Abstract
Cartilaginous fishes, comprising the chimeras, sharks, skates, and rays, split from the common ancestor with other jawed vertebrates approx. 450 million years ago. Being the oldest extant taxonomic group to possess an immunoglobulin (Ig)-based adaptive immune system, examination of this group has taught us much about the evolution of adaptive immunity, as well as the conserved and taxon-specific characteristics of Igs. Significant progress has been made analyzing sequences from numerous genomic and transcriptomic data sets. These findings have been supported by additional functional studies characterizing the Igs and humoral response of sharks and their relatives. This review will summarize what we have learned about the genomic organization, protein structure, and in vivo function of these Ig isotypes in cartilaginous fishes and highlight the areas where our knowledge is still lacking.
Keywords: cartilaginous fish, shark, antibody, IgM, IgNAR, memory, B cells
1. Introduction
Since adaptive immunity arose in the common ancestor of all vertebrate lineages, it has diversified into a complex arsenal of cellular and molecular defenses that each phylogenetic branch of both the ectotherms (cold-blooded organisms) and endotherms (warm-blooded organisms) has preserved or altered to varying degrees. However, the fundamental molecular armament of humoral immunity in all gnathostomes (jawed vertebrates) are immunoglobulins (Igs) or antibodies (Abs). It is these molecules, expressed as heterodimers of heavy (H) and light (L) chains on the surface of B cells and secreted by plasma cells, that provide the host adaptive immune system with exquisite specificity to protect against pathogens via a myriad of functional roles.
Cartilaginous fish (Chondrichthyes) split from the common ancestor with other jawed vertebrates approx. 450 million years ago (Inoue et al., 2010) and have two extant subclasses, the holocephalans (chimeras, such as elephant sharks and rat fishes) and the better known elasmobranchs (sharks, rays, and skates). Being the oldest extant taxonomic group to possess an Ig-based adaptive immune system (Flajnik and Rumfelt, 2000) examination of this group has taught us much about the evolution of adaptive immunity, as well as the conserved and taxon-specific characteristics of Igs. Sharks and their relatives have three IgH chain isotypes: the first two, IgM (μ) (Clem et al., 1967; Marchalonis and Edelman, 1966) and IgW (ω) (Anderson et al., 1999; Berstein et al., 1996; Greenberg et al., 1996; Kunihiko and Susumu, 1988), are orthologous to IgM and IgD (respectively) in other vertebrate groups (Ohta and Flajnik, 2006). In addition to these ‘conventional’ IgH-IgL isotypes elasmobranchs possess a third isotype IgNAR, an IgH chain homodimer that does not associate with L chain (Greenberg et al., 1995; Roux et al., 1998). Further, cartilaginous fishes have four IgL chain isotypes: kappa (κ), lambda (λ), sigma (σ), and sigma-2 (σ-2; also called σ-cart prior to its discovery in lobe-finned fishes) (Criscitiello and Flajnik, 2007; Saha et al., 2014). This review will summarize what we have learned about the genomic organization, protein structure, and in vivo function of these Ig isotypes in cartilaginous fishes in the context of immune system evolution.
2. Cartilaginous fish immune organs
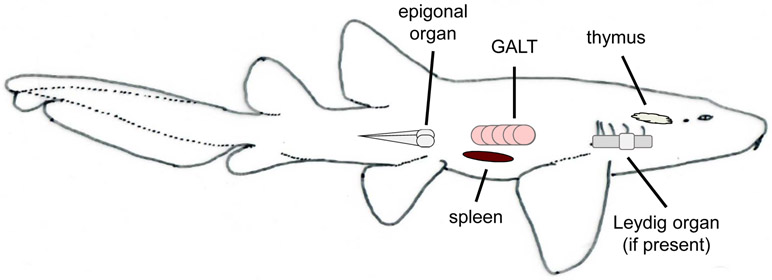
Like mammals, T cell maturation occurs in the cartilaginous fish thymus, which is paired and located dorsomedial to the gills (Fig.1) (Luer et al., 1995; Miracle et al., 2001. Lacking bone marrow, cartilaginous fish B cell lymphopoiesis primarily occurs in special sites known as the epigonal organ, associated with the gonads, and the Leydig organ, embedded within the wall of the esophagus (Fig. 1). Not all elasmobranch species have a Leydig organ, for example, while found in little skate (Leucoraja erinacea) it is not present in nurse sharks (Ginglymostoma cirratum). Histologically both organs are similar in structure, with the spongy appearance of mammalian bone marrow (Luer et al., 2004). Cartilaginous fishes, like other ectothermic vertebrates, lack lymph nodes (Zapata and Amemiya, 2000). Consequently, adaptive immune responses occur primarily in the spleen, although there is some evidence of lymphocyte activity in the gills, gut, and several other tissues (Hart et al., 1986; Rumfelt et al., 2002; Tomonaga et al., 1986). The shark spleen is highly vascularized and contains both red pulp and defined white pulp regions. Organized zones of B cells are evident and may facilitate clonal selection and somatic hypermutation (SHM) (Castro et al., 2013; Rumfelt et al., 2002, although true germinal centers (GCs) have not been identified in any ectotherms to date (Zapata and Amemiya, 2000; Zapata et al., 1996). As the spleen houses both naive B cells and differentiated plasma cells, it is the major site of Ig expression and secretion (Castro et al., 2013; Rumfelt et al., 2002). While the epigonal organ is hypothesized to be the home of long-lived plasma cells (Castro et al., 2013) secretory Ig transcript has also found in the pancreas, gill, liver, kidney, and olfactory organ of nurse sharks (Rumfelt et al., 2004a). Thus, as in mammals, Igs seemingly play a defensive role throughout all cartilaginous fish tissues.
Figure 1:
The major immunological organs of cartilaginous fishes and their relative positions.
3. Genomic organization of cartilaginous fish Igs and repertoire generation
Most vertebrate Ig genes are organized in a translocon configuration (Fig. 2), in which multiple variable (V), diversity (D; only present in IgH chains), and joining (J) segments are located upstream of constant (C) domains for all Ig isotypes. In contrast, cartilaginous fish IgH and IgL chain genes are arranged in a cluster configuration (Fig. 2), in which a single V segment, one or more D segments (again, IgH chains only), and a single J segment lie upstream of C region exons of a single isotype (Hinds and Litman, 1986). The number of clusters varies between isotypes and between cartilaginous fish species: for example, nurse sharks have ~15 IgM clusters (each having V, D, J segments and a set of Cμ domains) and 3 IgNAR clusters (V, multiple Ds, J, and CNAR domains) while the spiny dogfish (Squalus acanthias) has >100 IgM and 10-20 IgNAR clusters (Malecek et al., 2005; Smith et al., 2012). While most Ig segments are unjoined in the cartilaginous fish germline genome, a small portion are found to be partially (VD-J) or fully (VDJ or VJ) pre-rearranged or “germline-joined”; e.g. nurse sharks have both germline-joined IgM and IgNAR clusters (Diaz et al., 2002; Kokubu et al., 1988; Rumfelt et al., 2001). These germline-joined nurse shark IgH genes are preferentially expressed early in ontogeny and are hypothesized to play unique protective and/or homeostatic roles in neonates (Diaz et al., 2002; Rumfelt et al., 2001).
Figure 2:
Schematic showing the different Ig gene arrangements in mammals and sharks. Mammalian Igs are found in a translocon arrangement, with multiple V segments, D segments, and J segments arranged upstream of the C regions for all the different Ig isotypes. In contrast, cartilaginous fish Igs are found in a cluster configuration, where a single V segment, two or more D segments, a J segment, and the C regions for a single isotype are present in each cluster, and multiple clusters are present for each isotype. C regions are shown in grey, the segments that make up the variable region are colored by isotype.
Cartilaginous fishes use the same recombination mechanisms as mammals to generate a primary Ig repertoire, with recombination-activation gene (RAG1 and RAG2) enzymes active in the lymphopoietic organs (epigonal and Leydig organ) along with terminal deoxynucleotidyl transferase (TdT), which inserts N-nucleotides at the rearrangement junctions (Rumfelt et al., 2002). Rearrangement is primarily restricted within a cluster, and rarely occurs between different clusters (Malecek et al., 2008). Unlike mice and humans, where there is a strict order of segmental rearrangement (always D to J first, then V to DJ for Ig heavy chains) the V, D, and J segments of shark Ig clusters can recombine in any order (e.g. V to D1, D1 to D2, and D3 to J first round rearrangements have all been observed; H. Dooley, unpublished data), and multiple Ig genes are accessible for recombination at one time (Malecek et al., 2008). Despite this, single cell PCRs amplified only one IgH V region transcript per B cell in the clearnose skate (Raja eglanteria) (Eason and Litman, 2002) and no overlap is observed between IgM- and IgNAR-secreting cells in nurse sharks (Rumfelt et al., 2002), indicating both isotypic and allelic exclusion occur. More recent studies have shown that shark IgM clusters operate autonomously during V(D)J recombination. This stochastic activation of IgH genes for rearrangement initiation, combined with a restricted time window of accessibility, likely help maintain IgH chain exclusion (Zhu et al., 2011). It is hypothesized that once a productive V region is produced from any of the accessible clusters it prevents rearrangement and expression of the remaining Ig clusters, thereby maintaining B cell clonality (Zhu et al., 2011).
Based upon the limited number of V, (D) and J segments within a single cartilaginous fish cluster it might be concluded that the shark primary immune repertoire is restricted in diversity. However, several mechanisms compensate for this. First, as noted above, every Ig isotype has multiple clusters with extensive germline-encoded heterogeneity across complementarity determining regions (CDR)1 and 2 (Rast et al., 1998; Rumfelt et al., 2004b). Second, each heavy chain cluster contains multiple D segments and thus requires multiple rearrangement events (e.g. 4 for IgNAR), with associated trimming and N/P-addition, to generate a functional V region (Malecek et al., 2008). Finally, cartilaginous fish have four IgL chain isotypes that are among the most heterogenous of any vertebrate studied to date (Criscitiello and Flajnik, 2007; Fleurant et al., 2004). Thus, the weight of evidence suggests the primary repertoire of cartilaginous fishes is just as diverse (or possibly even more diverse) than that of mammals. Study of random IgNAR cDNAs obtained from peripheral blood lymphocyte samples showed a much lower mutation rate in transmembrane transcripts compared to secretory transcripts, and only mutations in the latter were biased towards replacement mutations in the CDRs (Diaz et al., 1998). This indicates that shark Igs undergo activation induced cytidine deaminase (AID)-mediated somatic diversification following antigen stimulation akin to mammals, rather than using mutation to generate the primary repertoire like birds and rabbits. Interestingly, the mutation frequency of IgNAR is exceptionally high, surpassing even the upper limits reported for mammalian V regions (Diaz et al., 1999). Study of random nurse shark IgNAR and IgL transcripts showed mutations accumulate in a pattern similar to mammalian IgG, targeting AGC/T hotspots, but with a high proportion of tandem mutations rather than singlets (Diaz et al., 1999; Greenberg et al., 1995; Lee et al., 2002). The fact that high replacement to synonymous (R/S) ratios were observed in the complementarity determining regions (CDRs) of some clones suggests positive selection can occur despite the apparent absence of GCs (Diaz et al., 2002). Thus, the ability of cartilaginous fishes to generate and diversify a primary Ig repertoire is by no means sub-optimal. Indeed, by all measures, sharks are as capable as mammals of combating the potential universe of pathogens they may encounter.
The cluster organization of cartilaginous fish Ig genes and absence of recognizable classical (i.e. mammalian-like) switch (S) regions (Zhu et al., 2012) suggests this group lacks tetrapod-like class switch recombination, which so far has only been identified as far back as amphibians (Mußmann et al., 1997). However, recent studies of nurse shark Ig transcripts have shown that the V regions from one cluster may be expressed fused to the C domains of another, despite being separated by large (>120Kb) distances (Zhang et al., 2013; Zhu et al., 2012). Unlike mammals this form of ‘switching’ is adirectional, i.e. IgW V regions can be expressed with IgM C regions and vice versa (Zhu et al., 2012). As the number of switched transcripts is higher in immunized animals it is hypothesized to occur via AID-initiated DNA lesions that prompt recombination between the clusters (Zhu et al., 2012). We do not yet know how this process is directed or its biological consequence; however, it is possible this is the predecessor of conventional CSR as found in other vertebrates. Thus, most of the fundamental components of Ig repertoire diversification, including V(D)J recombination, somatic hypermutation (SHM), and a potential precursor of CSR were all present in the common ancestor of jawed vertebrates.
4. The Ig isotypes of cartilaginous fishes
4.1. IgM
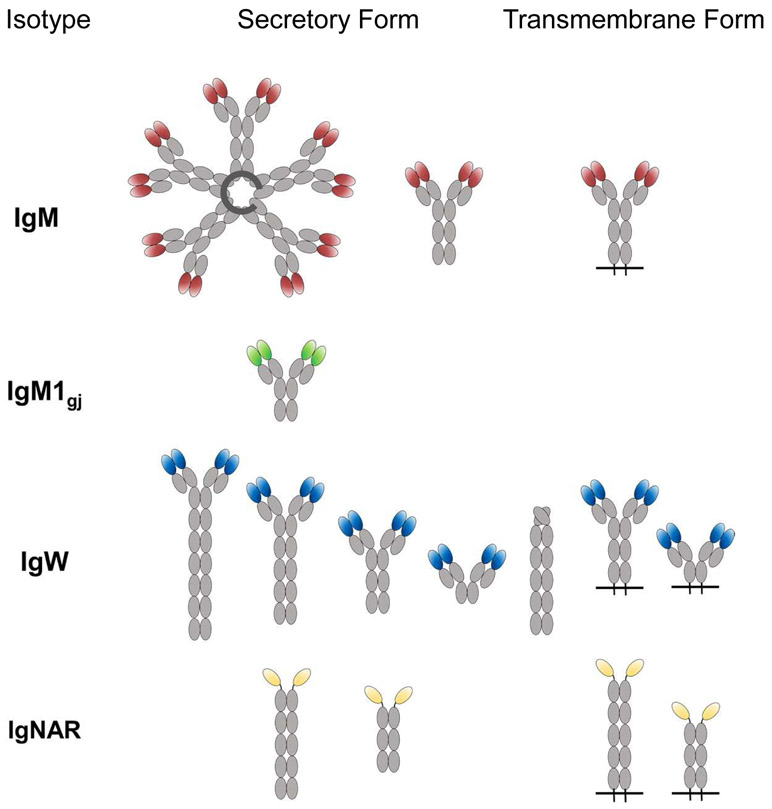
A protein with characteristics similar to IgM was first discovered in the plasma of cartilaginous fishes in the mid-1960s (Clem and Small Jr, 1967; Clem et al., 1967; Marchalonis and Edelman, 1966) and was originally thought to be the only isotype present. Indeed, in nurse shark IgM makes up roughly half of the total serum protein and is present in two forms, a monomeric form (mIgM or 7S) and a pentameric (pIgM or 19S) form (Fig. 3) in approximately equal amounts (Clem et al., 1967; Small et al., 1970). The two forms do not interconvert (Small et al., 1970) and are hypothesized to be produced by different B cell lineages (Dooley and Flajnik, 2005).
Figure 3:
Illustration of the transmembrane and secretory immunoglobulin isoforms found in cartilaginous fishes to date. Constant domains are shown in grey while the variable domains of each are colored by isotype. Secreted IgM pentamers are stabilized by joining (J) chain.
Secretion of pIgM is coordinated with J chain expression in adult nurse sharks, and it has also been found that pIgM/J chain+ nurse shark splenic B cells do not express the transcription factor Blimp-1, which controls plasma cell differentiation, and express lower levels of IgM transcript than mIgM B cells (Castro et al., 2013). In contrast, mIgM is expressed in Blimp-1+/J chain- B cells (Castro et al., 2013) and is likely produced in a T cell-dependent manner (Clem et al., 1967; Dooley and Flajnik, 2005; Marchalonis and Edelman, 1966), with antigen specific titers of mIgM rising over the course of an immune response. Coupled with its non-specific nature, pIgM is thought to act as an innate-like, ‘first line of defense’ against invading pathogens until a strong antigen-specific response can be developed. A lack of serum albumin in sharks suggests pIgM may also act as a carrier molecule or in osmoregulation, in addition to acting as an innate defender (Dooley and Flajnik, 2006; Fellows and Hird, 1981; Fellows et al., 1980). Like IgM in other vertebrates, the large size of pIgM restricts it to the vasculature, while mIgM is capable of penetrating tissues similar to mammalian IgG (Small et al., 1970)
Both the transmembrane and secreted forms of IgM contain 4 C domains (Rumfelt et al., 2004a) but, interestingly, a third form with only 3 C domains (expressed from a separate cluster where Cμ2 is deleted, making it structurally convergent with mammalian IgG) predominates in the plasma of neonatal animals and pups (Rumfelt et al., 2001). This IgM form, IgM1gj, forms both monomers and dimers, has an entirely germline-joined (VDJ) V domain (Rumfelt et al., 2001) and preferentially associates with a germline-joined L chain ((Lee et al., 2000) Flajnik & Hsu, unpublished data), giving this Ig a completely predetermined binding site. As pups age the levels of pIgM and mIgM gradually increase to become the dominant Ig forms. Like pIgM secreting cells, IgM1gj B cells are J chain+/Blimp-1-, and while they can be found in the spleen of nurse shark pups, they are only found in the epigonal organ of adult animals (Castro et al., 2013).
There are two proposed models for how the pIgM and mIgM B cells arise in the shark: model 1 suggests that these cells types are determined at the stem cell stage, and that pIgM and mIgM-secreting cells are derived from distinct lineages akin to B1 and B2 cell populations in mammals. Model 2 suggests that pIgM cells are a distinct developmental stage preceding mIgM cells, akin to plasmablasts (Castro et al., 2013; Dooley and Flajnik, 2005). The early appearance of pIgM B cells in ontogeny coupled with their lack of Blimp-1 expression, suggesting a potential for self-renewal, is similar to the phenotype of mammalian B1 cells, which do not require Blimp-1 upregulation for antibody secretion (Tumang et al., 2005) supporting model 1. However, mouse preplasmablasts also lack Blimp-1 expression (Kallies et al., 2007), and thus the absence of Blimp-1 in pIgM B cells would support model 2. However, if pIgM are truly a transitional stage preceding mIgM cells their high proportion and presence in the shark spleen regardless of immune state would suggest that this stage is more long-lived than seen in mammals (Castro et al., 2013). More work is required to prove which of these two models, or a combination of both, explains the role of pIgM and mIgM shark B cell lineages.
4.2. IgNAR
First reported in 1995, IgNAR is a novel Ig isotype that has so far only been found in elasmobranchs. Like the novel heavy chain antibodies found in Camelidae (camels, llamas, and their relatives), IgNAR is also a homodimer of IgH chains that does not associate with IgL chains (Greenberg et al., 1995; Roux et al., 1998), although the two isotypes evolved convergently (Nguyen et al., 1999; Nguyen et al., 1998). In nurse shark there is a single secretory form of IgNAR where an N-terminal V domain (VNAR) is joined to five C (C1-C5) domains (Fig. 3) (Greenberg et al., 1996; Rumfelt et al., 2004a). A second form of secretory IgNAR, where C1 is spliced directly to C4 (i.e. ΔC2-C3), has recently been found in the whitespotted bamboo shark (Chiloscyllium plagiosum) (Zhang et al., 2020). Membrane bound IgNAR is also expressed as long (five C domains) and short forms (three C domains), however here the short form is missing the C4 and C5 domains (Rumfelt et al., 2004a). Phylogenetic and structural analyses revealed that VNARs share greater structural homology with T cell receptor (TCR) and IgL V domains than conventional IgH V regions (Greenberg et al., 1995; Richards and Nelson, 2000; Stanfield et al., 2004). VNAR-like domains are also found in NAR-TCR, a doubly rearranging T cell receptor unique to cartilaginous fishes, suggesting a common ancestral origin for these domains (Criscitiello et al., 2006). As the constant domains (C2-C5) of IgNAR are homologous to those of IgW, it is hypothesized that IgNAR originated through the invasion of an IgW cluster by the V region of NAR-TCR (Criscitiello et al., 2006; Greenberg et al., 1996). Lacking light chains IgNAR does not have the combinatorial diversity generated through VH-VL pairing in conventional antibodies. However, as mentioned above, this deficiency is more than compensated by the multiple rearrangement events required to assemble their three D regions (Fig. 2). This generates CDR3s that are highly diverse in both length (7–34 aa) and sequence composition, bringing considerable heterogeneity to the primary repertoire (Diaz et al., 1998; Flajnik and Dooley, 2009; Greenberg et al., 1995). Upon exposure to antigen VNARs are further diversified by extremely high levels of SHM (Diaz et al., 1999).
IgNAR levels in nurse shark serum are approximately 10x lower than those observed for IgM, likely due to the presence of fewer IgNAR clusters and the apparent susceptibility of IgNAR proteins to proteolysis (Dooley and Flajnik, 2005; Roux et al., 1998). In nurse shark, IgNAR is found only as a monomer, but in spiny dogfish and small spotted catshark (Scyliorhinus canicula) it has been found as both a monomer and a multimer (Crouch et al., 2013; Smith et al., 2012).
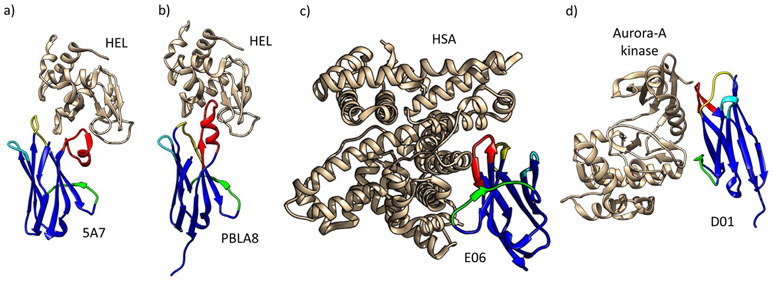
The role of IgNAR in the adaptive response is undoubtedly enhanced by the diversity of VNAR structures which enable this isotype to bind a wide array of epitopes. VNAR domains act as independent soluble units, that are attached to the C1 domain by an extended flexible tether rather than a conventional Ig hinge region (Roux et al., 1998). While VNARs adopt the typical conformation of Ig superfamily domains, a large portion of framework region 2 (FR2) and CDR2 is deleted (Greenberg et al., 1995) giving this domain its characteristically small size (approx. 12 kDa). The loss of CDR2 is compensated by two loops of high amino acid diversity, hypervariable region 2 (HV2) that forms a belt-like structure around the VNAR domain and hypervariable region 4 (HV4) that lies at the top of the domain next to CDR1 (Stanfield et al., 2004). In addition to the cysteines that typify Ig domains, VNARs also contain non-canonical cysteines that can form additional disulphide bonds and dramatically alter the structural topology of the variable loops. Based upon the number and position of non-canonical cysteine residues, VNARs are categorized into several ‘types’ (Fig. 4). Type I VNARs, so far identified only in nurse sharks (Greenberg et al., 1995; Matz and Dooley, 2019), carry an even number of cysteines (2, 4, or 6) in CDR3 and one each in FR2 and FR4. Disulphide bond formation between these residues causes the CDR3 loop to be pinned tightly against the side of the molecule (Stanfield et al., 2004). Type II VNAR domains, identified in several shark species (Liu et al., 2007; Nuttall et al., 2001; Zielonka et al., 2014), carry additional cysteines in CDR1 and CRD3 which results in an intra-molecular disulphide bond that brings the loops closer together, forming a protrusive ‘finger-like’ structure at the top of the domain (Streltsov et al., 2005). Type III VNARs, so far found only in nurse sharks, also carry extra non-canonical cysteines in CRD1 and CDR3. However, because two of the three diversity regions of type III VNARs are germline joined, they show less diversity in CDR3 compared to type I or II VNARs (Diaz et al., 2002). Like IgM1gj, type III VNARs are mainly expressed during neonatal development, before maturation of the antigen-driven response; however, some expression is maintained in the epigonal organ into adulthood (Diaz et al., 2002). Type IV domains (alternately called type IIb) only have the canonical cysteines possessed by all Ig domains, which may make their paratope topology more flexible (Kovalenko et al., 2013). The VNAR-antigen complexes crystalized thus far indicate that VNARs have a propensity to target clefts and recesses on their target antigens. Indeed, VNARs appear to exploit this binding strategy to increase their surface area of interaction with target, burying as much surface area in the interaction as a conventional H-L chain antibody, and facilitating high affinity interactions (Fig. 4). Finally, VNARs exist that do not conform to any of the classical types described above; for example, in a recent analysis of 1.2 million VNAR transcripts isolated from naive nurse sharks, a small percentage (~5%) could not be classified into any of the traditional types based upon cysteine number or location (Feng et al., 2019). Further structural and functional characterization is needed to understand the biophysical properties of these previously undescribed VNAR types.
Figure 4:
Structural analyses show that VNARs (blue) can complex their target antigens (gold) using a range of different binding modalities. The VNAR CDR1 is shown in yellow, CDR3 in red, HV2 in green, and HV4 in cyan in all panels. As can be seen in (a) the type I VNAR 5A7 contacts HEL via CDR1 and a folded CDR3 that is constrained by disulfide bonding to the FR2 and FR4 (PDB 1T6V) while (b) the type II VNAR PBLA8 contacts hen egg lysozyme (HEL) via CDR1 and an extended CDR3 loop projecting deep into HELs enzymatic cleft (PDB 2I25). In contrast, (c) the type IV/IIb VNAR E06 binds its target human serum albumin (HSA) through CDR3 and HV2 (PDB 4HGK), and (d) the synthetic VNAR D01 contacts Aurora-A kinase via CDR1, CDR3, and HV2 (PDB 5L8L). From Matz and Dooley, 2018.
The inherent attributes of VNARs, i.e., their small size, unique structural topologies, propensity to bind clefts and pockets on target antigens, and their high thermostability, make VNARs interesting prospects as future diagnostic and therapeutic agents (reviewed in (Matz and Dooley, 2019).
4.3. IgW
IgW was first identified in ocellate spot skate (Raja kenojei) and little skate (Raja erinacea) (Harding et al., 1990; Kunihiko et al., 1984) where it was named IgR and IgX, and later in nurse shark as IgNARC (Greenberg et al., 1996) then sandbar shark (Carcharhinus plumbeus) as IgW (Berstein et al., 1996). With the discovery of an additional ortholog in lungfish (Ota et al., 2003) the cartilaginous fish Igs were reconciled into one isotype, IgW, and recognized as orthologs of tetrapod IgD (Ohta and Flajnik, 2006). Thus, both IgM-like and IgW/D-like molecules must have been present in the ancestor of all jawed vertebrates (Ohta and Flajnik, 2006). Curiously, IgW appears to be lost from the elephant shark (Callorhinchus milii) genome, a holocephalan and ancient chondrichthyan (Venkatesh et al., 2014), but has been found in every elasmobranch species yet examined.
Several forms of IgW have been discovered. Based on transcript and protein S35 labeling, there exists both long (6 and 8Cω) and short (2 and 4Cω) secreted forms of IgW (Fig. 3) (Greenberg et al., 1996; Rumfelt et al., 2004a; Zhang et al., 2013). Transcript data has also confirmed the existence of two transmembrane forms of IgW (2 and 4Cω) (Rumfelt et al., 2004a; Zhang et al., 2013). The short secretory form (2Cω) has a long, cysteine-rich tail, unlike that of any other Ig, suggesting its effector function is different from other isoforms (Rumfelt et al., 2004a). It is likely that the different forms are generated through alternative splicing (Zhang et al., 2013). Additionally, transcript for a 6Cω form in which the leader is spliced directly to the C1, resulting in a molecule lacking the V domain (IgWΔV), was discovered in spiny dogfish and nurse shark (Smith et al., 2012). The existence of IgWΔV in two species of shark separated by ~200 million years of evolution, at least at the transcript level, suggests this form fulfils a unique functional role. It has been speculated that the C domains of IgW/D can act as pattern recognition molecules (Edholm et al., 2010; Seifert et al., 2009), binding directly to pathogens then triggering antimicrobial and immunostimulatory responses (Chen et al., 2009). The discovery of IgWΔV in sharks can help us put this hypothesis to the test.
A lack of monoclonal Abs (mAbs) for IgW has made it difficult to study its function at the protein level, but the plethora of transcript forms suggests IgW (along with IgNAR) has several diverse functions. Serum protein levels of IgW are low in adult nurse shark, however transcript levels are high in spleen, epigonal, pancreas, thymus, and gill, suggesting IgW plays a role in mucosal protection (Rumfelt et al., 2004a; Smith et al., 2012; Zhang et al., 2013). The short secreted form of IgW varies greatly in expression levels, both between juvenile and adult nurse sharks, and between individuals in the same cohort (Rumfelt et al., 2004a). In situ hybridization shows transcription of the IgW long secretory form overlaps with that of J chain in the spleen and epigonal organ of nurse sharks, suggesting it may form multimers (Castro et al., 2013).
We hypothesize that the diverse forms of secretory IgW generated through splicing provide a means to control the effector functions triggered (as has been observed for the full-length and short (ΔFc) forms of mallard duck IgY; (Humphrey et al., 2004)), however this requires formal testing. The presence of two IgW Tm forms also remains a mystery, although the Tm forms have fewer flexible hinge regions, possibly helping reduce their proteolytic shedding from the B cell surface (Smith et al., 2012; Zhang et al., 2013). Thus, it is possible cells expressing different versions of IgW-Tm have different activation requirements (Zhang et al., 2013). So far, no germline-joined versions of IgW have been discovered, suggesting there are not innate roles early in ontogeny for this isotype (Rumfelt et al., 2004a). Unlike IgM and IgD in mammals, IgM and IgW do not appear to be expressed simultaneously on the surface of cartilaginous fish B cells (Eason et al., 2004). While the role of IgD is not fully understood even in mammals, comparative studies have already increased our knowledge of this enigmatic isotype (Chen et al., 2009; Edholm et al., 2010). We believe that further investigation of shark IgW, which would be greatly facilitated by the development of antibodies for this isotype, will yield further insights regarding the evolution of this isotype and its contribution to humoral immunity.
4.4. IgL chains
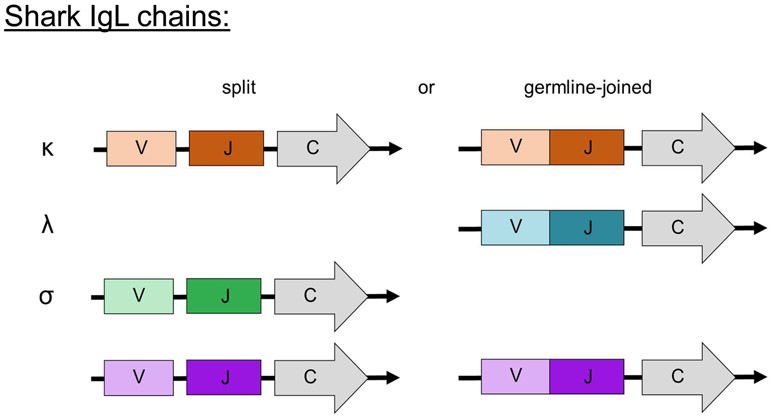
Early immunization studies recognized that shark Igs contained L chains akin to canonical mammalian Igs (Clem et al., 1967). It is now known that cartilaginous fishes have four L chain isotypes. The nomenclature of Ig L chains in chondrichthyans was confusing for many years, as it was difficult to determine the classification of L chains across vertebrate taxa due to large phylogenetic distances (Dooley and Flajnik, 2006; Smith et al., 2014). It is now established that sharks possess λ (formerly Type II/NS3), κ (formerly Type III/NS4), σ, and σ-cart/σ-2 (formerly Type I/NS5) L chains (Criscitiello and Flajnik, 2007). Like the H chains, shark Ig L chain genes are organized in a cluster configuration (Greenberg et al., 1993; Shamblott and Litman, 1989). Of these loci in nurse shark, κ and σ-cart contain both germline-joined and unjoined or split clusters, σ contains no germline-joined clusters, and all λ clusters are germline-joined (Fig. 5) (Criscitiello and Flajnik, 2007; Fleurant et al., 2004; Lee et al., 2000; Rast et al., 1994). The number of clusters of each isotype, like Ig H chains, varies between shark species, as does the proportion of split versus germline-joined clusters (Fleurant et al., 2004; Hohman et al., 1992; Rast et al., 1994).
Figure 5:
Like IgH chains, cartilaginous fish IgL chains can be found in both split and germline-joined configurations. Notably, the λ chains of all species examined to date are all germline-joined and σ are split, while the other isotypes are present in varying ratios of spit and joined in different species.
It has been proposed that germline-joined Igs arose as a consequence of RAG activity in the germline (Lee et al., 2000), however, it should be noted that other scenarios have not been entirely ruled out; for example, retrotransposition of a rearranged VDJ transcript back into the germline is a possibility, but the presence of a split leader (Anderson et al., 1995) makes this explanation far less likely. While it might be thought that germline-joined L chains would restrict the antibody repertoire, λ L chain loci are found to ypermutate at high rates, like shark IgNAR, in an antigen-driven fashion that results in tandem mutations, leading to diverse sequences despite a lack of junctional diversity (Lee et al., 2002). For isotypes with split clusters that undergo V-J rearrangement (e.g. κ and σ-cart) the majority of clones examined carry N nucleotide additions, vastly increasing light chain CDR3 repertoire diversity (Fleurant et al., 2004).
The major outstanding question from L chain research in shark is, if these four clades are present in the oldest phylogenetic group with Ig-based immunity, what was the model for their evolution and the biological significance of each isotype? As might be predicted, neither of these questions has been simple to answer. Several models are proposed for the origins of these L chain isotypes and how Ig antigen receptors evolved (Criscitiello and Flajnik, 2007), but what is apparent is that σ-cart is a “dead-end” isotype found only in cartilaginous fishes, σ is maintained only in ectothermic vertebrate lineages (Criscitiello and Flajnik, 2007; Schwager et al., 1991), and only κ and λ are maintained in the endothermic vertebrate lineages. The functional difference between κ and λ is not even clear in mammals (Hershberg and Shlomchik, 2006; Sverremark et al., 2000; Townsend et al., 2016), so the benefit of four different L chain isotypes in cartilaginous fish is equally unclear.
5. Ig responses in sharks
Immunization studies performed in the mid-1960s suggested that the antibody responses of cartilaginous fishes differed substantially from those of mammals; following a long (2-3 month) lag period serum IgM levels climbed slowly. While the proportion of mIgM to pIgM changed in some studies, binding affinities did not appear to increase appreciably over the course of most responses (Clem and Small Jr, 1967; Clem et al., 1967; Marchalonis and Edelman, 1966). Thus, for a long time, it was thought that the antibody response in cartilaginous fishes was somewhat ‘lacking’ when compared to mammals. However, pioneering work in nurse sharks determined how to generate true antigen-specific humoral responses and revealed that sharks were capable of affinity maturing their Ig repertoires (Dooley and Flajnik, 2005). While the affinity maturation observed was 10 fold rather than the ~100 fold change seen in mice (Dooley et al., 2006), it is presumed to be no less effective at protecting the host against pathogens.
Functional studies indicate distinct functional roles for pIgM and mIgM in the shark humoral response. Low affinity, high avidity pIgM can be found prior to injection of antigen and affinities of antigen-specific pIgM do not increase during an antigen-driven immune response, suggesting that affinity maturation does not occur in this Ig form (Dooley and Flajnik, 2005; Leslie and Clem, 1970; Voss and Sigel, 1972). It has been shown that pIgM serum levels can increase exclusively in response to bacterial carbohydrate, suggesting a T cell independent response (Clem and Leslie, 1971; Shankey and Clem, 1980a, b). In contrast, mIgM affinity matures in an antigen-driven manner during the humoral response. This response includes a delay in mIgM production following antigen exposure, an increase in antibody affinity over the duration of the response, and maintenance of long term, antigen-specific mIgM memory (Dooley and Flajnik, 2005; Eve et al., 2020; Rumfelt et al., 2002). That mIgM undergoes affinity maturation in an antigen-driven manner suggests there is a mechanism in sharks for B cell selection reminiscent of mammalian GCs; however, as noted above, true GCs that would mediate this process have not yet been observed in cartilaginous fishes (Zapata and Amemiya, 2000).
Akin to mIgM, antigen specific IgNAR titers also rise slowly after immunization and exhibit affinity maturation over the course of a response. The lag-period necessary to generate an antigen-driven IgNAR response, coupled with a lack of IgNAR in neonatal serum, strongly suggests that IgNAR responses require T cell help (Dooley and Flajnik, 2005; Rumfelt et al., 2002).
The Ig titers achieved through immunization in nurse sharks persist up to 1-3 years before returning to initial pre-immunization levels, suggesting long-term circulating protection against antigens. Further, antigen-specific Ig expression can be re-stimulated long (>8 years) after primary antigen exposure, clearly demonstrating B cell memory (Dooley and Flajnik, 2005; Eve et al., 2020). Collectively, this data demonstrates that sharks are capable of Ig responses of the same caliber as endotherms, albeit on longer timescales.
6. Perspective and future directions
From what has been learned about Igs in sharks and their relatives, what conclusions can we draw about the evolution of adaptive immunity? The discovery that cartilaginous fishes employ V(D)J recombination and somatic hypermutation to generate a highly diverse repertoire of binding specificities, can affinity mature their Ig pool, and maintain antigen-specific clones to provide long-term immunological memory (as detailed earlier) indicates these traits - long-associated with Ig-mediated protection in mammals - were almost certainly present in the common ancestor of all jawed vertebrates. The apparent division of function between cartilaginous fish innate-like Igs (pIgM and IgM1gj) and adaptive Igs (mIgM and IgNAR) suggests B1-like and B2-like cell lineages arose early in vertebrate evolution. Segregation of these cells followed by sequencing of their V region repertoires would allow us to test this hypothesis, not a simple task when species-specific monoclonal antibodies are lacking.
Further, the differential use of multiple Ig isotypes, combined with the presence of multiple forms of some isotypes (Dooley and Flajnik, 2005; Rumfelt et al., 2004a), indicates that cartilaginous fishes can tailor their response to achieve different immunological outcomes like other vertebrates. However, significant work is required to understand the effector functions mediated by the different shark Ig isotypes. The development of additional isotype-specific mAbs would greatly advance these studies; currently, the research tools available only allow for limited study of IgM and IgNAR. Once the in vivo roles of each isotype have been established it should also become easier to understand the functional consequences of the atypical form of CSR found in sharks. Such mAbs would also facilitate flow cytometry experiments, permitting the isolation of isotype-specific B cells from their native sites for further characterization.
The ability of cartilaginous fishes to affinity mature their Ig repertoire (Dooley and Flajnik, 2005; Dooley et al., 2006) in the apparent absence of GCs is also a point that requires further exploration. We hypothesize the presence of alternative selective environments (perhaps evolutionary precursors to mammalian-like GCs?) that facilitate the selection and expansion of antigen-specific B cell clones. The cloning of cartilaginous fish AID (Conticello et al., 2005; Quinlan et al., 2017) and orthologs of other molecules key to the germinal center reaction in mammals, will allow this hypothesis to be put to the test.
Now that long term memory has unequivocally been proven in cartilaginous fishes (Eve et al., 2020), we next need to confirm where long-lived memory B cells reside while awaiting recall and the signaling molecules required to recruit and sustain them. It is hypothesized that long-lived memory B cells reside in the epigonal organ of nurse shark (Castro et al., 2013) however the work performed so far has been unable to prove this. It may be that the frequency and diversity of the antigen-specific B cells retained for memory are few in number, as has previously been observed in studies of mammalian immunological memory (Mesin et al., 2020) making these cells difficult to find. It would also be interesting to establish if there is a division in B cell fates in sharks, for example, between long-lived plasma cells and memory B cells as observed in mammals.
Moving forward we anticipate the growing number of sequence datasets for cartilaginous fishes and application of new technologies, such as single cell sequencing and high-throughput proteomics, will greatly facilitate our work. Such approaches should help overcome the paucity of molecular tools available for cartilaginous fishes and better our understanding of adaptive immunity in this key lineage. As our knowledge increases, it will become easier to distinguish lineage-specific traits from those shared across jawed vertebrates, and undoubtedly result in a better understanding of Ig-mediated adaptive immunity in both human and non-human animals.
Highlights:
Cartilaginous fishes are the oldest extant taxonomic group to possess immunoglobulins
Study of this group has taught us much about the evolution of immunoglobulin-based adaptive immunity
This review summarizes our current understanding and highlights areas where our knowledge is still lacking
Acknowledgements:
HM is supported by NIH-NIAID predoctoral fellowship F31AI147532. DM was supported by an Elphinstone PhD Scholarship awarded by the University of Aberdeen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MK, Shamblott MJ, Litman RT, Litman GW, 1995. Generation of immunoglobulin light chain gene diversity in Raja erinacea is not associated with somatic rearrangement, an exception to a central paradigm of B cell immunity. J. Exp. Med 182, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MK, Strong SJ, Litman RT, Luer CA, Amemiya CT, Rast JP, Litman GW, 1999. A long form of the skate IgX gene exhibits a striking resemblance to the new shark IgW and IgNARC genes. Immunogenetics 49, 56–67. [DOI] [PubMed] [Google Scholar]
- Berstein R, Schluter SF, Shen S, Marchalonis JJ, 1996. A new high molecular weight immunoglobulin class from the carcharhine shark: implications for the properties of the primordial immunoglobulin. Proceedings of the National Academy of Sciences 93, 3289–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro CD, Ohta Y, Dooley H, Flajnik MF, 2013. Noncoordinate expression of J-chain and B limp-1 define nurse shark plasma cell populations during ontogeny. European journal of immunology 43, 3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm E-S, Santini PA, Rath P, Chiu A, 2009. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nature immunology 10, 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem L, Small P Jr, 1967. Phylogeny of immunoglobulin structure and function: I. Immunoglobulins of the lemon shark. The Journal of experimental medicine 125, 893–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem LW, De Boutaud F, Sigel MM, 1967. Phylogeny of Immunoglobulin Structure and Function: II. Immunoglobulins of the Nurse Shark. The Journal of Immunology 99, 1226–1235. [PubMed] [Google Scholar]
- Clem LW, Leslie GA, 1971. Production of 19S IgM antibodies with restricted heterogeneity from sharks. Proceedings of the National Academy of Sciences 68, 139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticello SG, Thomas CFJ, Petersen-Mahrt SK, Neuberger MS, 2005. Evolution of the AID/APOBEC family of polynucleotide (Deoxy)cytidine deaminases. Mol. Biol. Evol 22, 367–377. [DOI] [PubMed] [Google Scholar]
- Criscitiello MF, Flajnik MF, 2007. Four primordial immunoglobulin light chain isotypes, including λ and κ, identified in the most primitive living jawed vertebrates. European journal of immunology 37, 2683–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscitiello MF, Saltis M, Flajnik MF, 2006. An evolutionarily mobile antigen receptor variable region gene: Doubly rearranging NAR-TcR genes in sharks. Proceedings of the National Academy of Sciences of the United States of America 103, 5036–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch K, Smith LE, Williams R, Cao W, Lee M, Jensen A, Dooley H, 2013. Humoral immune response of the small-spotted catshark, Scyliorhinus canicula. Fish & shellfish immunology 34, 1158–1169. [DOI] [PubMed] [Google Scholar]
- Diaz M, Greenberg AS, Flajnik MF, 1998. Somatic hypermutation of the new antigen receptor gene (NAR) in the nurse shark does not generate the repertoire: possible role in antigen-driven reactions in the absence of germinal centers. Proceedings of the National Academy of Sciences 95, 14343–14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Stanfield RL, Greenberg AS, Flajnik MF, 2002. Structural analysis, selection, and ontogeny of the shark new antigen receptor (IgNAR): identification of a new locus preferentially expressed in early development. Immunogenetics 54, 501–512. [DOI] [PubMed] [Google Scholar]
- Diaz M, Velez J, Singh M, Cerny J, Flajnik MF, 1999. Mutational pattern of the nurse shark antigen receptor gene (NAR) is similar to that of mammalian Ig genes and to spontaneous mutations in evolution: the translesion synthesis model of somatic hypermutation. International Immunology 11, 825–833. [DOI] [PubMed] [Google Scholar]
- Dooley H, Flajnik M, 2006. Antibody repertoire development in cartilaginous fish. Developmental & Comparative Immunology 30, 43–56. [DOI] [PubMed] [Google Scholar]
- Dooley H, Flajnik MF, 2005. Shark immunity bites back: affinity maturation and memory response in the nurse shark, Ginglymostoma cirratum. Eur. J. Immunol 35, 936–945. [DOI] [PubMed] [Google Scholar]
- Dooley H, Stanfield RL, Brady RA, Flajnik MF, 2006. First molecular and biochemical analysis of in vivo affinity maturation in an ectothermic vertebrate. Proc. Natl. Acad. Sci. U. S. A 103, 1846–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eason DD, Litman GW, 2002. Haplotype exclusion: the unique case presented by multiple immunoglobulin gene loci in cartilaginous fish, Seminars in immunology. Elsevier, pp. 145–152. [DOI] [PubMed] [Google Scholar]
- Eason DD, Litman RT, Luer CA, Kerr W, Litman GW, 2004. Expression of individual immunoglobulin genes occurs in an unusual system consisting of multiple independent loci. European journal of immunology 34, 2551–2558. [DOI] [PubMed] [Google Scholar]
- Edholm E-S, Bengtén E, Stafford JL, Sahoo M, Taylor EB, Miller NW, Wilson M, 2010. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. The Journal of Immunology 185, 4082–4094. [DOI] [PubMed] [Google Scholar]
- Eve O, Matz H, Dooley H, 2020. Proof of long-term immunological memory in cartilaginous fishes. Dev Comp Immunol 108, 103674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows F, Hird F, 1981. Fatty acid binding proteins in the serum of various animals. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 68, 83–87. [Google Scholar]
- Fellows FC, Hird F, McLean RM, Walker T, 1980. A survey of the non-esterified fatty acids and binding proteins in the plasmas of selected animals. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 67, 593–597. [Google Scholar]
- Feng M, Bian H, Wu X, Fu T, Fu Y, Hong J, Fleming BD, Flajnik MF, Ho M, 2019. Construction and next-generation sequencing analysis of a large phage-displayed VNAR single-domain antibody library from six naive nurse sharks. Antibody therapeutics 2, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik M, Rumfelt L, 2000. The immune system of cartilaginous fish, Origin and evolution of the vertebrate immune system. Springer, pp. 249–270. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Dooley H, 2009. The generation and selection of single-domain, v region libraries from nurse sharks, Antibody Phage Display. Springer, pp. 71–82. [DOI] [PubMed] [Google Scholar]
- Fleurant M, Changchien L, Chen C-T, Flajnik MF, Hsu E, 2004. Shark Ig light chain junctions are as diverse as in heavy chains. The Journal of Immunology 173, 5574–5582. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Avila D, Hughes M, Hughes A, 1995. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. nature 374, 168. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Hughes AL, Guo J, Avila D, McKinney EC, Flajnik MF, 1996. A novel “chimeric” antibody class in cartilaginous fish: IgM may not be the primordial immunoglobulin. European journal of immunology 26, 1123–1129. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Steiner L, Kasahara M, Flajnik MF, 1993. Isolation of a shark immunoglobulin light chain cDNA clone encoding a protein resembling mammalian kappa light chains: implications for the evolution of light chains. Proceedings of the National Academy of Sciences 90, 10603–10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding FA, Amemiya CT, Litman RT, Cohen N, Litman GW, 1990. Two distinct immunoglobulin heavy chain isotypes in a primitive, cartilaginous fish, Raja erinacea. Nucleic acids research 18, 6369–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart S, Wrathmell A, Harris J, 1986. Ontogeny of gut-associated lymphoid tissue (GALT) in the dogfishScyliorhinus canicula L. Veterinary immunology and immunopathology 12, 107–116. [DOI] [PubMed] [Google Scholar]
- Hershberg U, Shlomchik MJ, 2006. Differences in potential for amino acid change after mutation reveals distinct strategies for κ and λ light-chain variation. Proceedings of the National Academy of Sciences 103, 15963–15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds K, Litman G, 1986. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature 320, 546. [DOI] [PubMed] [Google Scholar]
- Hohman VS, Schluter SF, Marchalonis JJ, 1992. Complete sequence of a cDNA clone specifying sandbar shark immunoglobulin light chain: gene organization and implications for the evolution of light chains. Proceedings of the National Academy of Sciences 89, 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey BD, Calvert CC, Klasing KC, 2004. The ratio of full length IgY to truncated IgY in immune complexes affects macrophage phagocytosis and the acute phase response of mallard ducks (Anas platyrhynchos). Developmental & Comparative Immunology 28, 665–672. [DOI] [PubMed] [Google Scholar]
- Inoue JG, Miya M, Lam K, Tay B-H, Danks JA, Bell J, Walker TI, Venkatesh B, 2010. Evolutionary origin and phylogeny of the modern holocephalans (Chondrichthyes: Chimaeriformes): a mitogenomic perspective. Molecular biology and evolution 27, 2576–2586. [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, Nutt SL, 2007. Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26, 555–566. [DOI] [PubMed] [Google Scholar]
- Kokubu F, Hinds K, Litman R, Shamblott M, Litman G, 1988. Complete structure and organization of immunoglobulin heavy chain constant region genes in a phylogenetically primitive vertebrate. The EMBO journal 7, 1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko OV, Olland A, Piché-Nicholas N, Godbole A, King D, Svenson K, Calabro V, Müller MR, Barelle CJ, Somers W, 2013. Atypical antigen recognition mode of a shark immunoglobulin new antigen receptor (IgNAR) variable domain characterized by humanization and structural analysis. Journal of Biological Chemistry 288, 17408–17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunihiko K, Susumu T, 1988. The second immunoglobulin class is commonly present in cartilaginous fish belonging to the order Rajiformes. Molecular immunology 25, 115–120. [DOI] [PubMed] [Google Scholar]
- Kunihiko K, Susumu T, Tadashi K, 1984. A second class of immunoglobulin other than IgM present in the serum of a cartilaginous fish, the skate, Raja kenojei: isolation and characterization. Molecular immunology 21, 397–404. [DOI] [PubMed] [Google Scholar]
- Lee SS, Fitch D, Flajnik MF, Hsu E, 2000. Rearrangement of immunoglobulin genes in shark germ cells. The Journal of experimental medicine 191, 1637–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Tranchina D, Ohta Y, Flajnik MF, Hsu E, 2002. Hypermutation in shark immunoglobulin light chain genes results in contiguous substitutions. Immunity 16, 571–582. [DOI] [PubMed] [Google Scholar]
- Leslie GA, Clem LW, 1970. Reactivity of normal shark immunoglobulins with nitrophenyl ligands. The Journal of Immunology 105, 1547–1552. [PubMed] [Google Scholar]
- Liu JL, Anderson GP, Delehanty JB, Baumann R, Hayhurst A, Goldman ER, 2007. Selection of cholera toxin specific IgNAR single-domain antibodies from a naive shark library. Molecular immunology 44, 1775–1783. [DOI] [PubMed] [Google Scholar]
- Luer CA, Walsh CJ, Bodine A, Wyffels JT, Scott TR, 1995. The elasmobranch thymus: anatomical, histological, and preliminary functional characterization. Journal of Experimental Zoology 273, 342–354. [Google Scholar]
- Luer CA, Walsh CJ, Bodine AB, 2004. The immune system of sharks, skates, and rays, Biology of sharks and their relatives. CRC Press, pp. 377–403. [Google Scholar]
- Malecek K, Brandman J, Brodsky JE, Ohta Y, Flajnik MF, Hsu E, 2005. Somatic hypermutation and junctional diversification at Ig heavy chain loci in the nurse shark. The Journal of Immunology 175, 8105–8115. [DOI] [PubMed] [Google Scholar]
- Malecek K, Lee V, Feng W, Huang JL, Flajnik MF, Ohta Y, Hsu E, 2008. Immunoglobulin heavy chain exclusion in the shark. PLoS biology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J, Edelman G, 1966. Polypeptide Chaini of Immunoglobulins from the Smooth Dogfish (Mustelus canis). Science 154, 1567–1568. [DOI] [PubMed] [Google Scholar]
- Matz H, Dooley H, 2019. Shark IgNAR-derived binding domains as potential diagnostic and therapeutic agents. Developmental & Comparative Immunology 90, 100–107. [DOI] [PubMed] [Google Scholar]
- Mesin L, Schiepers A, Ersching J, Barbulescu A, Cavazzoni CB, Angelini A, Okada T, Kurosaki T, Victora GD, 2020. Restricted Clonality and Limited Germinal Center Reentry Characterize Memory B Cell Reactivation by Boosting. Cell 180, 92–106 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AL, Anderson MK, Litman RT, Walsh CJ, Luer CA, Rothenberg EV, Litman GW, 2001. Complex expression patterns of lymphocyte-specific genes during the development of cartilaginous fish implicate unique lymphoid tissues in generating an immune repertoire. International Immunology 13, 567–580. [DOI] [PubMed] [Google Scholar]
- Mußmann R, Courtet M, Schwager J, Du Pasquier L, 1997. Microsites for immunoglobulin switch recombination breakpoints from Xenopus to mammals. European journal of immunology 27, 2610–2619. [DOI] [PubMed] [Google Scholar]
- Nguyen VK, Hamers R, Wyns L, Muyldermans S, 1999. Loss of splice consensus signal is responsible for the removal of the entire C(H)1 domain of the functional camel IGG2A heavy-chain antibodies. Mol. Immunol 36, 515–524. [DOI] [PubMed] [Google Scholar]
- Nguyen VK, Muyldermans S, Hamers R, 1998. The specific variable domain of camel heavy-chain antibodies is encoded in the germline. J. Mol. Biol 275, 413–418. [DOI] [PubMed] [Google Scholar]
- Nuttall SD, Krishnan UV, Hattarki M, De Gori R, Irving RA, Hudson PJ, 2001. Isolation of the new antigen receptor from wobbegong sharks, and use as a scaffold for the display of protein loop libraries. Molecular immunology 38, 313–326. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Flajnik M, 2006. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. Proceedings of the National Academy of Sciences 103, 10723–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T, Rast JP, Litman GW, Amemiya CT, 2003. Lineage-restricted retention of a primitive immunoglobulin heavy chain isotype within the Dipnoi reveals an evolutionary paradox. Proceedings of the National Academy of Sciences 100, 2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, King JJ, Amemiya CT, Hsu E, Larijani M, 2017. Biochemical Regulatory Features of Activation-Induced Cytidine Deaminase Remain Conserved from Lampreys to Humans. Mol Cell Biol 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rast JP, Amemiya CT, Litman RT, Strong SJ, Litman GW, 1998. Distinct patterns of IgH structure and organization in a divergent lineage of chrondrichthyan fishes. Immunogenetics 47, 234–245. [DOI] [PubMed] [Google Scholar]
- Rast JP, Anderson MK, Litman RT, Margittai M, Litman GW, Ota T, Shamblott MJ, 1994. Immunoglobulin light chain class multiplicity and alternative organizational forms in early vertebrate phylogeny. Immunogenetics 40, 83–99. [DOI] [PubMed] [Google Scholar]
- Richards M, Nelson J, 2000. The evolution of vertebrate antigen receptors: a phylogenetic approach. Molecular biology and evolution 17, 146–155. [DOI] [PubMed] [Google Scholar]
- Roux KH, Greenberg AS, Greene L, Strelets L, Avila D, McKinney EC, Flajnik MF, 1998. Structural analysis of the nurse shark (new) antigen receptor (NAR): molecular convergence of NAR and unusual mammalian immunoglobulins. Proceedings of the National Academy of Sciences 95, 11804–11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumfelt L, McKinney E, Taylor E, Flajnik M, 2002. The Development of Primary and Secondary Lymphoid Tissues in the Nurse Shark Ginglymostoma cirratum: B-Cell Zones Precede Dendritic Cell Immigration and T-Cell Zone Formation During Ontogeny of the Spleen. Scandinavian journal of immunology 56, 130–148. [DOI] [PubMed] [Google Scholar]
- Rumfelt LL, Avila D, Diaz M, Bartl S, McKinney EC, Flajnik MF, 2001. A shark antibody heavy chain encoded by a nonsomatically rearranged VDJ is preferentially expressed in early development and is convergent with mammalian IgG. Proceedings of the National Academy of Sciences 98, 1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumfelt LL, Diaz M, Lohr RL, Mochon E, Flajnik MF, 2004a. Unprecedented multiplicity of Ig transmembrane and secretory mRNA forms in the cartilaginous fish. The Journal of Immunology 173, 1129–1139. [DOI] [PubMed] [Google Scholar]
- Rumfelt LL, Lohr RL, Dooley H, Flajnik MF, 2004b. Diversity and repertoire of IgW and IgM VH families in the newborn nurse shark. BMC immunology 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha NR, Ota T, Litman GW, Hansen J, Parra Z, Hsu E, Buonocore F, Canapa A, Cheng JF, Amemiya CT, 2014. Genome complexity in the coelacanth is reflected in its adaptive immune system. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 322, 438–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J, Bürckert N, Schwager M, Wilson M, 1991. Evolution of immunoglobulin light chain genes: analysis of Xenopus IgL isotypes and their contribution to antibody diversity. The EMBO journal 10, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert M, Steimle-Grauer SA, Goossens T, Hansmann ML, Brauninger A, Kuppers R, 2009. A model for the development of human IgD-only B cells: Genotypic analyses suggest their generation in superantigen driven immune responses. Mol. Immunol 46, 630–639. [DOI] [PubMed] [Google Scholar]
- Shamblott M, Litman G, 1989. Genomic organization and sequences of immunoglobulin light chain genes in a primitive vertebrate suggest coevolution of immunoglobulin gene organization. The EMBO journal 8, 3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankey TV, Clem LW, 1980a. Phylogeny of immunoglobulin structure and function—VIII: Intermolecular heterogeneity of shark 19S IgM antibodies to pneumococcal polysaccharide. Molecular immunology 17, 365–375. [DOI] [PubMed] [Google Scholar]
- Shankey TV, Clem LW, 1980b. Phylogeny of immunoglobulin structure and function. IX. Intramolecular heterogeneity of shark 19S IgM antibodies to the dinitrophenyl hapten. The Journal of Immunology 125, 2690–2698. [PubMed] [Google Scholar]
- Small PA, Klapper DG, Clem LW, 1970. Half-lives, body distribution and lack of interconversion of serum 19S and 7S IgM of sharks. The Journal of Immunology 105, 29–37. [PubMed] [Google Scholar]
- Smith LE, Crouch K, Cao W, Muller MR, Wu L, Steven J, Lee M, Liang M, Flajnik MF, Shih HH, 2012. Characterization of the immunoglobulin repertoire of the spiny dogfish (Squalus acanthias). Developmental & Comparative Immunology 36, 665–679. [DOI] [PubMed] [Google Scholar]
- Smith SL, Sim RB, Flajnik MF, 2014. Immunobiology of the Shark. CRC Press. [Google Scholar]
- Stanfield RL, Dooley H, Flajnik MF, Wilson IA, 2004. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science 305, 1770–1773. [DOI] [PubMed] [Google Scholar]
- Streltsov VA, Carmichael JA, Nuttall SD, 2005. Structure of a shark IgNAR antibody variable domain and modeling of an early-developmental isotype. Protein science 14, 2901–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverremark E, Rietz C, Fernández C, 2000. κ-deficient mice are non-responders to dextran B512: is this unresponsiveness due to specialization of the κ and λ Ig repertoires? International immunology 12, 431–438. [DOI] [PubMed] [Google Scholar]
- Tomonaga S, Kobayashi K, Hagiwara K, Yamaguchi K, Awaya K, 1986. Gut-associated lymphoid tissue in elasmobranchs. Zoological science 3, 453–458. [Google Scholar]
- Townsend CL, Laffy JM, Wu Y-CB, Silva O’Hare J, Martin V, Kipling D, Fraternali F, Dunn-Walters DK, 2016. Significant differences in physicochemical properties of human immunoglobulin kappa and lambda CDR3 regions. Frontiers in immunology 7, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumang JR, Frances R, Yeo SG, Rothstein TL, 2005. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol 174, 3173–3177. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, 2014. Elephant shark genome provides unique insights into gnathostome evolution. Nature 505, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss EW, Sigel MM, 1972. Valence and temporal change in affinity of purified 7S and 18S nurse shark anti-2, 4 dinitrophenyl antibodies. The Journal of Immunology 109, 665–673. [PubMed] [Google Scholar]
- Zapata A, Amemiya C, 2000. Phylogeny of lower vertebrates and their immunological structures, Origin and evolution of the vertebrate immune system. Springer, pp. 67–107. [DOI] [PubMed] [Google Scholar]
- Zapata A, Torroba M, Sacedon R, Varas A, Vicente A, 1996. Structure of the lymphoid organs of elasmobranchs. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 275, 125–143. [Google Scholar]
- Zhang C, Du Pasquier L, Hsu E, 2013. Shark IgW C region diversification through RNA processing and isotype switching. The Journal of Immunology 191, 3410–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Qin L, Cai X, Juma SN, Xu R, Wei L, Wu Y, Cui X, Chen G, Liu L, 2020. Sequence structure character of IgNAR Sec in whitespotted bamboo shark (Chiloscyllium plagiosum). Fish & Shellfish Immunology. [DOI] [PubMed] [Google Scholar]
- Zhu C, Feng W, Weedon J, Hua P, Stefanov D, Ohta Y, Flajnik MF, Hsu E, 2011. The multiple shark Ig H chain genes rearrange and hypermutate autonomously. The Journal of Immunology 187, 2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Lee V, Finn A, Senger K, Zarrin AA, Du Pasquier L, Hsu E, 2012. Origin of immunoglobulin isotype switching. Current Biology 22, 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka S, Weber N, Becker S, Doerner A, Christmann A, Christmann C, Uth C, Fritz J, Schafer E, Steinmann B, 2014. Shark Attack: High affinity binding proteins derived from shark vNAR domains by stepwise in vitro affinity maturation. Journal of biotechnology 191, 236–245. [DOI] [PubMed] [Google Scholar]