Abstract
Background:
Zika virus (ZIKV) infection during pregnancy can cause infant brain and eye abnormalities and has been associated with adverse neurodevelopmental outcomes in exposed infants. Evidence is limited on ZIKV’s effects on children infected postnatally within the first year of life.
Objective:
To determine whether any adverse neurodevelopmental outcomes occurred in early childhood for children infected postnatally with ZIKV during infancy, given the neurotoxicity of ZIKV infection and the rapid brain development that occurs in infancy and early childhood.
Methods:
The Colombia Instituto Nacional de Salud (INS) conducted health and developmental screenings between September and November 2017 to evaluate 60 children at ages 20–30 months who had laboratory-confirmed symptomatic postnatal ZIKV infection at ages 1–12 months. We examined the frequency of adverse neurologic, hearing, eye, and developmental outcomes as well as the relationship between age at Zika symptom onset and developmental outcomes.
Results:
Nine of the 60 (15.0%) children had adverse outcomes on the neurologic, hearing, or eye examination. Six of the 47 (12.8%) children without these adverse findings, and who received a valid developmental screening, had an alert score in the hearing-language domain which signals the need for additional developmental evaluation.
Conclusion:
Neurologic, hearing, eye, and developmental findings suggest reassuring results. Since the full spectrum of neurodevelopmental outcomes in children postnatally infected with ZIKV remains unknown, routine paediatric care is advised to monitor the development of these children to ensure early identification of any adverse neurodevelopmental outcomes.
Keywords: child development, eye, hearing, neurologic examination, Zika virus
1 |. BACKGROUND
Zika virus (ZIKV) infection during pregnancy can cause infant brain and eye abnormalities and has been associated with adverse neurodevelopmental outcomes in exposed infants.1 Evidence is limited on ZIKV’s effects on children infected postnatally within the first year of life. Clinical manifestations at the time of ZIKV infection among symptomatic children are generally mild, largely with symptoms of mild febrile illness with rash.2–4 While microcephaly among infants congenitally infected with ZIKV is well-documented, there is limited information on the prevalence of microcephaly among children infected postnatally.1,5,6 A study in infant rhesus macaques found no association between postnatal ZIKV infection and total brain volume.7 From a previous report of children aged 1 month to 18 years with postnatal ZIKV disease in Colombia, a small proportion had adverse outcomes at the time of ZIKV reporting, including 631 patients (3.4%) who were hospitalised and 96 (0.5%) who had a report of an accompanying neurologic diagnosis.2 Given the neurotoxicity of ZIKV infection and the rapid brain development that occurs in infancy and early childhood, it is plausible that postnatal ZIKV infection in infants might have an effect on neurodevelopment in early childhood.3
In August 2015, the Colombia Instituto Nacional de Salud (INS) mandated reporting of individuals with ZIKV symptoms (eg fever, rash, nonpurulent conjunctivitis, headache, pruritus, arthralgia, myalgia, or malaise), with no known alternative aetiology, with priority reporting and laboratory testing of high-risk groups, including infants less than one year of age.8 Serum specimens collected within one week of symptom onset were tested for the presence of ZIKV ribonucleic acid (RNA) using real-time reverse transcription-poly-merase chain reaction (rRT-PCR).
The main objective of the present study was to examine neurodevelopmental outcomes in early childhood after postnatal infection of ZIKV during infancy. INS conducted health screenings to evaluate 60 children at ages 20–30 months who previously had laboratory-confirmed symptomatic postnatal infection at ages 1–12 months. We analysed these data to determine if there were adverse neurodevelopmental outcomes among the sample group.
2 |. METHODS
2.1 |. Cohort selection
Between September 2015 and May 2016, there were 389 infants between the ages of 1 and 12 months reported to INS with symptoms of ZIKV infection and laboratory confirmation. All children who met the following eligibility criteria were invited to participate in the health brigades: (a) resided in one of three departments (Norte Santander, Huila, and Valle del Cauca) with the highest number of infant ZIKV disease (ZVD) cases reported to surveillance and (b) postnatal infection defined as positive for ZIKV RNA using a serum sample by rRT-PCR at 1–12 months of age among those reporting symptoms of ZVD. Parents of 76 children from three departments with the highest number of postnatal cases were invited to participate for evaluation at health brigades. Of those children, the parents of 60 (79%) accepted the invitation.
2.2 |. Exposure
Only children infected between the ages of 1–12 months were included to reduce the possibility of including congenital ZIKV infections. While the duration of detectable ZIKV RNA in infants congenitally infected with ZIKV is unknown and some variation has been reported, current recommendations are to test symptomatic patients within one week of symptom onset as a positive nucleic acid amplification test (NAAT) result typically provides evidence of acute infection.9
2.3 |. Outcomes
Health brigades were organised by INS and CDC in specific sites across Colombia in order to conduct comprehensive and free health evaluations from specialists for the cohort; participation was voluntary. The children participated in these health brigades during September-November 2017, 17–21 months after the reported ZIKV infection.
At the health brigades, children were examined by a multidisciplinary medical team and had a neurologic examination, anthropometric assessment consisting of height, weight, body mass index (BMI) and head circumference measurements, hearing evaluation (auditory brainstem response [ABR]), and retinal examination via indirect ophthalmoscopy conducted in addition to an examination of external eye structures and clinical observation of eye movement and alignment. Diagnostic ABR was conducted in two of the health brigade sites; screening by automated ABR was conducted in the third. Age-appropriate developmental screening was conducted using the Escala Abreviada de Desarrollo (EAD-1), a standardised, validated developmental screening tool normed on a diverse sample of Colombian children.10 Parents were encouraged to bring their child’s medical records to the health brigade for abstraction of clinical history.
Neurodevelopmental outcomes included results from the eye, hearing, and neurologic examinations in addition to the EAD-1. Three clinical subject matter experts reviewed the data from the children to evaluate the presence of adverse hearing, eye, or neurologic examination findings.
2.4 |. Statistical analysis
Growth percentiles for age and sex were calculated using the WHO Child Growth Standards.11 Potential hearing abnormalities were defined as a failed diagnostic or automated ABR. EAD-1 scores in the sample were converted to percentiles, which were derived from standardised scores based on the normed population.10 The scores from four EAD-1 screeners were excluded due to incorrect administration. An “alert” outcome in any domain was defined as possible developmental delay with additional evaluation needed. The relationship between age at symptom onset of ZVD and EAD-1 score percentiles was examined using Pearson correlation by developmental domain. SAS software (version 9.4; SAS Institute) was used to conduct analyses.
2.5 |. Ethics approval
Written informed consent to participate in the health brigades was obtained from the parent or guardian. The health brigades were deemed public health practice by CDC.
3 |. RESULTS
Infants in this investigation experienced onset of ZIKV symptoms at a median age of 5 months (IQR: 3–6). Three (5.0%) infants were hospitalised at the time of their ZIKV infection. Reasons for hospital admission were not reported, yet none of the three infants were known to have meningitis or encephalitis at time of infection. The majority of the 60 children evaluated were male (55.0%), from an urban residence (95.0%), had health insurance (93.3%), and were mestizo ethnicity (98.3%). Nine of the 60 (15.0%) children had adverse outcomes on the neurologic, hearing, or eye examination: hypotonia (n = 2), eye abnormalities (n = 4), and hearing abnormalities (n = 4), with one of the children presenting both hypotonia and sensorineural hearing loss (Table 1). At the health brigades, none of the children had a head circumference below the 5th percentile (Table 1). Among the 51 children without abnormalities, one (2.0%) had weight below the 5th percentile, and four (7.8%) had a length/height measurement below the 5th percentile. One child was reported to have epilepsy; however, there was no information on type and timing of diagnosis. Of note, two children with abnormalities identified at the brigade also had other conditions which may have contributed to the abnormalities. One child was reported to have Down syndrome, had a weight measurement below the 5th percentile, and presented with hypotonia at the health brigade. Another child was identified as having a structural eye abnormality and retinal scarring and was also reported to have had congenital toxoplasmosis (Table 1). Other comorbidities reported include a history of chronic respiratory infections (n = 1), history of cardiac arrhythmia (n = 1), and thorax abnormality with no further description (n = 1).
TABLE 1.
Neurologic, hearing, and eye examinations conducted at 20–30 mo of age among children postnatally infected with ZIKVa (N = 60) in Colombia, September-November 2017
| Evaluated at health brigades (N = 60) | |
|---|---|
| Preterm birthb | 8 (13.3%) |
| Age at time of health brigade (months) | |
| 20–22 | 12 (20.0%) |
| 23–25 | 26 (43.3%) |
| 26–30 | 22 (36.7%) |
| Median time since infection (months) | 20 (IQR: 19–20, Range: 17–21) |
| Growth measurements at health brigadec | |
| Weight for age and sex <5th percentile | 2 (3.3%) |
| Length/Height for age and sex <5th percentile | 4 (6.7%) |
| Head circumference for age and sex | |
| <5th percentile | 0 (0%) |
| <3rd percentile | 0 (0%) |
| Neurologic examination at health brigade | |
| Normal neurologic examination | 58 (96.7%) |
| Neurologic abnormality | 2 (3.3%) |
| Hypotoniad | 2 |
| Hearing screening or evaluation (N = 49) | |
| Passed or normal | 45 (91.8%) |
| Failed or hearing loss | 4 (8.2%) |
| Conductive hearing loss | 0 |
| Sensorineural hearing loss | 1 |
| Unknown typee | 3 |
| Ophthalmologic evaluation | |
| Normal | 56 (93.3%) |
| Structural abnormalityf | 2 (3.3%) |
| Possible visual impairmentg | 2 (3.3%h) |
Infants were laboratory-confirmed with ZIKV infection by rRT-PCR within 1 wk of symptom onset at 1–12 mo of age.
Preterm birth refers to an infant born at less than 37 wk of age.
Percentiles were based on sex- and age-adjusted using World Health Organization standards.
One of the children with hypotonia had a diagnosis of Down syndrome, and the other child had sensorineural hearing loss.
Two of these three children did not receive diagnostic ABR.
One child with a structural abnormality had retinal scarring and reported congenital toxoplasmosis, and one child had an optic nerve pit.
Two children with possible visual impairment had diagnoses of strabismus, independent from those children with structural abnormalities.
Percentages may not sum to 100 due to rounding.
Of the 47 children without abnormalities and with a valid score on the EAD-1, six (12.8%) received an alert score in the hearing-language domain of the screener. Within the other developmental domains covered by the EAD-1, the percentage of children falling in the alert range was less than 7% (Table 2). Age at symptom onset of ZVD was positively correlated with percentiles in the personal-social domain of the EAD-1 (r = 0.3), but there was no correlation in the gross motor, fine motor, or the hearing-language domain (Figure 1).
TABLE 2.
Escala Abreviada de Desarrollo, version 1a (EAD-1) screener results at 20–30 mo of age among children postnatally infected with ZIKVb (N = 60), Colombia, September-November 2017
| Developmental domains | Without adverse neurologic, eye, or hearing outcomes (N = 51) | With adverse neurologic, eye, or hearing outcomes (N = 9c) |
|---|---|---|
| Gross motor | (N = 51) | (N = 9) |
| Alertd | 0 (0%) | 1 (11.1%) |
| Fine motor | (N = 51) | (N = 9) |
| Alertd | 2 (3.9%) | 1 (11.1%) |
| Hearing and language | (N = 47e) | (N = 9) |
| Alertd | 6 (12.8%) | 2 (22.2%) |
| Personal-social | (N = 50e) | (N = 9) |
| Alertd | 3 (6.0%) | 1 (11.1%) |
The EAD-1 is a standardised, validated developmental screening tool in Colombia.
Participants were laboratory-confirmed with ZIKV infection by rRT-PCR within one week of symptom onset at 1–12 mo of age.
One child with hypotonia also had a diagnosis of Down syndrome; the other child with hypotonia also had a diagnosis of sensorineural hearing loss; three children had unknown hearing loss; and four children had an abnormal eye finding. The child with abnormal retinal scarring also had reported congenital toxoplasmosis.
An “alert” was based on cut points defined in the EAD-1 manual.
Children whose domain score could not be calculated due to incorrect EAD-1 application were excluded.
FIGURE 1.
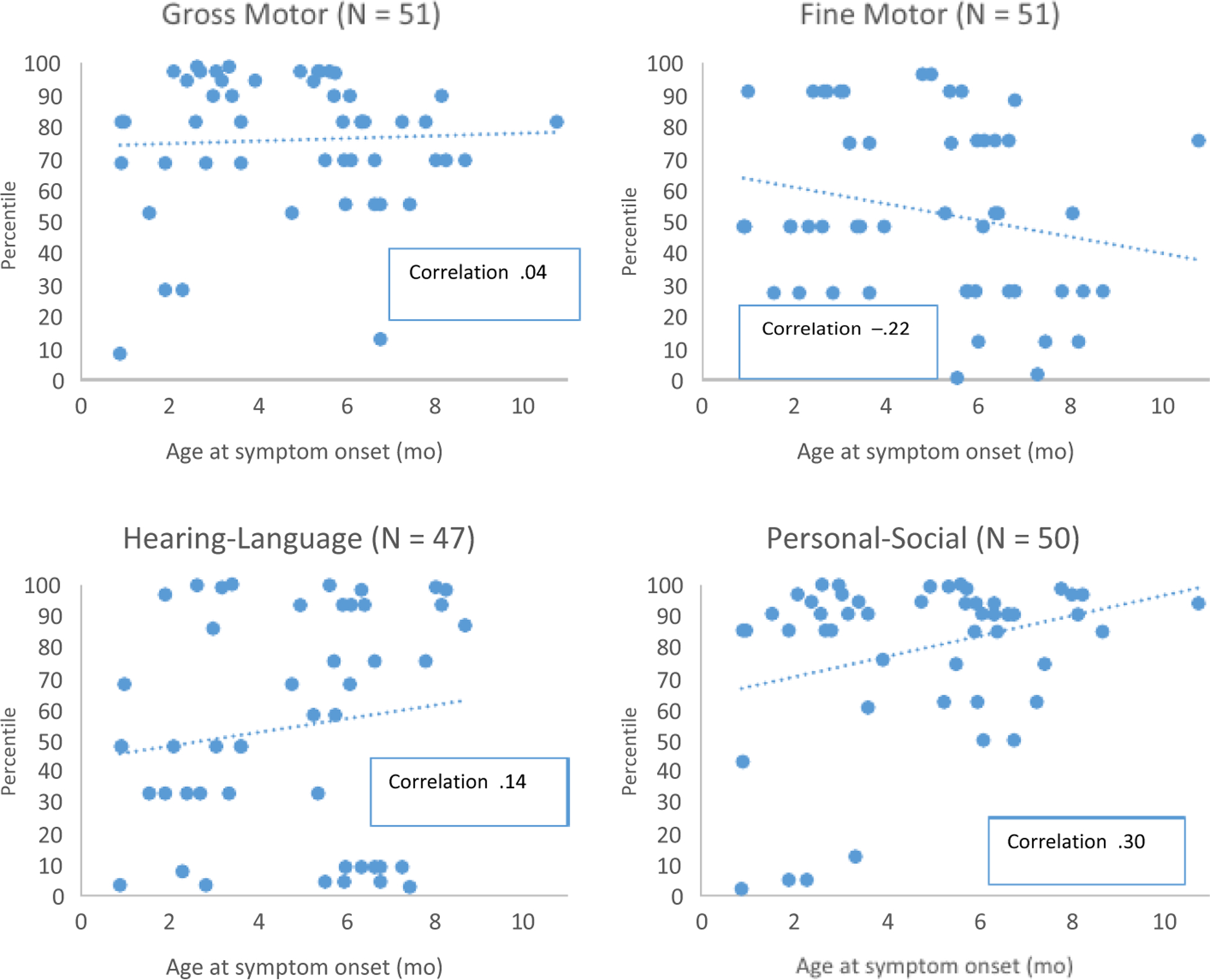
Escala Abreviada de Desarrollo, version 1a (EAD-1) percentilesb at 20 to 30 mo of age by domain and age at Zika symptom onset of children postnatally infected with ZIKVc and without adverse neurologic, eye, or hearing outcomes (N = 51d), Colombia, September-November 2017e. aThe EAD-1 is a standardised, validated developmental screening tool in Colombia. bEAD-1 scores from the sample were converted into percentiles based on the normed Colombian population. c Participants were laboratory-confirmed with ZIKV infection by serum rRT-PCR within one week of symptom onset at 1–12 mo of age. dFour children in the hearing-language domain and one child in the personal-social domain, whose respective domain scores could not be calculated due to incorrect EAD-1 application, were excluded. eThe four lowest percentiles in the personal-social domain are driving the correlation; if these four points were removed, the correlation is no longer statistically significant
4 |. COMMENT
4.1 |. Principal findings
Overall, among the 60 children with symptomatic, postnatal laboratory-confirmed ZIKV infection, neurodevelopmental outcomes 17–21 months post-infection suggest reassuring results. Nine children had adverse outcomes on the neurologic, hearing, or eye examination, but the lack of a comparison group and baseline estimates of the prevalence of these abnormalities in this population limits interpretation.
4.2 |. Strengths of the study
This study presents novel findings on children postnatally infected with ZIKV in infancy and neurodevelopmental outcomes in early childhood. In addition, data from the health brigades were collected both through clinicians and medical record abstraction, as well as parent report; this allowed for more complete and accurate data. Further, our study restricted participants to only those children confirmed ZIKV positive by rRT-PCR over the age of one month, thereby reducing the possibility of confounding from congenital ZIKV infections.
4.3 |. Limitations of the data
Participants were selected based on proximity to the health brigade site; therefore, a large proportion were from an urban setting. In addition, self-selection bias may be introduced by those parents choosing to participate in the health brigade. Results may not be generalisable to all Colombian infants with postnatal ZIKV infection; however, the characteristics of participants were similar for timing of infection, sex, insurance type, and hospitalisation at time of infection to the 389 infants with reported postnatal ZIKV infection in Colombia during our study period. One of the health brigade sites did not have the technology available for diagnostic ABR; therefore, two of the children with reported hearing loss of an unknown type only received screening by automated ABR. In addition, it could not be determined whether the child with sensorineural hearing loss had a history of hearing loss prior to ZIKV infection.
4.4 |. Interpretation
Despite not observing serious adverse health outcomes among this small sample, the potential neurodevelopmental effects of infection with a neurotropic virus during the first year of life are of particular concern given that the first two years of life involve dramatic brain development.12 A study in infant rhesus macaques found associations between postnatal ZIKV infection and neurotropism and alterations of the central nervous system.7 There are also examples of other viral infections in early childhood, such as varicella and measles, having associations with adverse neurodevelopmental outcomes.13,14 In this investigation, there were insufficient data to determine whether postnatal ZIKV infection was associated with EAD-1 alert scores. Children who had ZIKV infection at a younger age had lower scores in the personal-social domain of the EAD-1. However, the four lowest percentiles are driving this correlation; if these four points were removed, the correlation is no longer statistically significant. Further investigation with larger samples is required.
5 |. CONCLUSIONS
This investigation presents novel findings on neurodevelopmental outcomes in children with symptomatic, postnatal laboratory-confirmed ZIKV infection. Families and providers should follow recommended guidance on mosquito bite prevention,15 and routine paediatric care is advised to monitor the development of children postnatally infected with ZIKV for early detection of adverse neurodevelopmental outcomes.16
Synopsis.
Study question
After postnatal infection of Zika virus during infancy, are there adverse neurodevelopmental outcomes in early childhood?
What is already known
Congenital Zika virus (ZIKV) infection can cause brain and eye abnormalities and has been associated with adverse neurodevelopmental outcomes. Infancy and early childhood are times of dramatic brain development. Evidence is limited on ZIKV’s effects on children infected postnatally within the first year of life.
What this study adds
This investigation presents novel findings on neurodevelopmental outcomes in children with symptomatic, postnatal laboratory-confirmed ZIKV infection. Among a sample of 60 Colombian children aged 20–30 months who had symptomatic, postnatal laboratory-confirmed ZIKV infection at ages 1–12 months, neurodevelopmental outcomes suggest reassuring results.
Funding information
This publication was made possible through support provided by the US Centers for Disease Control and Prevention (CDC) and the Office of Infectious Disease, Bureau for Global Health, US Agency for International Development (USAID), under the terms of an Interagency Agreement with CDC. Support for national surveillance efforts was supported through a cooperative agreement to Vysnova Partners, Inc (NU2GGH001732). The opinions expressed herein are those of the authors and do not necessarily represent the official position of the funding agencies.
REFERENCES
- 1.Rice ME, Galang RR, Roth NM, et al. Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection - U.S. Territories and Freely Associated States, 2018. Morb Mortal Wkly Rep (MMWR). 2018;67(31):858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolosa N, Tinker SC, Pacheco O, et al. Zika virus disease in children in Colombia, August 2015 to May 2016. Paediatr Perinat Epidemiol. 2017;31(6):537–545. [DOI] [PubMed] [Google Scholar]
- 3.Asturias EJ. Uncovering the spectrum of postnatal Zika infection in children. JAMA Pediatrics. 2018;172(7):624–625. [DOI] [PubMed] [Google Scholar]
- 4.Lindsey NP, Porse CC, Potts E, et al. Uncovering the spectrum of postnatal Zika infection in children. JAMA Pediatrics. 2018;172(7):624–625. [DOI] [PubMed] [Google Scholar]
- 5.Franca GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388(10047):891–897 [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen SA. An update on Zika virus as a cause of birth defects. Birth Defects Res. 2018;110(9):722. [Google Scholar]
- 7.Mavigner M, Raper J, Kovacs-Balint Z, et al. Postnatal Zika virus infection is associated with persistent abnormalities in brain structure, function, and behavior in infant macaques. Sci Transl Med. 2018;10(435):eaao6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacheco O, Beltran M, Nelson CA, et al. Zika virus disease in Colombia – preliminary report. N Engl J Med. 2016. 10.1056/NEJMoa1604037 [DOI] [PubMed] [Google Scholar]
- 9.Sharp TM, Fischer M, Munoz-Jordan JL, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. Morbidity and Mortality Recommendations and Reports. 2019;68(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombia Ministerio de Salud Publica. Diseño y normalizacion de la escala abreviada de desarrollo EAD-1. Bogota: Unicef; 1993. [Google Scholar]
- 11.de Onis M 4.1 the WHO child growth standards. World Rev Nutr Diet. 2015;113:278–294. [DOI] [PubMed] [Google Scholar]
- 12.Knickmeyer RC, Gouttard S, Kang C, et al. A Structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Science M, MacGregor D, Richardson SE, Mahant S, Tran D, Bitnun A. Central nervous system complications of Varicella-Zoster virus. J Pediatrics. 2014;165(4):779–785. [DOI] [PubMed] [Google Scholar]
- 14.Mekki M, Eley B, Hardie D, Wilmshurst JM. Subacute sclerosing panencephalitis: clinical phenotype, epidemiology, and preventive interventions. Dev Med Child Neurol. 2019;61(10):1139–1144. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Prevent Mosquito Bites 2019. Available from: https://www.cdc.gov/features/stopmosquitoes/index.html.
- 16.Centers for Disease Control and Prevention. Zika in infants & children 2018. Available from: https://www.cdc.gov/pregnancy/zika/testing-follow-up/zika-in-infants-children.html.


