indicate poorer functioning. For PM2.5 exposure during the first trimester, decrements were consistent across adaptive subscale scores including Adaptability (β: −1.51, 95% CI: −2.72, −0.30), Social Skills (β: −1.63, 95% CI: −2.90, −0.36), and Functional Communication (β: −1.21, 95% CI: −2.21, −0.21). The association between 1st trimester PM2.5 and depression was stronger in males than females (β for males: 1.52, 95% CI: −0.41, 3.45; β for females: −0.13, 95% CI: −1.99, 1.72); p-int: 0.07).
Conclusions:
Exposure to PM2.5 during early pregnancy may be associated with impaired behavioral development in children, particularly for measures of adaptive skills. These results suggest that air pollution impacts behavioral domains as well as cognition, and that the timing of exposure may be critical.
Keywords: air pollution, behavior, childhood, neurodevelopment, particulate matter
1. Introduction
Air pollution is a growing public health concern worldwide, particularly for urban populations (WHO, 2018). Fine particulate matter (PM2.5) is one of the most common air pollutants globally and is associated with an increased risk of several adverse health outcomes including mortality, cardiovascular disease, and asthma (Lee et al., 2018; McGuinn et al., 2017). In recent years, a growing body of literature demonstrates the adverse impact of air pollution on the central nervous system, with a particular focus on the developing brain (Calderon-Garciduenas et al., 2015; Calderon-Garciduenas et al., 2014). Given the complexity of critical neurodevelopmental processes occurring in the first months of gestation, the prenatal period is thought to be a particularly vulnerable developmental window to the effects of air pollution on the brain (Rice and Barone, 2000; Sunyer and Dadvand, 2019). Systemic inflammatory responses to prenatal air pollution exposure may increase risk of neuroinflammation, neuron damage/loss, and microglia activation (Block and Calderon-Garciduenas, 2009), potentially increasing the risk of cognitive and behavioral deficits in children.
A number of recent epidemiologic studies demonstrate associations between early life air pollution exposure and diagnoses of attention deficit hyperactivity disorder (ADHD) (Aghaei et al., 2019) and autism spectrum disorder (ASD) (Kalkbrenner et al., 2015; McGuinn et al., 2020; Volk et al., 2013). While informative, studies focusing on clinical diagnoses may suffer from outcome misclassification and issues related to changes in diagnostic criteria (Sagiv et al., 2015). Further, studies focused on diagnostic outcomes may fail to capture subclinical effects on neurobiological systems that may be altered by chemical exposures (Rauh and Margolis, 2016). In this study, we focus on dimensional variation of a suite of behavioral outcomes measures using the BASC-2. Our approach focusing on behavioral symptoms rather than categorical diagnoses may be more sensitive to subtle sub-clinical toxic effects, permitting the development of dose response profiles, thus informing future testing of more complex functional relationships between brain and behavior (Rauh and Margolis, 2016). Notably, in addition to continuous measures of internalizing and externalizing behaviors, the BASC-2 includes assessments of adaptive skills and ASD-like behaviors including communication and social skills. Ultimately, assessing modifiable risk factors for childhood behavioral development is critical as impairments in adaptive skills and greater externalizing and internalizing symptoms in childhood has been linked to later adolescent and adult health outcomes, such as depression and substance abuse (Loth et al., 2014).
Several recent studies demonstrate the adverse impact of postnatal and childhood air pollution exposure on behavioral development in children (Forns et al., 2016; Harris et al., 2016; Newman et al., 2013). We build on this growing body of literature by focusing on the prenatal period, a vulnerable window of brain development (Sunyer and Dadvand, 2019) that is currently understudied with respect to air pollution exposure and behavioral outcomes (Perera et al., 2012). Our longitudinal study examines a broad range of clinical and adaptive behavioral outcomes in children. Finally, of the previous studies on this specific research topic, the majority have been based in the United States or Europe. The effects of early life air pollution exposure on behavioral development in children residing in low- and middle-income countries, often in the presence of high levels of environmental and social stressors, remain largely unknown.
In this study, we examine the impact of prenatal air pollution exposure and behavioral development in a cohort of children living in Mexico City, a megacity with comparatively high air pollution levels (Calderon-Garciduenas and Torres-Jardon, 2012). Specifically, we aim to assess the association between trimester-specific prenatal PM2.5 exposure and behavioral development in 4–6 year old children. Additionally, given previous sex-specific PM2.5 findings (Chiu et al., 2014), we additionally aim to assess sex-specific associations between PM2.5 and behavioral development in children.
2. Methods
2.1. Study sample
This study takes place among mothers and their children enrolled in the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study, a prospective birth cohort study in Mexico City. Briefly, pregnant women were recruited between 2007 and 2011 at 12–24 weeks’ gestation through the Mexican Social Security System (IMSS). Women were eligible to participate in the study if they were 18 years or older, planned to live in Mexico City after their child’s birth, were less than 20 weeks gestation, had completed primary education, had no medical history of heart or kidney disease, and did not consume alcohol daily (Braun et al., 2014). In total, 948 women enrolled in the 2nd trimester and delivered a live child who was then followed longitudinally. For this analysis, we used data from 539 mother-child pairs with complete exposure, outcome, and covariate information.
Protocols were approved by the institutional review boards at the Icahn School of Medicine at Mount Sinai, Harvard School of Public Health, and Mexican National Institute of Public Health. All women provided informed consent.
2.2. Air pollution exposure assessment
Early life exposure to PM2.5 was estimated using a hybrid satellite-based exposure model developed by our team (Just et al., 2015). Briefly, we used satellite-derived Aerosol Optical Depth (AOD) measurements from the MODIS satellite, meteorological data, and land use regression variables (such as roadway density, temperature, relative humidity, planetary boundary layer height, and daily precipitation) to predict observations from ground monitoring sites. The hybrid land use regression approach used mixed effect models with temporal and spatial predictors and day-specific random effects to calibrate the satellite AOD and account for temporal variation in the PM2.5-AOD relationship as well as temporal and spatial predictors. Spatiotemporal smoothing was used for gap-filling with predictions output at a daily temporal and 1*1 km spatial resolution across the entire study area. Model performance was evaluated using monitor-level leave one out cross-validation with an R2 of 0.74. Further details on this model, including methods and performance, can be found elsewhere (Just et al., 2015).
The nearest 1km exposure grid was linked to each participant based on GPS coordinates collected at her residential address by study personnel during mid-pregnancy. Gestational age was used to link the air pollution exposures on time. Gestational age was based on last menstrual period, as reported by the mother, and by a standardized physical examination to determine gestational age at birth. Average levels of PM2.5 were calculated for each trimester of pregnancy (1st trimester: 1–13 weeks, 2nd trimester: 14–27 weeks, 3rd trimester: 28 weeks-delivery) by averaging the daily PM2.5 levels across these time periods.
2.3. Assessment of childhood behavior
Children’s behavior was assessed using the second edition of the Behavioral Assessment System for Children (BASC-2) Parent Rating Scale, validated in English and Spanish. The BASC assesses children’s adaptive and problem behaviors in the home and community settings (Reynolds and Kamphaus, 2004). The Spanish version of the BASC-2 was administered to mothers (or primary caregivers) at the 4–6 year study visit. Our primary analysis focused on relationship between PM2.5 and adaptive behavior using the Adaptive Skills composite score (which include subscales of Adaptability, Social Skills, Daily Living, and Functional Communication). In addition, we used the BASC-2 Developmental Social Disorders (DSD) content scale to assess associations with ASD-like behaviors, including difficulties with social skills and communication (Reynolds and Kamphaus, 2004). Previous studies have shown that the BASC-2 DSD scale has high sensitivity and specificity for identifying children with high-functioning ASD, when using a threshold of 60 (1 SD from the population mean) (Volker et al., 2010). We also examined associations between PM2.5 and the three BASC-2 composite clinical indices; Externalizing Problems, Internalizing Problems, and the Behavioral Symptoms Index (an overall level of behavioral problems). The Externalizing Problems composite score includes subscales of Aggression and Hyperactivity; Internalizing Problems include subscales of Anxiety, Depression, and Somatization; Behavioral Symptoms Index includes subscales of Attention, Atypicality, and Withdrawal (see Supplemental Table S1 for included BASC-2 measures). Higher scores on BASC-2 Attention and Hyperactivity subscales at this age have shown to be predictive of a later diagnosis of ADHD (Harvey et al., 2009).
Raw scores from all composite measures and subscales were converted to age- standardized T-scores (mean=50, SD=10). Spanish-speaking parents and children were included in the normative sample; however, separate Spanish norms are not available for the BASC-2. Higher scores on the BASC-2 DSD scale and three clinical scales (Externalizing, Internalizing and Behavioral Skills Index) indicate more problematic behaviors; lower scores on the Adaptive Skills composite score and subscales indicate poorer functioning.
2.4. Covariates
Covariate information was obtained from standardized questionnaires administered to mothers at baseline and follow-up study visits. Questionnaires collected sociodemographic information such maternal age at enrollment, years of education, and socioeconomic status (SES). Thirteen variables derived from questionnaire results were used to classify study participants into six levels based on the SES index created by the Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública (Carrasco, 2002). We further collapsed these six levels into low, medium, and high SES based on the distribution in our study population (Stroustrup et al., 2016). The Spanish version of the Edinburgh Postnatal Depression Scale (EPDS) was administered to women to screen for the presence of depressive symptoms during pregnancy (Alvarado-Esquivel et al., 2014; Murray and Cox, 2007). EPDS scores were dichotomized at a score of 13, based on previous studies in Spanish speaking populations (Alvarado-Esquivel et al., 2014; Murray and Cox, 2007). Finally, a subset of mothers completed the Home Observation for Measurement of the Environment (HOME) at the 24-month study visit (Bradley and Caldwell, 1979). The HOME evaluation measures the quality and support of the child’s home environment and was measured in the participant’s home during a separate visit.
A directed acyclic graph (DAG) was used to identify the minimally sufficient adjustment set; we additionally used the DAG to avoid adjustment for potential mediating variables on the causal pathway. Our DAG-identified adjustment set includes the following variables: maternal age in years, maternal education (less than high school, high school, or greater than high school), maternal SES (low, medium, high), maternal EPDS score (<13, ≥13), maternal IQ (using the Wechsler Adult Intelligence Scale, Spanish version), child’s age at BASC-2 assessment, and season of conception. Season of conception was categorized as cold-dry (November-February), warm-dry (March-April), and rainy (May-October).
2.5. Statistical analyses
We examined the distribution and summary statistics for all variables using univariate and bivariate models. The distributions of trimester-specific and entire pregnancy PM2.5 concentrations and BASC-2 scores (including the DSD scale, and adaptive skills, externalizing, internalizing, and BSI composite scores and subscales) measured at age 4–6 were examined.
We estimated associations between trimester-specific PM2.5 averages and changes in childhood behavioral development using both linear and logistic regression approaches. For linear models, we included the continuous BASC-2 measure. The estimates from these analyses can be interpreted as the difference in each BASC-2 measure per 5-μg/m3 increase in PM2.5. Logistic regression was used to assess associations between increases in PM2.5 and the odds of clinically-relevant BASC-2 scores on the composites and subscales outcomes, using the cutpoints provided in the BASC-2 manual. Children were categorized as being in the at risk/clinically significant group for the Externalizing Problems, Internalizing Problems, Behavioral Symptoms Index, and DSD scale if the t-scores were ≥ 60; a cut-off of ≤ 40 was used to identify children in the at risk/clinically significant group in terms of the Adaptive Skills composite score and subscale scores (Reynolds and Kamphaus, 2004). These cut-offs represent 1 SD from the population mean of 50.
Analyses were run separately for each BASC-2 composite and subscale outcome measure. Trimester-specific associations were reported separately for single models and mutually adjusted for exposures during the other trimesters (Wilson et al., 2017); for example, estimates for first trimester PM2.5 exposure were mutually adjusted for PM2.5 concentrations during the second and third trimesters.
Previous studies have found differences in effects of PM2.5 exposure on males and females (Chiu et al., 2014; Lee et al., 2018). Therefore, we assessed if child sex modified PM2.5-BASC-2 associations. In order to adjust for the quality of the caregiving environment, in sensitivity analyses we also adjusted for HOME scores for the subset of participants for whom scores were available. Finally, we assessed the confounding effects of prenatal lead exposure by adjusting for maternal lead exposure in the subset of participants who had prenatal blood lead concentrations available (Renzetti et al., 2017).
3. Results
3.1. Study population
Sociodemographic, exposure, and outcome measures for our study population are shown in Tables 1 and 2. Children were on average 4.8 years at the 4–6-year follow-up visit; there was an even distribution of females and males in our study sample. Mothers were 28 years old (± 5.6) at enrollment and primarily of low education and of low socioeconomic status. About 30% of mothers were categorized as having EPDS scores above 13. Average trimester-specific PM2.5 levels ranged from 22.1 – 22.9 μg/m3; the entire pregnancy average PM2.5 level was 22.7 μg/m3 (Table S2). Women in our study population were exposed to considerably higher levels than the Mexican Air Quality Standard of 12 μg/m3.
Table 1.
Demographics for 539 mother-child dyads participating in the PROGRESS study.
| Characteristic | N (%) or mean ± SD |
|---|---|
| Sex of the child | |
| Male | 272 (50) |
| Female | 267 (50) |
| Age of child at assessment (years) | 4.8 ± 0.5 |
| Gestational age (weeks) | 38 ± 1.7 |
| Maternal age at delivery (years) | 28 ± 5.6 |
| Maternal IQ | 85 ± 12 |
| Maternal depression | |
| EPDS score < 13 | 377 (70) |
| EPDS score ≥ 13 | 162 (30) |
| Socioeconomic status | |
| Low | 279 (52) |
| Medium | 205 (38) |
| High | 55 (10) |
| Maternal education | |
| <High school | 221 (41) |
| High school | 185 (34) |
| >High school | 133 (25) |
| Season of conception | |
| Cold-dry (Nov - Feb) | 154 (29) |
| Warm-dry (March - Apr) | 115 (21) |
| Rainy (May - Oct) | 268 (50) |
Abbreviations: EPDS, Edinburgh Postnatal Depression Scale; IQ, intelligence quotient; PROGRESS, Programming Research in Obesity, Growth, Environment and Social Stressors Study.
Table 2.
Distribution of BASC-2 T-scores (means ± SDs), overall and by sex of the child for 539 4–6 year children enrolled in the PROGRESS study.
| BASC-2 Measures | Overall | Females | Males |
|---|---|---|---|
| Adaptive Skills | 48.6 ± 9.5 | 50.2 ± 9.0 | 47.1 ± 9.8 |
| Adaptability | 50.4 ± 9.8 | 51.0 ± 9.7 | 49.8 ± 9.9 |
| Social skills | 50.4 ± 10.4 | 51.7 ± 10.1 | 49.2 ± 10.5 |
| Daily living | 48.8 ± 10.2 | 51.0 ± 9.2 | 46.6 ± 10.7 |
| Functional communication | 46.2 ± 8.2 | 46.9 ± 8.2 | 45.5 ± 8.1 |
| Developmental Social Disorders | 52.2 ± 9.9 | 51.0 ± 9.1 | 53.4 ± 10.5 |
| Externalizing Problems | 51.3 ± 10.1 | 49.2 ± 8.6 | 53.3 ± 11.1 |
| Aggression | 49.1 ± 10.0 | 47.1 ± 8.3 | 51.1 ± 11.1 |
| Hyperactivity | 53.2 ± 10.2 | 51.3 ± 9.2 | 55.0 ± 10.9 |
| Internalizing Problems | 54.2 ± 10.5 | 53.5 ± 10.0 | 54.9 ± 11.0 |
| Anxiety | 57.2 ± 9.9 | 57.3 ± 10.0 | 57.0 ± 9.9 |
| Depression | 51.0 ± 11.1 | 49.8 ± 10.2 | 52.2 ± 11.9 |
| Somatization | 51.5 ± 10.3 | 50.8 ± 10.0 | 52.2 ± 10.6 |
| Behavioral Symptoms Index | 52.2 ± 10.9 | 50.0 ± 9.6 | 54.2 ± 11.7 |
| Attention Problems | 52.4 ± 10.1 | 50.7 ± 9.6 | 54.1 ± 10.4 |
| Atypicality | 53.3 ± 12.3 | 51.3 ± 10.4 | 55.3 ± 13.7 |
| Withdrawal | 49.7 ± 10.2 | 49.3 ± 9.6 | 50.1 ± 10.8 |
Abbreviations: BASC-2, Behavior Assessment System for Children, Second Edition; SD, standard deviation.
3.2. Distribution of BASC-2 scores
The distributions (mean ± SD) of BASC-2 T-scores are shown in Table 2, overall and by sex. Compared to females, males tended to have higher mean scores on the Externalizing Problems, Behavioral Symptoms Index (including Attention Problems), and DSD scales, and lower mean scores on the Adaptive Skills measures. While the distributions of scores in our study sample were fairly similar to the age-standardized population (i.e., means and SDs around 50 and 10, respectively), our study sample tended to have higher mean values for Externalizing Problems, Internalizing Problems (particularly for the Anxiety subscale), and the Behavioral Symptoms Index, and lower scores for Adaptive Skills (most notably for Functional Communication) compared to the age-standardized population.
3.3. Association between prenatal PM2.5 and childhood behavior
Figure 1 shows the difference in continuous BASC-2 Adaptive Skills composite and Developmental Social Disorders scores per 5-μg/m3 increase in PM2.5. Higher concentrations of PM2.5 during the first trimester were associated with lower Adaptive Skills composites scores (β: −1.45, 95% CI: −2.60, −0.30). We did not observe significant associations between either 2nd and 3rd trimester PM2.5 and Adaptive Skills (Figure 1, Table S3). First trimester PM2.5 was similarly associated with lower scores of the three Adaptive subscales; Adaptability (β: −1.51, 95% CI: −2.72, −0.30), Social Skills (β: −1.63, 95% CI: −2.90, −0.36), and Functional Communication (β: −1.21, 95% CI: −2.21, −0.21) (Table S3). About 20% of children scored in the at-risk/ clinical range for Adaptive Skills (i.e., < 40); percentages for subscales ranged from 16–23% (Table S4). PM2.5 concentration during the first trimester was associated with an increased odds of being in the at-risk/ clinical range for Adaptive Skills (OR: 1.41, 95% CI: 1.00, 1.98) and Functional Communication (OR: 1.35, 95% CI: 0.98, 1.85) subscales (Table S5).
Figure 1.
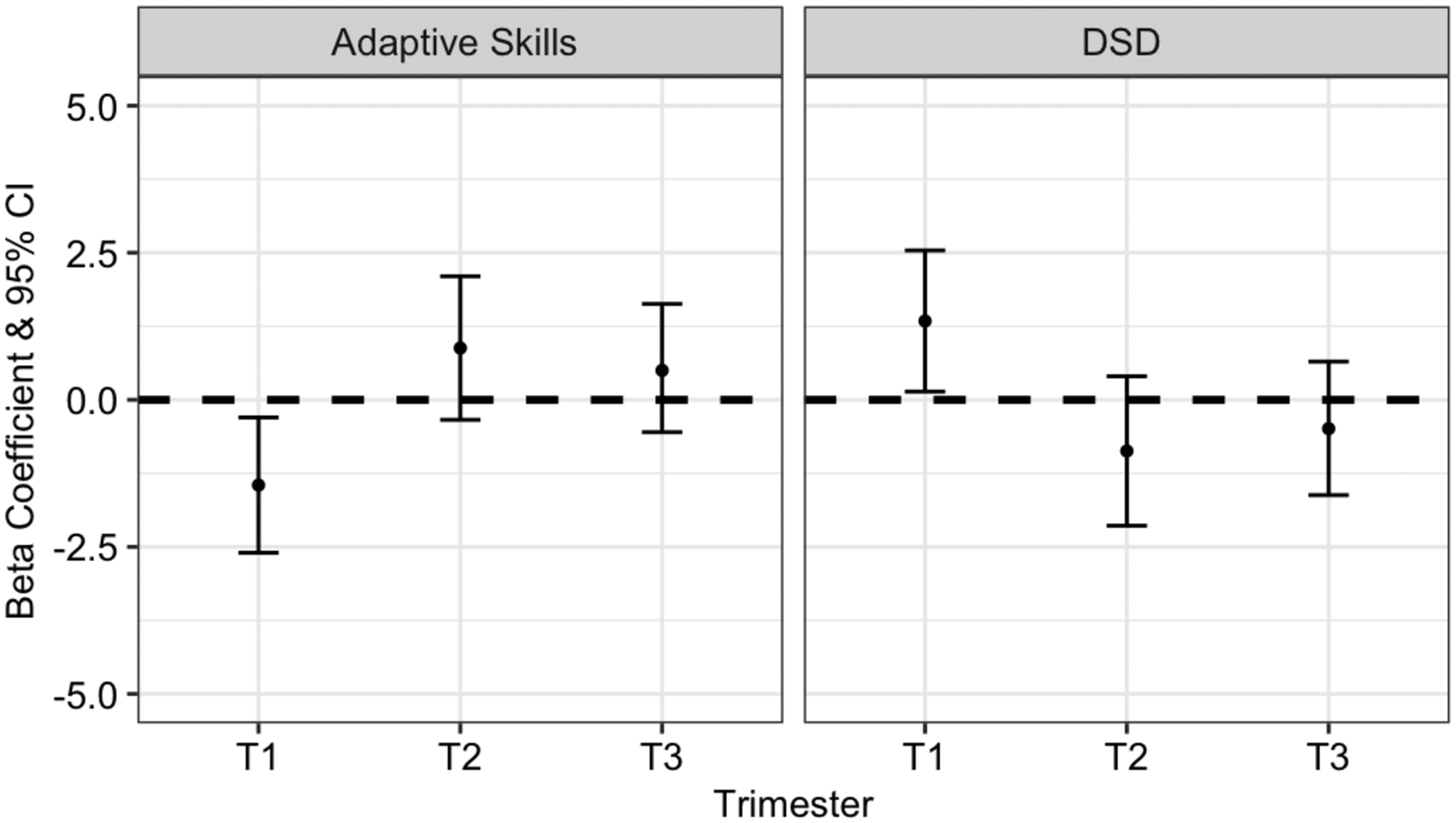
Adjusted beta coefficients and 95% confidence intervals (CI) demonstrating associations between trimester-specific PM2.5 exposure and continuous measures of BASC-2 Adaptive Skills composite and Developmental Social Disorder scores. All models are adjusted for maternal education, maternal age, maternal SES, maternal depression, maternal IQ, age of child at study visit, season of conception, and PM2.5 exposures during the other trimesters.
Consistent with results from the Adaptive Skills composite score and subscales, first trimester PM2.5 exposure was associated with deficits in social skills and communication, as measured by the Developmental Social Disorders content scale (β: 1.34, 95% CI: 0.14, 2.54) (Figure 1, Table S3).
For the clinical composite scores (Externalizing, Internalizing, and BSI), we observed similar trends. Although greater first trimester air pollution exposure was associated with more behavioral problems, confidence intervals for these results included the null (Figure 2). We did observe associations between first trimester PM2.5 exposure and increases in a few of the behavioral subscales, including Attention Problems (β: 1.44, 95% CI: 0.17, 2.71) and Withdrawal subscales (β: 1.40, 95% CI: 0.12, 2.67) (Table S3). We did not observe associations between composite scores or DSD outcomes and PM2.5 averaged over the entire pregnancy period (Table S6).
Figure 2.
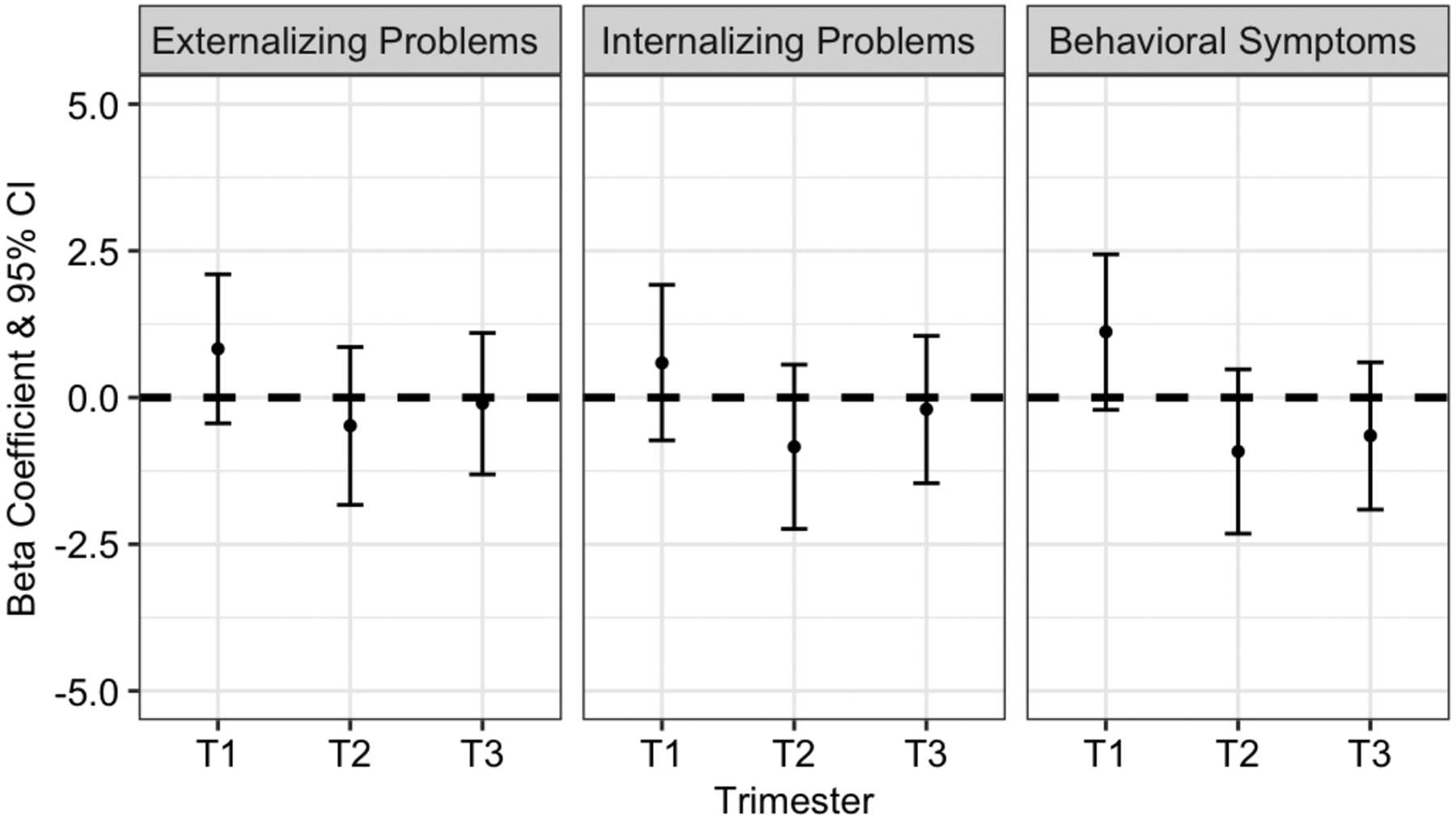
Adjusted beta coefficients and 95% confidence intervals (CI) demonstrating associations between trimester-specific PM2.5 exposure and continuous measures of BASC-2 Externalizing, Internalizing, and BSI composite T-scores. All models are adjusted for maternal education, maternal age, maternal SES, maternal depression, maternal IQ, age of child at study visit, season of conception, and PM2.5 exposures during the other trimesters.
Our main finding of associations between first trimester PM2.5 and Adaptability was not sex-specific (β for females: −2.45, 95% CI: −4.13, −0.77; β for males: −0.80, 95% CI: −2.47, 0.86; p-int=0.25). While we did not observe an association between PM2.5 and the Internalizing Problems subscale of depression, child sex did modify the association between first trimester PM2.5 and depression. In PM2.5-sex interaction models, we observed an association between PM2.5 and higher depression scores for males (β: 1.52, 95% CI: −0.41, 3.45) but not females (β: −0.13, 95% CI: −1.99, 1.72) (p-int: 0.07) (Table S7).
Among the subset of participants with available maternal blood lead concentrations (n=465), adjusting for maternal blood lead did not change any estimates significantly (Table S8). Finally, adjusting for HOME scores (n=376) did not significantly alter our results (Table S9), nor did adjusting for maternal blood lead and HOME scores in the same model.
4. Discussion
In this study, higher first trimester PM2.5 exposure was associated with poorer BASC-2 Adaptive Skills composite score and subscales, suggesting this life stage is a critical exposure window during which air pollution can affect the developing brain. This association was not observed with second and third trimester PM2.5, nor with PM2.5 averaged across the entire pregnancy. First trimester PM2.5 was also associated with the increased DSD content scale and higher reporting of Attention and Withdrawal symptoms. Overall, our findings did not differ significantly by sex; however, first trimester PM2.5 exposure was associated with higher depression scores for males compared to females.
Our study adds to the accumulating body of literature examining associations between early life air pollution and children’s neurodevelopment by a) focusing on behavioral rather than cognitive outcomes and b) addressing air pollution exposure throughout all of pregnancy, including the first trimester. Our most robust findings were for associations between early pregnancy PM2.5 exposure and lower adaptive functioning, including worse Communication and Social Skills scores. Early studies linking air pollution and children’s neurodevelopment focused largely on cognitive outcomes (Block et al., 2012; Calderon-Garciduenas et al., 2014) with more recent studies shifting to focus on behavioral outcomes (Guxens et al., 2014; Lubczynska et al., 2017; Stingone et al., 2017; Sunyer et al., 2015). Deficits in social skills and communication, as measured using the DSD, are hallmarks of ASD (Bradstreet et al., 2017; Robertson et al., 1999). Several previous studies have found an increased risk of ASD among children with greater prenatal and/or early postnatal air pollution exposures (Flores-Pajot et al., 2016; McGuinn et al., 2020). In a population-based study of typically developing children, early prenatal PM2.5 was associated with worse communication scores (Ha et al., 2019). Among children with ASD compared to typically developing children, first postnatal year nitrogen dioxide (a proxy for air pollution) exposure was associated with reduced adaptive skills (Kerin et al., 2018).
To date, only a limited number of studies focus on associations between prenatal air pollution exposure and child neurodevelopment. In a New York City based cohort, polycyclic aromatic hydrocarbons (PAH) measured in third trimester personal air monitors were associated with increased anxiety, depression and attention in young children (Perera et al., 2012). Third trimester black carbon exposure was not associated with behavioral outcomes (Harris et al., 2016). Neither study included estimates of first or second trimester air pollution exposure. To our knowledge, ours is one of the first epidemiologic studies to assess associations with childhood behavior using each trimester-specific PM2.5 average including the first trimester.
A growing body of toxicologic literature exists supporting the biologic plausibility of an association between early life air pollution exposure and adverse impact on the developing brain (Costa et al., 2014). A number of animal studies have found increases in neuroinflammation, oxidative stress, and markers of microglial activation in response to air pollution exposure (Block and Calderon-Garciduenas, 2009). Notably, and consistent with epidemiologic literature, outcomes appear to be sex-specific, with effects often only present in male mice (Bolton et al., 2017). In humans, systemic inflammatory responses from early life air pollution exposure may reach the developing brain, increasing risk of neuroinflammation, neuron damage/loss, and microglia activation (Block and Calderon-Garciduenas, 2009). Our most consistent findings were for exposures during the first trimester, a period of rapid brain development (Rice and Barone, 2000). Prior studies have found associations between first trimester PM2.5 exposure and fetal growth restriction (Michikawa et al., 2017), which has been implicated in childhood behavioral disorders (Wiles et al., 2006).
While mechanistic studies linking air pollution exposure to neurodevelopmental outcomes in humans are rare, magnetic resonance imaging (MRI) offer non-invasive in vivo insight into structural and functional changes in the brain that may be associated with environmental exposures (Horton et al., 2014). Higher air pollution was associated with increased white matter brain lesions in Mexico City children compared to those children in less polluted areas (56% vs 8% respectively) (Calderon-Garciduenas et al., 2008). Another recent study observed associations between prenatal PM2.5 and decreases in childhood corpus callosum (CC) volume (Mortamais et al., 2019). Structural changes in the CC may be one of the mechanisms linking early life air pollution exposure to childhood behavioral problems.
Our study has several limitations. We used each participant’s residential location during mid-pregnancy to assign PM2.5 exposures for the entire pregnancy period, and is possible that relocation throughout pregnancy and childhood would impact PM2.5 exposure. However, several studies leveraging full prenatal residential address history suggest minimal exposure misclassification, and that when women do move they often stay in the same exposure category (Bell and Belanger, 2012). Personal air sampling during pregnancy, the gold standard for air pollution exposure assessment (Larkin and Hystad, 2017) was not available for these subjects. We also note that personal air sampling, while more precise, is not feasible to conduct beyond very short time periods from several days to weeks. We adjusted for HOME scores in sensitivity analyses to control for the caretaking environment, as this may impact behavioral development of the children. Adjusting for HOME scores in the subset of participants who completed the in-person home visit did not substantially impact the resulting estimates. Further, housing conditions, ventilation in the house, and other occupational exposures could potentially confound our associations. We did not have access to this data in the current analyses and we note this as a limitation. Finally, although we adjusted for several key covariates, we did not have information on other urban correlates such as environmental noise exposure. Future studies will consider additional measures of other urban correlates.
We used the BASC-2 parent reported measure to assess behavioral development in 4–6 year children. It is possible that some parents may have misreported their child’s behavioral functioning; however, given the age of the children, the parent reported measure would likely be more valid at this younger age, compared to the self-reported measures (Reynolds and Kamphaus, 2004). Further, maternal mental health status may impact both the reporting of their child’s behavior as well as the child’s development of behavioral problems. (Leis et al., 2014). We adjusted for maternal depression in our analyses in order to control for these potential relationships. As children in the PROGRESS cohort age, we will have both self- and parent-reported BASC-2 measures, so we will be able to assess the impact of informant on the associations between air pollution and children’s behavioral outcomes. Finally, given the young age of the children, instead of using strict diagnostic criteria, we assessed associations using broad behavioral symptoms.
5. Conclusions
This is one of the first studies to assess the question of whether prenatal exposure to fine particulate matter impacts behavioral development in children residing in a middle-income country with considerably high levels of air pollution. We assessed this research question in a well-characterized, prospective birth cohort using state-of-the-art satellite-based air pollution exposure modeling. Adjusting for several important confounders such as maternal SES and depression, we demonstrated an inverse association between first trimester air pollution exposure and increased behavioral problems in children at 4–6 years of age.
Supplementary Material
Financial support
This work was supported by funding from the NIH T32HD049311, R01ES013744, R01ES021357, and R24ES028522.
References
- Aghaei M, Janjani H, Yousefian F, Jamal A, Yunesian M, 2019. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ Res 173, 135–156. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Sifuentes-Alvarez A, Salas-Martinez C, 2014. Validation of the edinburgh postpartum depression scale in a population of adult pregnant women in Mexico. J Clin Med Res 6(5), 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Belanger K, 2012. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol 22(5), 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L, 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9), 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, Cory-Slechta DA, Costa D, Diaz-Sanchez D, Dorman DC, Gold DR, Gray K, Jeng HA, Kaufman JD, Kleinman MT, Kirshner A, Lawler C, Miller DS, Nadadur SS, Ritz B, Semmens EO, Tonelli LH, Veronesi B, Wright RO, Wright RJ, 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33(5), 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD, 2017. Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. Front Synaptic Neurosci 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, 1979. Home observation for measurement of the environment. American Journal of Mental Deficiency 84(3), 235–244. [PubMed] [Google Scholar]
- Bradstreet LE, Juechter JI, Kamphaus RW, Kerns CM, Robins DL, 2017. Using the BASC-2 Parent Rating Scales to Screen for Autism Spectrum Disorder in Toddlers and Preschool-Aged Children. J Abnorm Child Psychol 45(2), 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo YOM, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM, 2014. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health 13(1), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Kulesza RJ, Doty RL, D’Angiulli A, Torres-Jardon R, 2015. Megacities air pollution problems: Mexico City Metropolitan Area critical issues on the central nervous system pediatric impact. Environ Res 137, 157–169. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, Gomez-Garza G, Barragan-Mejia G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, Henriquez-Roldan C, Perez-Guille B, Torres-Jardon R, Herrit L, Brooks D, Osnaya-Brizuela N, Monroy ME, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Solt AC, Engle RW, 2008. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn 68(2), 117–127. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Torres-Jardon R, 2012. Air Pollution, Socioeconomic Status, and Children’s Cognition in Megacities: The Mexico City Scenario. Front Psychol 3, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Torres-Jardon R, Kulesza RJ, Park SB, D’Angiulli A, 2014. Air pollution and detrimental effects on children’s brain. The need for a multidisciplinary approach to the issue complexity and challenges. Front Hum Neurosci 8, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco AJE, 2002. The Amai System of Classifying Households by Socio-economic Level: the Experience of Mexico and Its Comparison with Brazil and Argentina. www.esomar.org.
- Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, Wright RJ, 2014. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol 133(3), 713–722 e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P, 2014. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int 2014, 736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ, 2016. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environ Res 151, 763–776. [DOI] [PubMed] [Google Scholar]
- Forns J, Dadvand P, Foraster M, Alvarez-Pedrerol M, Rivas I, Lopez-Vicente M, Suades-Gonzalez E, Garcia-Esteban R, Esnaola M, Cirach M, Grellier J, Basagana X, Querol X, Guxens M, Nieuwenhuijsen MJ, Sunyer J, 2016. Traffic-Related Air Pollution, Noise at School, and Behavioral Problems in Barcelona Schoolchildren: A Cross-Sectional Study. Environ Health Perspect 124(4), 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Garcia-Esteban R, Giorgis-Allemand L, Forns J, Badaloni C, Ballester F, Beelen R, Cesaroni G, Chatzi L, de Agostini M, de Nazelle A, Eeftens M, Fernandez MF, Fernandez-Somoano A, Forastiere F, Gehring U, Ghassabian A, Heude B, Jaddoe VW, Klumper C, Kogevinas M, Kramer U, Larroque B, Lertxundi A, Lertxuni N, Murcia M, Navel V, Nieuwenhuijsen M, Porta D, Ramos R, Roumeliotaki T, Slama R, Sorensen M, Stephanou EG, Sugiri D, Tardon A, Tiemeier H, Tiesler CM, Verhulst FC, Vrijkotte T, Wilhelm M, Brunekreef B, Pershagen G, Sunyer J, 2014. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology 25(5), 636–647. [DOI] [PubMed] [Google Scholar]
- Ha S, Yeung E, Bell E, Insaf T, Ghassabian A, Bell G, Muscatiello N, Mendola P, 2019. Prenatal and early life exposures to ambient air pollution and development. Environ Res 174, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, Schwartz JD, Gryparis A, Kloog I, Koutrakis P, Bellinger DC, Belfort MB, Webster TF, White RF, Sagiv SK, Oken E, 2016. Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicol Teratol 57, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EA, Youngwirth SD, Thakar DA, Errazuriz PA, 2009. Predicting attention-deficit/hyperactivity disorder and oppositional defiant disorder from preschool diagnostic assessments. J Consult Clin Psychol 77(2), 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Margolis AE, Tang C, Wright R, 2014. Neuroimaging is a novel tool to understand the impact of environmental chemicals on neurodevelopment. Curr Opin Pediatr 26(2), 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AC, Wright RO, Schwartz J, Coull BA, Baccarelli AA, Tellez-Rojo MM, Moody E, Wang Y, Lyapustin A, Kloog I, 2015. Using High-Resolution Satellite Aerosol Optical Depth To Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environ Sci Technol 49(14), 8576–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, Thayer BP, Daniels JL, 2015. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 26(1), 30–42. [DOI] [PubMed] [Google Scholar]
- Kerin T, Volk H, Li W, Lurmann F, Eckel S, McConnell R, Hertz-Picciotto I, 2018. Association Between Air Pollution Exposure, Cognitive and Adaptive Function, and ASD Severity Among Children with Autism Spectrum Disorder. J Autism Dev Disord 48(1), 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin A, Hystad P, 2017. Towards Personal Exposures: How Technology Is Changing Air Pollution and Health Research. Curr Environ Health Rep 4(4), 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, Wilson A, Schwartz J, Cohen S, Coull BA, Wright RO, Wright RJ, 2018. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J Allergy Clin Immunol 141(5), 1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis JA, Heron J, Stuart EA, Mendelson T, 2014. Associations between maternal mental health and child emotional and behavioral problems: does prenatal mental health matter? J Abnorm Child Psychol 42(1), 161–171. [DOI] [PubMed] [Google Scholar]
- Loth AK, Drabick DA, Leibenluft E, Hulvershorn LA, 2014. Do childhood externalizing disorders predict adult depression? A meta-analysis. J Abnorm Child Psychol 42(7), 1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubczynska MJ, Sunyer J, Tiemeier H, Porta D, Kasper-Sonnenberg M, Jaddoe VWV, Basagana X, Dalmau-Bueno A, Forastiere F, Wittsiepe J, Hoffmann B, Nieuwenhuijsen M, Hoek G, de Hoogh K, Brunekreef B, Guxens M, 2017. Exposure to elemental composition of outdoor PM2.5 at birth and cognitive and psychomotor function in childhood in four European birth cohorts. Environ Int 109, 170–180. [DOI] [PubMed] [Google Scholar]
- McGuinn LA, Ward-Caviness C, Neas LM, Schneider A, Di Q, Chudnovsky A, Schwartz J, Koutrakis P, Russell AG, Garcia V, Kraus WE, Hauser ER, Cascio W, Diaz-Sanchez D, Devlin RB, 2017. Fine particulate matter and cardiovascular disease: Comparison of assessment methods for long-term exposure. Environ Res 159, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinn LA, Windham GC, Kalkbrenner AE, Bradley C, Di Q, Croen LA, Fallin MD, Hoffman K, Ladd-Acosta C, Schwartz J, Rappold AG, Richardson DB, Neas LM, Gammon MD, Schieve LA, Daniels JL, 2020. Early Life Exposure to Air Pollution and Autism Spectrum Disorder: Findings from a Multisite Case-Control Study. Epidemiology 31(1), 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa T, Morokuma S, Fukushima K, Kato K, Nitta H, Yamazaki S, 2017. Maternal exposure to air pollutants during the first trimester and foetal growth in Japanese term infants. Environ Pollut 230, 387–393. [DOI] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, Martinez-Vilavella G, Fenoll R, Reynes C, Sabatier R, Rivas I, Forns J, Vilor-Tejedor N, Alemany S, Cirach M, Alvarez-Pedrerol M, Nieuwenhuijsen M, Sunyer J, 2019. Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ Res 178, 108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D, Cox JL, 2007. Screening for depression during pregnancy with the edinburgh depression scale (EDDS). Journal of Reproductive and Infant Psychology 8(2), 99–107. [Google Scholar]
- Newman NC, Ryan P, Lemasters G, Levin L, Bernstein D, Hershey GK, Lockey JE, Villareal M, Reponen T, Grinshpun S, Sucharew H, Dietrich KN, 2013. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect 121(6), 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Camann D, Rauh V, 2012. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect 120(6), 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Margolis AE, 2016. Research Review: Environmental exposures, neurodevelopment, and child mental health - new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry 57(7), 775–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, Just AC, Burris HH, Oken E, Amarasiriwardena C, Svensson K, Mercado-Garcia A, Cantoral A, Schnaas L, Baccarelli AA, Wright RO, Tellez-Rojo MM, 2017. The association of lead exposure during pregnancy and childhood anthropometry in the Mexican PROGRESS cohort. Environ Res 152, 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW, 2004. BASC-2: Behavior Assessment System for Children, 2. Pearson, Bloomington, MN. [Google Scholar]
- Rice D, Barone S Jr., 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108 Suppl 3, 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Tanguay PE, L’Ecuyer S, Sims A, Waltrip C, 1999. Domains of social communication handicap in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 38(6), 738–745. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Kalkbrenner AE, Bellinger DC, 2015. Of decrements and disorders: assessing impairments in neurodevelopment in prospective studies of environmental toxicant exposures. Environ Health 14, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingone JA, Pandey OP, Claudio L, Pandey G, 2017. Using machine learning to identify air pollution exposure profiles associated with early cognitive skills among U.S. children. Environ Pollut 230, 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup A, Hsu HH, Svensson K, Schnaas L, Cantoral A, Solano Gonzalez M, Torres-Calapiz M, Amarasiriwardena C, Bellinger DC, Coull BA, Tellez-Rojo MM, Wright RO, Wright RJ, 2016. Toddler temperament and prenatal exposure to lead and maternal depression. Environ Health 15(1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J, Dadvand P, 2019. Pre-natal brain development as a target for urban air pollution. Basic Clin Pharmacol Toxicol 125 Suppl 3, 81–88. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Esnaola M, Alvarez-Pedrerol M, Forns J, Rivas I, Lopez-Vicente M, Suades-Gonzalez E, Foraster M, Garcia-Esteban R, Basagana X, Viana M, Cirach M, Moreno T, Alastuey A, Sebastian-Galles N, Nieuwenhuijsen M, Querol X, 2015. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med 12(3), e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R, 2013. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70(1), 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker MA, Lopata C, Smerbeck AM, Knoll VA, Thomeer ML, Toomey JA, Rodgers JD, 2010. BASC-2 PRS profiles for students with high-functioning autism spectrum disorders. J Autism Dev Disord 40(2), 188–199. [DOI] [PubMed] [Google Scholar]
- WHO, 2018. Ambient (outdoor) air pollution. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health.
- Wiles NJ, Peters TJ, Heron J, Gunnell D, Emond A, Lewis G, 2006. Fetal growth and childhood behavioral problems: results from the ALSPAC cohort. Am J Epidemiol 163(9), 829–837. [DOI] [PubMed] [Google Scholar]
- Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA, 2017. Potential for Bias When Estimating Critical Windows for Air Pollution in Children’s Health. Am J Epidemiol 186(11), 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


