Abstract
Objective
Thalamus models of psychosis implicate association nuclei in the pathogenesis of psychosis and mechanisms of cognitive impairment. Studies to date have provided conflicting findings for structural deficits specific to these nuclei. The authors characterized thalamic structural abnormalities in psychosis and a neurodevelopmental cohort, and determined if nuclear volumes were associated with cognitive function.
Methods
Thalamic nuclei volumes were tested in a cross-sectional sample of 472 adults (293 with psychosis) and the Philadelphia Neurodevelopmental Cohort (PNC), consisting of 1393 youth (398 psychosis spectrum youth, 609 youth with other psychopathologies), using a recently developed, validated method for segmenting thalamic nuclei and complementary voxel-based morphometry. Cognitive function was measured with the Screen for Cognitive Impairment in Psychiatry (SCIP) in the psychosis cohort and the Penn Computerized Neurocognitive Battery (CNB) in the PNC.
Results
The psychosis group had smaller pulvinar, mediodorsal, and to a lesser extent, ventrolateral nuclei volumes compared to the healthy control group. Psychosis spectrum youth also had smaller pulvinar volumes, compared to both typically developing youth and youth with other psychopathologies. Pulvinar volumes were positively correlated with general cognitive function.
Conclusions
Our findings demonstrate that smaller thalamic association nuclei represent a neurodevelopmental abnormality associated with psychosis, risk for psychosis in youth and cognitive impairment. Identifying specific thalamic nuclei abnormalities in psychosis has implications for early detection of psychosis risk and treatment of cognitive impairment in psychosis.
INTRODUCTION
Multiple lines of evidence implicate the thalamus in psychotic disorders, including smaller thalamus volume, decreased activation during task performance, abnormal functional and anatomical connectivity with the cortex, reduced expression of biochemical markers of neuronal integrity, abnormal sleep spindles and lower cell numbers in some thalamic nuclei (1–13). These findings contributed to the development of several models of psychosis, many of which propose a neurodevelopmental basis for thalamus pathology and emphasize thalamic abnormalities in the mechanisms of cognitive impairment (14–18).
Several models further propose selective dysfunction of specific thalamic nuclei, particularly thalamic association nuclei, including the mediodorsal and pulvinar nuclei (14–18). There is abundant evidence that connectivity of some association nuclei (e.g. mediodorsal nucleus) are abnormal (3, 4); however, evidence of selective anatomical abnormalities is sparse and inconsistent. For instance, while post-mortem studies consistently find smaller volume and fewer cell numbers in the pulvinar (19–21), mediodorsal nucleus findings are mixed (22, 23) and small sample sizes raise broader concerns about replicability and generalization from post-mortem studies (10, 23). Similarly, the handful of neuroimaging studies examining specific thalamic nuclei report both smaller and normal mediodorsal and pulvinar volumes (24–27). Inconsistent neuroimaging results are likely due to a combination of factors including modest sample sizes and use of idiosyncratic methods for quantifying thalamic nuclei that have not been widely adopted by the neuroimaging community.
The recent development of a novel method for segmenting the thalamus has created a new opportunity to investigate the thalamus in psychosis. Specifically, adapting an approach developed to segment hippocampal subfields (28), Iglesias and colleagues (29) built a probabilistic atlas from ex vivo MRI and histological data which can be applied to standard T1-weighted in vivo MRI to segment thalamic nuclei using Bayesian inference. Critically, their method is able to recover the 3D structure of histological data from ex vivo MRI; yields volumes that are in good agreement with other histological atlases of the thalamus; has excellent test-retest reliability for most thalamic nuclei (inter-class correlation coefficients ranging from 0.86–0.99); is robust against changes in MRI contrast; and demonstrates good correspondence between in vivo MRI and established neuropathology in neurological disorders (i.e. Alzheimer’s disease). Moreover, their method is included in FreeSurfer, one of the most widely used software packages for quantifying brain anatomy, ensuring dissemination to the broader neuroimaging community (29).
We applied the method described above to a large cohort of individuals with a psychotic disorder (n>450) and the Philadelphia Neurodevelopmental Cohort (PNC: n=1601), which includes youth with psychosis spectrum symptoms, in order to clarify the anatomical specificity, neurodevelopmental basis and cognitive correlates of thalamic pathology in psychosis. Our investigation had three aims. 1. Characterize thalamic nuclei volumes in psychosis. We hypothesized that thalamic mediodorsal and pulvinar nuclei will be smaller in psychosis. 2. Determine if thalamic abnormalities extend to youth with psychosis spectrum symptoms and establish whether thalamic volume abnormalities are specific to psychosis or are related to psychopathology more broadly. Consistent with a neurodevelopmental basis for thalamic pathology in psychosis, we hypothesized that psychosis spectrum youth, but not youth with other psychopathologies, would demonstrate a similar pattern of volume abnormality as individuals with a psychotic disorder. 3. Establish the cognitive correlates of thalamic nuclei volumes in individuals with a psychotic disorder and psychosis spectrum youth. In keeping with models proposing thalamic dysfunction in the mechanisms of cognitive impairment in psychosis, and abundant evidence demonstrating the importance of the mediodorsal and pulvinar nuclei in higher cognitive abilities (30–32), we hypothesized that the volume of the mediodorsal and pulvinar nuclei would correlate with cognitive function across typically developing individuals, people with psychosis and psychosis spectrum youth.
METHODS
Study Participants
Psychosis cohort:
Our psychosis cohort was drawn from a repository study comprised of 593 individuals that participated in one of 3 neuroimaging projects (CT00762866; 1R01MH070560; 1R01MH102266). Detailed study procedures and clinical characterizations are described in the Supplemental Materials. After excluding 121 individuals that did not meet study criteria, including neuroimaging quality assurance, our final sample included 179 healthy individuals and 293 people with a psychotic disorder (schizophrenia spectrum: n=199, psychotic bipolar disorder: n=94). Demographics are presented in Table 1. Neuropsychological functioning was assessed with the Screen for Cognitive Impairment in Psychiatry (SCIP) (33). A SCIP composite z-score was created by averaging accuracy scores across the 5 subtests of the SCIP: working memory, processing speed, verbal fluency, immediate and delayed word list recall.
Table 1.
Demographics of study participants in the psychosis cohort.
| Healthy Individuals | Psychosis | Statistics | |||||
|---|---|---|---|---|---|---|---|
| (n=179) | (n=293) | df | x2/t/F | P | |||
| Gender (F:M) | 73:106 | 115:178 | 1 | 0.11 | .741 | ||
| Ethnicity (W:AA:O) | 124:46:9 | 205:74:14 | 2 | 0.03 | .985 | ||
| Mean | SD | Mean | SD | ||||
| Age | 29.2 | 10.2 | 30.1 | 11.7 | 470 | −0.86 | .388 |
| Education | 15.4 | 2.1 | 13.6 | 2.2 | 441 | 8.96 | <.001 |
| Parental Education | 14.7 | 2.4 | 14.5 | 2.6 | 433 | 0.68 | .496 |
| Neuropsychological Functioning | |||||||
| SCIP Composite z-score | 0.12 | 0.65 | −0.92 | 0.96 | 456 | 12.81 | <.001 |
| Estimated Premorbid IQ | 111.1 | 11.2 | 101.9 | 15.2 | 458 | 6.85 | <.001 |
| Clinical Characteristics | |||||||
| Duration of Illness (years) | -- | -- | 8.2 | 10.6 | -- | -- | -- |
| PANSS Positive | -- | -- | 16.7 | 8.6 | -- | -- | -- |
| PANSS Negative | -- | -- | 13.8 | 6.4 | -- | -- | -- |
| PANSS General | -- | -- | 29.4 | 8.5 | -- | -- | -- |
| YMRS | -- | -- | 6.1 | 9.4 | -- | -- | -- |
| APD Dose (in CPZ equivalents) | -- | -- | 325.4 | 216.9 | -- | -- | -- |
Abbreviations: AA=African American; APD=Antipsychotic Drug; CPZ=Chlorpromazine; F=Female; M=Male; O=Other; PANSS=Positive and Negative Syndrome Scale; SCIP=Screen for Cognitive Impairment in Psychiatry; W=White; YMRS=Young Mania Rating Scale
Philadelphia Neurodevelopmental Cohort (PNC):
The PNC was obtained from the database of Genotypes and Phenotypes (dbGaP). Of note, we used the most recent version of the PNC available on dbGaP (Study Accession phs000607.v3.p2), which consists of 9498 children and youth aged 8–21 and includes the complete baseline neuroimaging sample (n=1601). Of the 1601 participants, 1393 were included in the current study after excluding participants that did not meet our inclusion criteria, including neuroimaging quality assurance. Using similar procedures as prior PNC studies (34), individuals were classified as typically developing (n=386), psychosis spectrum (n=398), or other psychopathology (n=609). Demographics are presented in Table 2. Youth with other psychopathologies were defined as those that had suprathreshold psychopathology symptoms but did not meet psychosis spectrum criteria. Participant selection and clinical characterization details are provided in the Supplemental Materials.
Table 2.
Demographics of study participants in the Philadelphia Neurodevelopmental Cohort.
| Typically Developing | Psychosis Spectrum | Other Psychopathology | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=386) | (n=398) | (n=609) | df | x2/t/F | p | Contrasts | ||||
| Gender (F:M) | 189:197 | 211:187 | 332:277 | 2.97 | .227 | -- | ||||
| Ethnicity (W:AA:O) | 213:128:43 | 121:228:48 | 293:248:64 | 59.39 | <.001 | -- | ||||
| Mean | SD | Mean | SD | Mean | SD | |||||
| Age | 14.1 | 3.7 | 15.9 | 3.1 | 14.7 | 3.6 | 2,1390 | 26.01 | <.001 | PS > OP > TD |
| Education | 7.1 | 3.6 | 8.2 | 27 | 7.7 | 3.5 | 2,1390 | 11.30 | <.001 | PS > OP > TD |
| Parental Education | 14.5 | 2.5 | 13.5 | 2.2 | 14.1 | 2.2 | 2,1382 | 20.07 | <.001 | TD > OP > PS |
| Neuropsychological Functioning | ||||||||||
| CNB Composite z-score | 0.07a | 0.02b | −0.02a | 0.02b | 0.05a | 0.02b | 2,1379 | 4.63a | .010 | TD > OP > PS |
| WRAT Standard Score | 105.6 | 15.8 | 98.5 | 16.9 | 102.5 | 15.9 | 2,1387 | 19 20 | <.001 | TD > OP > PS |
Abbreviations: AA=African American; F=Female; M=Male; O=Other; OP= OtherPsychapathology; PS=Psychosis Spectrum; TD=Typically Developing; W=White; WRAT=Wide Range Achievement Test
Adjusted for age, sex and parental education
Standard Error
Cognition was assessed with the Penn Computerized Neurocognitive Battery (CNB) (35), which consists of 14 tests covering 5 main cognitive domains: executive function, episodic memory, social cognition, complex cognition and sensorimotor ability. Penn CNB was scored as previously described (35) and a CNB composite score was created by averaging accuracy scores across the 4 non-motor cognitive domains.
Neuroimaging
Image data storage and processing took place on the Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT (36, 37). The processing pipelines were containerized using Singularity and built at SingularityHub (38) (https://singularity-hub.org).
MRI Data Acquisition and Thalamic Nuclei Segmentation:
MRI acquisition parameters, thalamic segmentation details, quality control measures and FreeSurfer segmentation examples in selected participants are presented in the Supplemental Materials. Briefly, T1-weighted images were processed using the FreeSurfer 6 image analysis suite with standard parameters (39, 40) and the thalamus segmentation module to quantify thalamic nuclei volumes (29). We studied the following five nuclei groups, mediodorsal, pulvinar, ventrolateral, ventral anterior and ventral posterolateral nuclei, due to: 1) their large size relative to neuroimaging data resolution (i.e. 1 mm3; 2) reliable segmentation (29); and 3) putative involvement in psychosis (e.g. mediodorsal, pulvinar). Several nuclei, including some relevant to psychosis, were not examined due to their small size and/or lack of contrast with surrounding white matter (e.g. lateral and medial geniculate nuclei).
Voxel-based morphometry (VBM) analysis of the thalamus:
To further localize thalamic structural abnormalities and enhance the scientific rigor of our study by examining the consistency of results across methods, we complemented the FreeSurfer-based segmentation approach described above with a voxel-based morphometry (VBM) analysis using the Computational Anatomy Toolbox 12 (CAT12: Version 12.5) in Statistical Parametric Mapping 12 (SPM12: Version 7487). See Supplemental Materials for a detailed description of the VBM analysis, including quality control measures. Group analyses were restricted to only voxels within the thalamus using a whole thalamus mask. The resulting statistical parametric maps were cluster-level corrected at p<.05FWE, for a voxel-wise threshold of p<.001. Additionally, to determine if volume loss was more pronounced in specific nuclei, the statistical parametric maps were overlaid on the Krauth atlas of thalamic nuclei (41) and the percentage overlap was calculated for each nucleus across statistical thresholds ranging from p=10−3 to 10−5.
Statistical Analyses
Group analyses:
FreeSurfer thalamic nuclei volumes were analyzed using mixed ANCOVA models in SPSS (Version 26) with the 5 thalamic nuclei as within-subject variables and group as the between subject variable. Nuclei were combined across the left and right hemispheres. Significant nuclei by group interactions were followed up with separate ANCOVAs for each nucleus. Critical alpha was Bonferroni correction for the 5 nuclei tested, resulting in an alpha of .01. For all statistical analyses, age, sex, intracranial volume (ICV) and project for the psychosis cohort were included as covariates of no interest.
Associations with cognition:
A priori planned associations between the mediodorsal and pulvinar volumes and overall cognitive function (i.e. SCIP composite and CNB composite z-scores) were calculated separately in the psychosis cohort and PNC. Associations between cognition and mediodorsal and pulvinar nuclei volumes in each cohort were tested with linear regression models predicting cognition from the volume of each nucleus. In each model, group was included as a fixed factor to account for mean group differences and age, sex, ICV, and project for psychosis cohort were included as covariates of no interest. The critical alpha was Bonferroni corrected for 2 nuclei tested, resulting in an alpha of .025.
RESULTS
Thalamic Nuclei Volumes in Psychosis
Group differences:
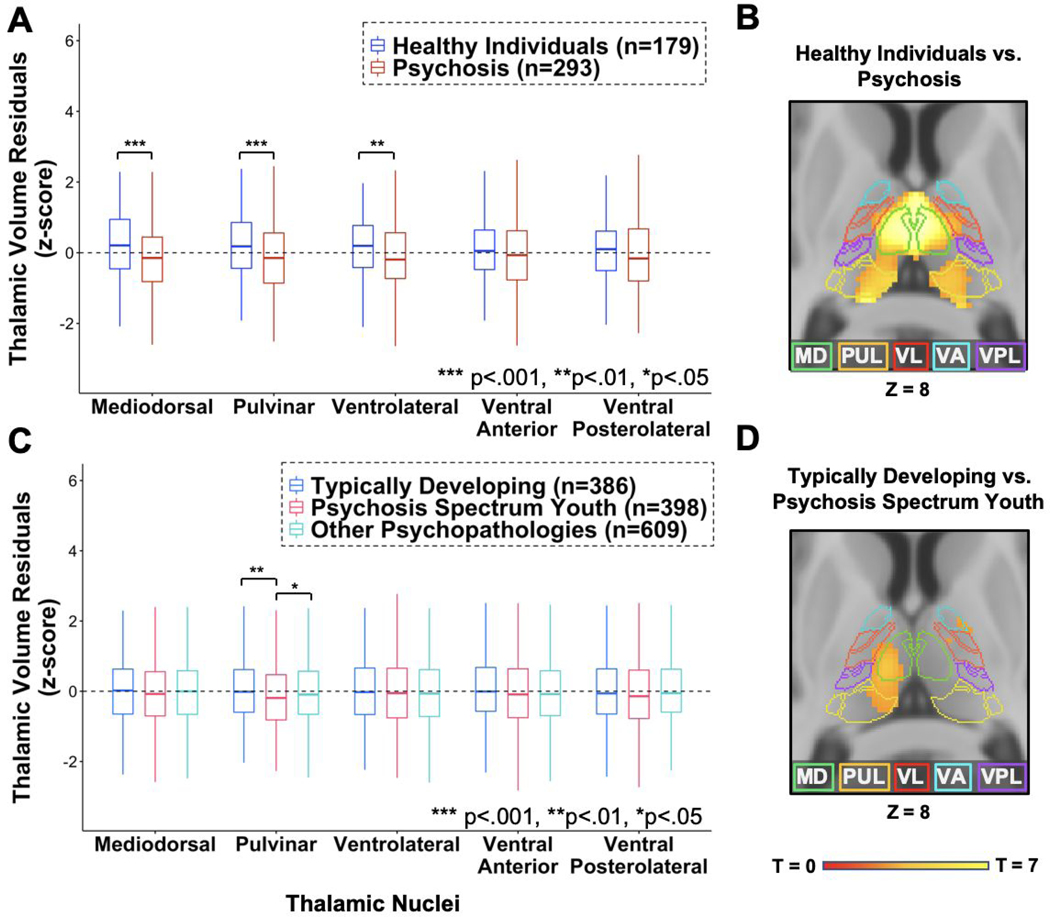
There was a significant nuclei by group interaction (F(4,1860)=6.894, p<.001) and a main effect of group (F(1,465)=14.573, p<.001), indicating differential volume reduction across the 5 thalamic nuclei superimposed on a general decrease in thalamic volumes in psychosis. As shown in Figure 1A and Table 3, mediodorsal (2.60% smaller, p<.001), pulvinar (2.74% smaller, p<.001) and ventrolateral (1.94% smaller, p=.006) nuclei volumes were smaller in the psychosis group. See Supplemental Materials for complete statistical results.
Figure 1.
A) Individuals with psychosis showed significantly smaller thalamus volumes in mediodorsal (MD), pulvinar (PUL) and ventrolateral (VL) nuclei, but not ventral anterior (VA) or ventral posterolateral (VPL) nuclei observed in standardized nuclei volumes from FreeSurfer. B) Smaller thalamus volumes in individuals with psychosis was supported by a voxel-based morphometry (VBM) analysis. C) Psychosis spectrum youth showed smaller pulvinar volumes compared to both typically developing youth and youth with other psychopathologies in standardized thalamic volumes from FreeSurfer. D) VBM analyses showed smaller thalamic volume in psychosis spectrum compared to typically developing youth. This cluster was less extensive than that observed in the psychosis cohort. VBM analyses were presented at cluster corrected at p<.05FWE, voxel-wise threshold of p<.001.
Table 3.
Mean volumes of thalamic nuclei in the psychosis cohort and the Philadelphia Neurodevelopmental Cohort.
| Psychosis Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy Individuals | Psychosis | Statistics | |||||||
| Mean (mm3) | SE | Mean (mm3) | SE | Cohen’s f | % reduction in psychosis | P | |||
| Mediodorsal | 2041 | 10.7 | 1988 | 8.3 | -- | 0.182 | 2.60 | -- | <.001 |
| Pulvinar | 3391 | 18.7 | 3298 | 14.6 | -- | 0.182 | 2.74 | -- | <.001 |
| Ventrolateral | 2892 | 15.9 | 2836 | 12,4 | -- | 0.128 | 1,94 | -- | .006 |
| Ventral Anterior | 859 | 5.4 | 847 | 4.2 | -- | 0.084 | 1,40 | -- | .071 |
| Ventral Posterolateral | 1621 | 9.2 | 1599 | 7.2 | -- | 0.090 | 1.36 | -- | .059 |
| Philadelphia Neurodevelopmental Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Typically Developing | Psychosis Spectrum | Other Psychopathology | Statistics | |||||||
| (TD) | (PS) | (OP) | ||||||||
| Mean (mm3) | SE | Mean (mm5) | SE | Mean (mm3) | SE | Cohen’s f | % reduction in PS | % reduction in OP | P | |
| Mediodorsal | 2228 | 9.5 | 2210 | 9.4 | 2218 | 7.5 | 0.032 | 0.08 | 0.45 | .429 |
| Pulvinar | 3518 | 17.4 | 3442 | 17.3 | 3498 | 13.7 | 0.084 | 2.16 | 0.57 | .006 |
| Ventrolateral | 3040 | 11.5 | 3027 | 11.4 | 3030 | 9.1 | 0.032 | 0.43 | 0.33 | .681 |
| Ventral Anterior | 894 | 3.7 | 888 | 3.7 | 888 | 2.9 | 0.032 | 0.67 | 0.67 | .448 |
| Ventral Posterolateral | 1769 | 8.8 | 1753 | 8.7 | 1767 | 6.9 | 0.045 | 0.90 | 0.11 | .343 |
Supplemental analyses examining the effects of psychotic disorder diagnosis (i.e. schizophrenia spectrum, bipolar disorder with psychotic features), hemisphere, age, and sex, as well as group by age and group by sex interactions were performed and reported in the Supplemental Materials. Briefly, results were similar across psychotic disorders and there was no evidence of group by hemisphere, group by age or group by sex interactions. While no associations were hypothesized, we also examined the correlations between thalamus nuclei volumes and clinical symptoms of psychosis, including positive and negative symptoms. Briefly, none of the thalamus volumes correlated with clinical symptoms. See Supplemental Materials for complete statistical results.
Voxel-based morphometry analysis:
As shown in Figure 1B and consistent with the FreeSurfer-based results presented above, VBM analyses revealed lower gray matter volume in psychosis in a cluster encompassing bilateral mediodorsal, pulvinar and left ventrolateral nuclei. Overlaying the results on the Krauth thalamus atlas indicated that the cluster covered large portions of the bilateral mediodorsal (left: 84.5%, right: 92.9%), pulvinar (left: 67.8%, right: 60.6%), but much less of the ventrolateral (left: 16.2%, right: 11.7%) nuclei at a standard p=.001 threshold. With increasingly stringent thresholds, the cluster continued to cover a portion of the mediodorsal nucleus and pulvinar, but not the ventrolateral nucleus. Detailed results from the VBM analysis are presented in the Supplemental Materials.
Thalamic Nuclei Volumes in Psychosis Spectrum Youth
Group differences:
There was a significant nuclei by group interaction (F(8,5548)=3.216, p=.001), though not a significant main effect of group (F(2,1387)=2.646, p=.07). As shown in Figure 1C and Table 3, post-hoc tests indicated that only the pulvinar exhibited a main effect of group (F(2,1387)=5.206, p=.006). Pulvinar volume was significantly smaller in psychosis spectrum youth compared to both typically developing youth (2.16% smaller; p=.002), and youth with other psychopathologies (1.60% smaller; p=.01), but did not differ between typically developing and youth with other psychopathologies (0.57% smaller in youth with other psychopathologies; p=.38). See Supplemental Materials for complete statistical results.
Supplemental analyses examining the effects of hemisphere, age and sex, as well as group by age and group by sex interactions were performed and are presented in the Supplemental Materials. Briefly, there was no evidence for group by hemisphere, group by age, or group by sex interactions in any nucleus.
Sensitivity Analysis:
To ensure that smaller pulvinar volumes in psychosis spectrum youth was not driven by group differences in demographic variables (i.e. age, sex, race), we performed a sensitivity analysis by creating sub-samples of the psychosis spectrum and typically developing youth matched for age, sex and race. Matching was conducted using the MatchIt package (42) (Version 3.0.2) in R (Version 3.6.1; R Core Team, 2019) and resulted in a final sample of 150 psychosis spectrum and 133 typically developing youth. The main effect of group remained significant for the pulvinar, reflecting smaller pulvinar volume in psychosis spectrum youth (2.56% smaller, p=.03). No other nucleus showed significant group effects. See Supplemental Materials for complete details of the sensitivity analysis.
Voxel-based morphometry analysis:
As shown in Figure 1D, VBM analysis revealed significantly lower gray matter volume in psychosis spectrum youth in a cluster in the left thalamus encompassing mediodorsal, pulvinar and anterior nuclei, and a cluster in the right thalamus with a peak in the anterior thalamus. Overlaying the results on the Krauth thalamus atlas indicated that lower thalamus volume in psychosis spectrum youth covered the left mediodorsal (34.3%), pulvinar (10.7%) and ventrolateral nuclei (22.6%). Detailed results of the VBM analysis are presented in the Supplementary Materials.
Thalamic Nuclei Volumes and Cognition in Psychotic Disorders and Psychosis Spectrum Youth
Psychosis cohort:
As shown in Figure 2A, overall cognitive function (i.e. SCIP composite z-score) correlated with pulvinar (Rpartial=.111, p=.02), but not mediodorsal nuclei volumes (Rpartial=.087, p=.06) after correction for multiple comparisons.
Figure 2.
Pulvinar volumes were significantly associated with overall cognitive function as measured with: A) The Screen for Cognitive Impairment in Psychiatry (SCIP) composite z-score in the psychosis cohort and B) The Computerized Neurocognitive Battery (CNB) composite z-score in the Philadelphia Neurodevelopmental Cohort.
PNC:
As shown in Figure 2B, overall neuropsychological functioning (i.e. CNB composite z-score) was positively correlated with pulvinar volumes (Rpartial=.121, p<.001) at the corrected statistical threshold, but not mediodorsal nuclei volumes (Rpartial=.035, p =.193).
DISCUSSION
The current investigation examined thalamic nuclei volumes in a large cohort of individuals with a psychotic disorder and a community-ascertained neurodevelopmental cohort, the PNC. We used a recently developed, validated method included in the FreeSurfer software package for segmenting thalamic nuclei on conventional T1-weighted MRI and complemented this approach with a VBM analysis.
The current findings contribute to our understanding of thalamic pathology in psychosis in several ways. First, they clarify the anatomical specificity of thalamic structural abnormalities in psychosis. Using complementary segmentation and voxel-based approaches, we found that lower thalamic volume was most pronounced in the mediodorsal and pulvinar nuclei. As touched upon earlier, findings from post-mortem (10) and neuroimaging studies (24–27) are mixed, likely due to a combination of several factors including small sample sizes and, in the case of neuroimaging studies, variable methods used to measure specific thalamic nuclei. Our results support thalamic models of psychotic disorders that emphasize dysfunction among association nuclei.
Second, our finding that psychosis spectrum youth also demonstrate smaller thalamic volumes is consistent with a neurodevelopmental basis for thalamic abnormalities in psychosis (14, 15). Disruption of the thalamus during development may affect cortical development in regions involved in the pathogenesis of psychosis (e.g. prefrontal cortex). For instance, animal studies show that disrupted thalamus development is associated with lower cell density and volume in neuroanatomically connected cortical regions (43, 44). In our neurodevelopmental cohort, smaller pulvinar volume was specific to psychosis spectrum youth. Smaller pulvinar volumes were constant across age in both samples, suggesting that structural abnormalities in the pulvinar is present prior to the onset of psychosis. VBM analysis indicated that smaller volume in psychosis spectrum youth also encompassed a portion of the mediodorsal nucleus. Indeed, qualitative comparison of VBM results indicates that there is significant overlap in thalamic volume loss in psychosis and psychosis spectrum youth; although, not surprisingly, volume loss is less extensive in psychosis spectrum youth.
While prior studies have identified smaller thalamic volume in youth exhibiting symptoms of psychosis, including clinical high risk individuals (45, 46), the current results extend these findings in critical ways. First, we included a large cohort of individuals with a psychotic disorder to compare concordance across the psychosis spectrum. Second, we examined volumes of specific thalamic nuclei, in contrast to most prior studies which examined whole thalamus volume only. Third, the current sample size is considerably larger than many prior studies, including a recent study that used an earlier release of the PNC that did not include the complete neuroimaging sample (n=997 vs. n=1601 in the current study) and separated those with psychosis-spectrum symptoms only from youth with psychosis and bipolar spectrum symptoms (47). Fourth, in contrast to all prior studies (47, 48), we established that smaller thalamic volume is specific to psychosis spectrum youth by including a large sample of youth with other psychopathology. Finally, we examined associations between thalamus volume and cognitive function.
One major implication of smaller thalamic nuclei volumes in both psychosis spectrum youth and psychosis is that this pathological abnormality contributes to the cognitive impairment considered to be a core feature of psychotic disorders (49). This is supported in our data, as thalamic nuclei volumes, pulvinar specifically, is associated with general cognitive function. Moreover, the association, while modest, was strikingly similar across psychosis and youth cohorts. Previous studies have found that whole thalamus volume, and pulvinar–cortex covariance predict cognition in schizophrenia (50, 51). The pulvinar is intimately involved in cognitive functions, particularly as it relates to flexible, goal oriented direction of attention (30). The pulvinar also has a role in synchronizing cortical activity during attention (52, 53), and lesions of the pulvinar reduce attention-related signals in the cortex (53), suggesting that it may influence cognition through thalamo-cortical interactions. Inappropriate modulation of attention through cortical networks that include the thalamus (e.g. the salience network) is hypothesized as a core deficit in schizophrenia that impairs the integrity of sensory information and contextprocessing to control goal-directed behavior (54). Furthermore, positron emission tomography (PET) studies in schizophrenia have demonstrated reduced dopamine D2 receptor binding in the pulvinar and mediodorsal nucleus specifically, with lower binding being associated with more severe psychotic symptoms (55, 56).
Strengths and Limitations
Our study had several strengths including: large sample sizes, complementary methods for measuring thalamic volumes, and convergent findings across both methods and cohorts. Nevertheless, there are several limitations. While our use of an automated segmentation technique allowed us to obtain a well validated segmentation of larger thalamic nuclei, we did not include several nuclei due to their small size and poor contrast in standard mm3 T1 imaging, some of which may be relevant to psychosis, such as the lateral and medial geniculate nuclei. Another limitation was our use of cross-sectional samples, which limits our ability to investigate changes in the volumes of thalamic nuclei over time in individuals with a psychotic disorder and psychosis spectrum youth.
Conclusion
Thalamic association nuclei, including pulvinar and mediodorsal nuclei, are smaller in individuals with a psychotic disorder. Our findings also demonstrate that in a large cohort of youth with psychosis risk, as compared to youth with other psychopathology and taking into account specific thalamic nuclei, smaller thalamic association nuclei volumes represent a neurodevelopmental abnormality associated with cognitive impairment and higher risk for developing a psychotic disorder. Identification of specific thalamic nuclei affected in psychosis provides potential targets in the treatment of psychosis and cognitive impairment in psychosis with new neuromodulation technology, such as focused ultrasound therapy (57).
Supplementary Material
Acknowledgements:
The authors thank the individuals who participated in the study. The authors would also like to thank Kristan Armstrong, Erin Brosey, Molly Boyce, Victoria Fox, Yasmeen Iqbal, Margo Menkes, Austin Woolard, Katherine Seldin, and Margee Quinn for their assistance recruiting and screening study participants.
Funding/Support: This work was supported by NIMH grants R01 MH102266 (awarded to NDW), R01 MH115000 (awarded to NDW and AA), R01 MH070560 (awarded to SH); the Charlotte and Donald Test Fund, the Jack Martin, MD, Research Professorship in Psychopharmacology (awarded to JUB); and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH). This work was conducted in part using the resources of the Center for Computational Imaging at the Vanderbilt University Institute of Imaging Sciences and the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University, Nashville, TN.
Footnotes
Conflicts of Interest Disclosures: No commercial support was received for the preparation of this manuscript. AA consults, holds equity and is a scientific board member for BlackThorn Therapeutics. All other authors have no conflicts of interest to report.
REFERENCES
- 1.Brugger S, Davis JM, Leucht S, et al. : Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol Psychiatry 2011; 69:495–503 [DOI] [PubMed] [Google Scholar]
- 2.Giraldo-Chica M, Rogers BP, Damon SM, et al. : Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol Psychiatry 2018; 83:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giraldo-Chica M, Woodward ND: Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res 2017; 180:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsay IS: An Activation Likelihood Estimate Meta-analysis of Thalamocortical Dysconnectivity in Psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4:859–869 [DOI] [PubMed] [Google Scholar]
- 5.Minzenberg MJ, Laird AR, Thelen S, et al. : Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 2009; 66:811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang AS, Rogers BP, Woodward ND: Disrupted modulation of thalamus activation and thalamocortical connectivity during dual task performance in schizophrenia. Schizophr Res 2019; 210:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adriano F, Spoletini I, Caltagirone C, et al. : Updated meta-analyses reveal thalamus volume reduction in patients with first-episode and chronic schizophrenia. Schizophr Res 2010; 123:1–14 [DOI] [PubMed] [Google Scholar]
- 8.van Erp TGM, Hibar DP, Rasmussen JM, et al. : Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2016; 21:547–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibar DP, Westlye LT, van Erp TGM, et al. : Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry 2016; 21:1710–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorph-Petersen K-A, Lewis DA: Postmortem structural studies of the thalamus in schizophrenia. Schizophr Res 2017; 180:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parnaudeau S, O’Neill P-K, Bolkan SS, et al. : Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 2013; 77:1151–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchmann A, Dentico D, Peterson MJ, et al. : Reduced mediodorsal thalamic volume and prefrontal cortical spindle activity in schizophrenia. Neuroimage 2014; 102 Pt 2:540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baran B, Karahanoğlu FI, Mylonas D, et al. : Increased Thalamocortical Connectivity in Schizophrenia Correlates With Sleep Spindle Deficits: Evidence for a Common Pathophysiology. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4:706–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreasen NC: The role of the thalamus in schizophrenia. Can J Psychiatry 1997; 42:27–33 [DOI] [PubMed] [Google Scholar]
- 15.Jones EG: Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 1997; 23:483–501 [DOI] [PubMed] [Google Scholar]
- 16.Sim K, Cullen T, Ongur D, et al. : Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm 2006; 113:907–928 [DOI] [PubMed] [Google Scholar]
- 17.Pergola G, Selvaggi P, Trizio S, et al. : The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev 2015; 54:57–75 [DOI] [PubMed] [Google Scholar]
- 18.Steullet P: Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis [Internet]. Schizophr Res 2019; Available from: 10.1016/j.schres.2019.05.027 [DOI] [PubMed] [Google Scholar]
- 19.Byne W, Buchsbaum MS, Mattiace LA, et al. : Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 2002; 159:59–65 [DOI] [PubMed] [Google Scholar]
- 20.Highley JR, Walker MA, Crow TJ, et al. : Low medial and lateral right pulvinar volumes in schizophrenia: a postmortem study. Am J Psychiatry 2003; 160:1177–1179 [DOI] [PubMed] [Google Scholar]
- 21.Byne W, Fernandes J, Haroutunian V, et al. : Reduction of right medial pulvinar volume and neuron number in schizophrenia. Schizophr Res 2007; 90:71–75 [DOI] [PubMed] [Google Scholar]
- 22.Pakkenberg B: Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47:1023–1028 [DOI] [PubMed] [Google Scholar]
- 23.Young KA, Holcomb LA, Yazdani U, et al. : Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry 2004; 161:1270–1277 [DOI] [PubMed] [Google Scholar]
- 24.Gilbert AR, Rosenberg DR, Harenski K, et al. : Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry 2001; 158:618–624 [DOI] [PubMed] [Google Scholar]
- 25.Kemether EM, Buchsbaum MS, Byne W, et al. : Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry 2003; 60:983–991 [DOI] [PubMed] [Google Scholar]
- 26.Horga G, Bernacer J, Dusi N, et al. : Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2011; 261:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pergola G, Trizio S, Di Carlo P, et al. : Grey matter volume patterns in thalamic nuclei are associated with familial risk for schizophrenia. Schizophr Res 2017; 180:13–20 [DOI] [PubMed] [Google Scholar]
- 28.Iglesias JE, Augustinack JC, Nguyen K, et al. : A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015; 115:117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iglesias JE, Insausti R, Lerma-Usabiaga G, et al. : A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage 2018; 183:314–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saalmann YB, Kastner S: Cognitive and perceptual functions of the visual thalamus. Neuron 2011; 71:209–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rikhye RV, Wimmer RD, Halassa MM: Toward an Integrative Theory of Thalamic Function. Annu Rev Neurosci 2018; 41:163–183 [DOI] [PubMed] [Google Scholar]
- 32.Ouhaz Z, Fleming H, Mitchell AS: Cognitive Functions and Neurodevelopmental Disorders Involving the Prefrontal Cortex and Mediodorsal Thalamus. Front Neurosci 2018; 12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purdon SE: The Screen for Cognitive Impairment in Psychiatry (SCIP): Instructions and three alternate forms. PNL Inc, Edmonton, Alberta: 2005; [Google Scholar]
- 34.Roalf DR, Quarmley M, Calkins ME, et al. : Temporal Lobe Volume Decrements in Psychosis Spectrum Youths. Schizophr Bull 2017; 43:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gur RC, Richard J, Calkins ME, et al. : Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology 2012; 26:251–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrigan RL, Yvernault BC, Boyd BD, et al. : Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT: A multimodal data archive and processing environment. Neuroimage 2016; 124:1097–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huo Y, Blaber J, Damon SM, et al. : Towards Portable Large-Scale Image Processing with High-Performance Computing. J Digit Imaging 2018; 31:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sochat VV, Prybol CJ, Kurtzer GM: Enhancing reproducibility in scientific computing: Metrics and registry for Singularity containers. PLoS One 2017; 12:e0188511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dale AM, Fischl B, Sereno MI: Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9:179–194 [DOI] [PubMed] [Google Scholar]
- 40.Fischl B, Salat DH, Busa E, et al. : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355 [DOI] [PubMed] [Google Scholar]
- 41.Krauth A, Blanc R, Poveda A, et al. : A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage 2010; 49:2053–2062 [DOI] [PubMed] [Google Scholar]
- 42.Ho DE, Imai K, King G, et al. : MatchIt: nonparametric preprocessing for parametric causal inference [Internet]. Journal of Statistical Software, http://gkingharvardedu/matchit2011; Available from: https://r.iq.harvard.edu/docs/matchit/2.4-18/matchit.pdf [Google Scholar]
- 43.Ouhaz Z, Ba-M’hamed S, Mitchell AS, et al. : Behavioral and cognitive changes after early postnatal lesions of the rat mediodorsal thalamus. Behav Brain Res 2015; 292:219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selemon LD, Wang L, Nebel MB, et al. : Direct and indirect effects of fetal irradiation on cortical gray and white matter volume in the macaque. Biol Psychiatry 2005; 57:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lunsford-Avery JR, Orr JM, Gupta T, et al. : Sleep dysfunction and thalamic abnormalities in adolescents at ultra high-risk for psychosis. Schizophr Res 2013; 151:148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrisberger F, Buechler R, Smieskova R, et al. : Alterations in the hippocampus and thalamus in individuals at high risk for psychosis. NPJ Schizophr 2016; 2:16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jalbrzikowski M, Freedman D, Hegarty CE, et al. : Structural Brain Alterations in Youth With Psychosis and Bipolar Spectrum Symptoms. J Am Acad Child Adolesc Psychiatry 2019; 58:1079–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs GR, Ameis SH, Ji JL, et al. : Developmentally divergent sexual dimorphism in the cortico-striatal-thalamic-cortical psychosis risk pathway. Neuropsychopharmacology 2019; 44:1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elvevag B, Goldberg TE: Cognitive Impairment in Schizophrenia Is the Core of the Disorder [Internet]. CRNA 2000; 14[cited 2020 Jan 31] Available from: http://www.dl.begellhouse.com/journals/7b004699754c9fe6,492097963b0961c1,742755367b0d9340.html [PubMed] [Google Scholar]
- 50.Ramsay IS, Fryer S, Boos A, et al. : Response to Targeted Cognitive Training Correlates with Change in Thalamic Volume in a Randomized Trial for Early Schizophrenia. Neuropsychopharmacology 2018; 43:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitelman SA, Byne W, Kemether EM, et al. : Correlations between volumes of the pulvinar, centromedian, and mediodorsal nuclei and cortical Brodmann’s areas in schizophrenia. Neurosci Lett 2006; 392:16–21 [DOI] [PubMed] [Google Scholar]
- 52.Saalmann YB, Pinsk MA, Wang L, et al. : The pulvinar regulates information transmission between cortical areas based on attention demands. Science 2012; 337:753–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou H, Schafer RJ, Desimone R: Pulvinar-Cortex Interactions in Vision and Attention. Neuron 2016; 89:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palaniyappan L, Simmonite M, White TP, et al. : Neural primacy of the salience processing system in schizophrenia. Neuron 2013; 79:814–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasuno F, Suhara T, Okubo Y, et al. : Low Dopamine D2 Receptor Binding in Subregions of the Thalamus in Schizophrenia. AJP 2004; 161:1016–1022 [DOI] [PubMed] [Google Scholar]
- 56.Kessler RM, Woodward ND, Riccardi P, et al. : Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry 2009; 65:1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legon W, Ai L, Bansal P, et al. : Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum Brain Mapp 2018; 39:1995–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.