Abstract
In-vitro fertilization (IVF) is a popular method of resolving complications such as endometriosis, poor egg quality, a genetic disease of mother or father, problems with ovulation, antibody problems that harm sperm or eggs, the inability of sperm to penetrate or survive in the cervical mucus and low sperm counts, resulting human infertility. Nevertheless, IVF does not guarantee success in the fertilization. Choosing IVF is burdensome for the reason of high cost and uncertainty in the result. As the complications and fertilization factors are numerous in the IVF process, it is a cumbersome task for fertility doctors to give an accurate prediction of a successful birth. Artificial Intelligence (AI) has been employed in this study for predicting the live-birth occurrence. This work mainly focuses on making predictions of live-birth occurrence when an embryo forms from a couple and not a donor. Here, we compare various AI algorithms, including both classical Machine Learning, deep learning architecture, and an ensemble of algorithms on the publicly available dataset provided by Human Fertilisation and Embryology Authority (HFEA). Insights on data and metrics such as confusion matrices, F1-score, precision, recall, receiver operating characteristic (ROC) curves are demonstrated in the subsequent sections. The training process has two settings Without feature selection and With feature selection to train classifier models. Machine Learning, Deep learning, ensemble models classification paradigms have been trained in both settings. The Random Forest model achieves the highest F1-score of 76.49% in without feature selection setting. For the same model, the precision, recall, and area under the ROC Curve (ROC AUC) scores are 77%, 76%, and 84.60%, respectively. The success of the pregnancy depends on both male and female traits and living conditions. This study predicts a successful pregnancy through the clinically relevant parameters in In-vitro fertilization. Thus artificial intelligence plays a promising role in decision making process to support the diagnosis, prognosis, treatment etc.
Subject terms: Computational biology and bioinformatics, Origin of life
Introduction
More than 80 million couples are affected by infertility. An 'unsuccessful conception' after almost 12 months of having unprotected intercourse can be caused due to infertility1. To reduce the number of unsuccessful conception, ovum from the female ovary and sperm from the male are fused outside the body, i.e., in the laboratory resulting in an embryo, which is then placed in the female's ovary for development is In-Vitro Fertilization (IVF). In some cases, artificial insemination results in conception by injecting sperm into the uterus directly. It has been reported that more than 5 million babies have been born from IVF around the world. IVF is used to overcome the male and female infertility caused due to various problems related to both the sexs' reproductive characteristics. IVF works by combining various medical and surgical procedures to help in fertilization. The whole process has more than one round and can take several months to get a pregnancy. Easy accessibility of IVF treatments rose the usage of IVF but not due to infertility couples2. Opting for IVF treatment is also considered a very challenging task due to its high cost, no guarantee of the success, and the stress of the treatment3,4. Patients generally discontinue IVF treatment due to the physical and psychological burden of the treatment5,6.
Several medical practitioners have been predicting the possibility of pregnancy by a trial and error method through their expertise. Therefore, conventional prediction methods are dependent on the level of experience of an individual medical practitioner, which does not employ any systematic statistical approach. Hence, they are more subjective. Medical practitioners and patients are eagerly looking for a measurement to guide them for decision making about IVF treatment. Recent advancements in technologies such as Artificial Intelligence (AI), Machine Learning (ML), Deep learning (DL) promises in solving some of the endemic problems with statistical data-driven approaches. Highly accurate analysis driven by AI can lead to radically solve most of the challenges with vast amounts of data by interpreting them in a meaningful way. The statistical approach has attracted researchers' attention to develop prediction models for fertility by which a medical practitioner can get an accurate prediction if a successful birth happens in the IVF setup.
Machine Learning is a field of study that teaches computers/systems to think in a similar way and outputs predictions by learning/training upon past experiences7. It explores data in a meaningful, pattern-oriented manner that gives the systems' robustness to mimic a human decision-making capability. Deep learning is a subset of ML that works on the principles of human neural networks8. Analyzing huge data records with lots of parameters can make humans miss some crucial patterns in the data. ML and DL get you covered in this aspect and can identify these patterns easily, which then helps a human in decision-making.
With continued improvements in ML, several healthcare domains have already adopted it to improve decision-making, including enabling personalized care, surgery simulations, drug discovery, and accelerating disease diagnosis9–12. In reproductive science, ML was applied for predicting implantation after blastocyst transfer in IVF13. ML has also been applied to build a prediction model for embryo selection to evaluate the live-birth live-birth predictors and predict twins14–16. Contemporary use of DL techniques to predict fatal heart pregnancy and human blastocyst selection have also been witnessed17,18. Hence, ML can be applied to the clinical datasets to develop risk assessment, diagnostic, prognostic models, and improve patient healthcare.
Machine Learning Some studies in the past use ML techniques to predict the live-birth chances of women undergoing IVF treatment. One of the earlier and most accepted prediction models is the McLernon Model19,20, which utilizes only discrete logistic regression to predict the chances of live-birth for a couple having up to six complete IVF cycles. Two prediction models, a pre-treatment model (predicts before the IVF treatment starts) and a post-treatment model (predicts the chances of live-birth after the first attempt at embryo transfer), are developed. Data was collected from The Human Fertilisation and Embryology Authority (HFEA) of 253,417 women who started IVF treatment in the United Kingdom from 1999 to 2008 using their own eggs and partner sperms. C-index was used to assess the performance of both models. The C-index for pre-treatment model was 0.69 (0.68–0.69) and C-index for post-treatment model was 0.76 (0.75–0.77).
Rafiul Hassan et al.21 proposes a hill-climbing feature selection algorithm with five different ML models to analyze and predict IVF pregnancy in greater accuracy. Data for this study were collected from an infertility clinic in Istanbul, Turkey, for about three years from March 2005 to January 2008 and consists of infertility treatment of 1048 patients. This study used 27 attributes like age, diagnosis, Antral Follicle Counts (AFC), sperm quality, etc. It is found that age is the most influential IVF attribute that affects pregnancy outcome. Performance of all classifiers improved when hill climbing feature selection techniques (electing only important features by the classifiers) was employed. Overall, Support Vector Machine (SVM) attains the highest accuracy of 98.38%, F1-score of 98.4%, and AUC score of 99.5% considering 19 IVF attributes.
A survey done by Guvenir et al.22 on the ML models, namely SVM, Decision trees, Naïve Bayes, K nearest neighbor (KNN), etc. showed that different models require a various number of features to perform well. Patient attributes such as age, Body Mass Index (BMI), sperm count, etc. were used to train these models. SVM in this survey considers up to 64 features to resulting in an accuracy of 84%, while others considered as low as 5–6 features like artificial neural networks (ANN) by Kaufmann et al.23 that resulted in 59% accuracy24. focuses on developing a model that helps the couple decide whether to take IVF treatment or not. In this survey, two problems are addressed: one to check the probability of having pregnancy in the IVF treatment and another in helping doctors choose the most viable embryos.
Jiahui Qiu et al.25 predicts live birth before the IVF using four models: logistic regression, Random forest, Extreme gradient boosting (XGBoost), and SVM. Data is collected from 7188 women who underwent their first IVF treatment from the Medical Center of Shengjing Hospital of China Medical University during 2014–2018. Attributes like age, AMH, BMI, duration of infertility, previous live birth, previous miscarriage, etc. and type of infertility (tubal, male factor, anovulatory, unexplained, and others) are considered. Calibration and receiver operating characteristic (ROC) curves are employed as performance metrics. XGBoost achieved the highest area under ROC curve (ROC AUC) score of 0.73 on the validation dataset and exhibited the best calibration model among all models.
Predicting the live-birth occurrence belongs to the binary classification problem determining whether a female gives birth or not is predicted based on the given IVF parameters. The present work aims to compare various models on predicting live-birth occurrence after the complete IVF cycle. The work mainly focuses on making predictions of live-birth occurrence when an embryo forms from a couple and not a donor. A complete IVF cycle refers to the fresh cycle and the following freeze–thaw cycles from one round of ovarian stimulation. There are several reproductive characteristics related to both males and females that cause infertility. It has been found that factors related to female-like age (decrease in quantity and quality of the eggs), menstrual disorder, uterine factor, cervical factor, previous pregnancies, duration of infertility, female primary (if the patient is unable to get pregnant after at least one year), female secondary (if the patient able to get pregnant at least once but now unable to) and unexplained factors have a significant impact on causing infertility26–30. Factors related to males, such as semen concentration, semen motility, semen morphology, sperm volume, and semen count, are essential for testing infertility in males31,32. All the above-said important reproductive characteristics of males and females have been considered in this study. A public dataset33 that contains all the above-said parameters, provided by Human Fertilisation and Embryology Authority, is the longest-running fertility treatment database in the world. Data of 495,630 records with 94 clinical features are considered in this study acquired from 2010 to 2016 from IVF centers across the UK. After performing data cleaning 141,160 records with 25 essential clinical features are considered for training and testing in which both positive and negative classes contain 70,580 records each.
ML, DL, Ensemble learning are employed in this study. Models such as Logistic Regression34, K nearest neighbor35, Multi-Layer Perceptron36, Decision Tree37, 1-D Deep learning model38 are used for training purpose. Ensemble learning39 is used to make a collective decision on predictions from the above-said models. Random forest40, AdaBoost41, voting classifiers42 are employed in this strategy. Predominantly, two settings are followed in this study: one training model without feature selection and the other using two feature selection techniques. Feature selection techniques such as Linear support vector classifier (Linear SVC) based selection from model43, Tree-based feature selection44 are considered. The performance of models in both these settings was measured based on metrics such as F1-score, precision, recall, and ROC-AUC curve.
The remainder of this article is organized as follows: in the Methodology section describes the dataset, pre-processing techniques used to make train-ready data and models trained. A comprehensive comparison between the performance metrics of various models is demonstrated in Results & Discussion. The conclusion section explains about the insights that can be drawn and future work from this study.
Methodology
Dataset description
The dataset used in this study is anonymized register data collected from the year 2010–2016 obtained from the Human Fertilisation & Embryology Authority33. It holds the longest-running database register of fertility treatments globally to improve patient care while ensuring reliable protection of patient, donor, and offspring confidentiality. This dataset contains 495,630 patient records with 94 features on treatment cycles collected from various patient studies between 2010 and 2016. Focusing on the aim of this study, the dataset after filtration contains 141,160 patient records. It includes three types of data that are numerical, categorical, and text. There was no medical intervention in the couples' behavioral and biomedical routine for this work. Furthermore, this work involves only analyzing the couples' data; hence no permission was taken from the Institutional Review Board (IRB). All relevant guidelines were followed for the study.
Many factors influence the live-birth occurrence (target variable) after IVF treatment. In this study, the original dataset contains 94 features, but not all features significantly affect the outcome. So, only 30 features are considered. Hence, feature engineering has been performed based on the subject knowledge, recommended by Dr. Bharti Bansal and NICE clinical guideline (The National Institute of Health and Care Excellence, UK). In this study, features such as fresh cycles and the following freeze–thaw cycles from the same stimulation for a woman undergoing IVF (including ICSI) are considered. Donor oocytes/sperm cycles and PGD/PCS cycles are excluded. Features selected in this study are age, the total number of previous cycles, the total number of previous IVF pregnancies, number of eggs mixed with partner sperm, number of embryos transferred in this cycle, type and cause of infertility that includes male factor, female factors, ovulatory, endometriosis, tubal, cervical, etc. Table 1 summarizes a detailed description of the dataset features considered in the approach.
Table 1.
IVF attributes of our dataset.
| Field | Type | Description |
|---|---|---|
| Patient age at treatment | Categorical | Patient age at treatment, banded as follows: 18–34, 35–37, 38–39, 40–42, 43–44, 45–50 |
| Total number of previous cycles | Numerical | How many treatment cycles of IVF the patient has previously had |
| Total number of IVF pregnancies | Numerical | How many patients have been pregnant through IVF |
| Total number of live births- conceived through IVF | Numerical | How many live births the patients have had through IVF |
| Type of infertility—female primary | Categorical | 1 if the patient unable to get pregnant after at least 1 year, 0 otherwise |
| Type of Infertility—female secondary | Categorical | 1 if the patient able to get pregnant at least once but now unable to, 0 otherwise |
| Type of infertility—male primary | Categorical | 1 if the leading cause of the infertility is patient, 0 otherwise |
| Type of infertility—male secondary | Categorical | 1 if the secondary cause of infertility is due to the patient, 0 otherwise |
| Type of infertility—couple primary | Categorical | 1 if the leading cause of the infertility is patient/partner, 0 otherwise |
| Type of infertility—couple secondary | Categorical | 1 if the secondary cause of infertility is due to the patient/partner, 0 otherwise |
| Cause of infertility—tubal disease | Categorical | 1 if there is damage in the fallopian tubes that prevents sperm from reaching the ovary, 0 otherwise |
| Cause of infertility—ovulatory disorder | Categorical | 1 if the primary cause of this infertility is due to ovulation disorder, 0 otherwise |
| Cause of infertility—male factor | Categorical | 1 if the primary cause of this infertility is due to male patients, 0 otherwise |
| Cause of infertility—patient unexplained | Categorical | 1 if the primary cause of infertility in the patient is unknown, 0 otherwise |
| Cause of infertility—endometriosis | Categorical | 1 if the primary cause of this infertility is due to endometriosis, 0 otherwise |
| Cause of infertility—cervical factors | Categorical | 1 if the primary cause of this infertility is due to the Cervical factor, 0 otherwise |
| Cause of infertility—female factors | Categorical | 1 if the primary cause of this infertility is due to female factors, 0 otherwise |
| Cause of infertility—partner sperm concentration | Categorical | 1 if the primary cause of this infertility is due to low sperm count, 0 otherwise |
| Cause of infertility—partner sperm morphology | Categorical | 1 if the primary cause of this infertility is an abnormality in sperm morphology, 0 otherwise |
| Cause of infertility—partner sperm motility | Categorical | 1 if the primary cause of this infertility is poor sperm motility, 0 otherwise |
| Cause of infertility—partner sperm immunological factors | Categorical | 1 if the primary cause of this infertility is due to sperm immunological factors, 0 otherwise |
| Stimulation used | Categorical | 1 if the stimulation medication is used, 0 otherwise |
| Egg source | Text | Indicates whether the eggs used in this cycle came from Patient (P) or a Donor (D) |
| Sperm source | Text | Indicates whether the eggs used in this cycle came from Patient (P) or a Donor (D) |
| Fresh cycle | Categorical | 1 if this cycle using fresh embryos, 0 otherwise |
| Frozen cycle | Categorical | 1 if the cycle used from frozen embryos, 0 otherwise |
| Eggs thawed | Numerical | If this cycle frozen eggs, the number of eggs thawed |
| Fresh eggs collected | Numerical | The number of eggs collected in this cycle |
| Eggs mixed with partner sperm | Numerical | The number of eggs mixed with sperm from the partner |
| Embryos transferred | Numerical | The number of embryos transferred into the patient in this cycle |
Pre-processing of dataset
The raw dataset contains 94 attributes, out of which few do not significantly affect predicting live-birth occurrence. The filtration of the dataset depends on the stimulation used, sperm source, egg source features. If the source of sperm and egg is from the same couple, i.e., Partner and Patient, then those patient records are considered, the rest are eliminated. In IVF, injectable medication containing both follicle-stimulating hormone (FSH) and luteinizing hormone (LH) is injected into females to stimulate more than one egg developing at a time45. It is described as "Stimulation Used" in the dataset; this study considers only patient records where stimulation is done.
In the field "Patient Age at Treatment," few patient records contain value 999 that are eliminated. Text and age ranges are converted into categorical data. For instance, in the field "Patient Age at Treatment."
18–34 is converted to 0
35–37 is converted to 1
38–39 is converted to 2
40–42 is converted to 3
43–44 is converted to 4
45–50 is converted to 5
The field "Live-birth Occurrence" is the target variable, which is numerical and contains values ranging from 0 to 5 where 0 represents no birth (negative class) and greater than 1 represents birth occurrence (positive class). To make the classification binary, all the patient records whose Live-birth Occurrence are more significant than 1 are set to 1 and remaining to 0.
After the above filtration, the negative samples are 5 × more than positive samples that make data imbalance. Few patient records from negative samples are removed to encounter the problem of imbalance in the dataset. Now the dataset contains 141,160 patient records and 25 features, which distributes 70,580 samples in each class. It is found that few fields such as sperm source, egg source, cause of infertility partner sperm immunological factors, stimulation used, cause of female infertility factors is homogenous (contains same value) in both positive and negative cases, which adds up no significance in classification hence these fields are removed. The samples or patient records are then split by 34% in the validation and 66% in training sets.
The training set contains 93,165 samples
The validation set contains 47,995 samples
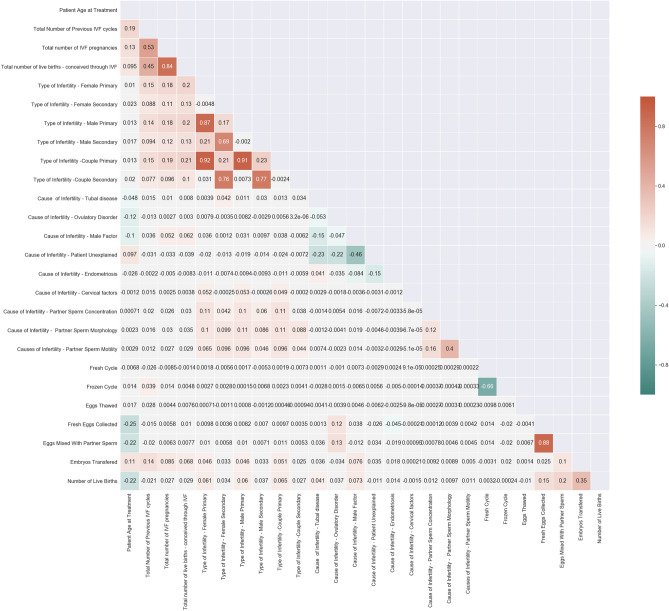
Data is normalized as it contains values which are distributed in an extensive range of integers. Features with very similar trends are also likely to carry very similar information. In this case, only one of them will suffice to feed the Machine Learning model. A correlation matrix constructed is shown in Fig. 1 contains 25 features that reveal the importance of each of the parameters on the model developed. Here we calculate the correlation coefficient between numerical and nominal columns as the Coefficient and the Pearson's chi-square46 value. Pairs of columns with a correlation coefficient higher than a threshold are reduced to only one.
Figure 1.
Correlation matrix of 25 features.
Model training
Overview
In this study, Machine Learning , Deep learning, and ensemble models are trained for the purpose. Machine Learning ML models such as Logistic Regression (LR), K nearest neighbor (KNN), Multi-Layer Perceptron (MLP), Decision Tree are used for training. A Deep learning model, especially a 1-D Neural Network with a sigmoid activation neuron at the output layer, is proposed. Ensemble learning is also employed to get a concrete decision from a list of Machine Learning models. Random Forest, AdaBoost, Voting classifier hard/soft are considered in ensemble learning techniques.
Two settings
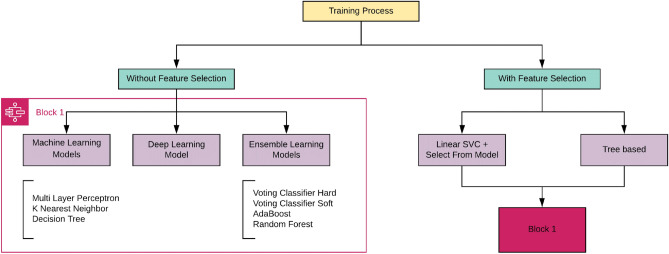
Training models in this study takes place in two settings, one With Feature Selection and the other Without Feature Selection. Feature selection techniques are applied to get the essential features from the dataset. In the Without Feature Selection setting, all the features (25 in total) are used in the training process. In the With Feature Selection setting, only certain features (important ones) are used in the training process based on the technique. This approach gives a comprehensive analysis of results where models have been trained on data with and without feature selection. A flowchart of the training protocols employed in this study is detailed in Fig. 2. The models trained under With feature selection & Without feature selection setting remain the same as explained in Fig. 2.
Figure 2.
Flowchart of the training process.
After taking suggestions from domain expert Dr. Bharti Bansal we selected the essential features, but statistical feature importance is employed later. Hence exploring different feature selection algorithms may help a lot in improving overall performance factors. Feature selection techniques selected are
Linear support vector classifier (Linear SVC) + Select From Model
Tree-based feature selection
Linear SVC + Select From Model
Linear models penalized with the L1 norm have sparse solutions: many of their estimated coefficients are zero. If the goal is to reduce the dimensionality of data and use another classifier, they can be used along with feature_selection.SelectFromModel in the scikit-learn to select the non-zero coefficients. Sparse estimators useful for this purpose are the Lasso for regression, Logistic Regression, and Linear SVC43. The sparse estimators used in this method are Logistic Regression, Decision Tree, Random Forest, K Nearest Neighbours classifier. The feature space reduces from 25 to 20 using this technique.
Linear SVC + Tree-based feature selection
Tree-based estimators such as Random Forests, once trained, the importance of each feature is computed with which we can filter and reduce the feature space. Every feature in random forests, while training is given with a Gini impurity or information gain/entropy using this measure, we calculate the feature importance44. After reducing the feature space, we can then train different estimators or classifiers on this new set. This study's sparse estimators are Logistic Regression, Decision Tree, Linear Discriminant Analysis, Random Forest, K Nearest Neighbours. The feature space reduces from 25 to 5 using this technique.
Deep learning: custom deep neural network
Along with the ML models, a Deep learning classifier (DL) architecture was trained on the same data. The neural network takes numerical values (array of size 25) as the input; hence it is 1-dimensional in the architectural perspective (1-D Model). The output layer contains one neuron with a sigmoid activation function to give a binary output (Birth occurrence or Not). The architecture contains a total of 9 dense layers, each neuron (in all dense layers) output values are passed through a Rectified Linear Unit (ReLU)47 activation function. In the first half of the DL classifier, neurons in each layer get increased precisely two times the previous layer; this is maintained uniform because of performance on this dataset. The second half follows a decreasing rate of two neurons per layer, making the last layer one. Adam optimizer48 is used for optimizing loss values while training the deep neural network. Due to its broader adoption in Deep learning applications and combining the AdaGrad and RMSProp algorithms' best properties to provide an optimization algorithm that can handle sparse gradients on noisy problems, Adam optimizer is chosen. Not just on the theory intuitions of Adam optimizer's performance, but also the performance on this dataset is checked across different optimizer algorithms such as Stochastic gradient descent, RMSProp, AdaGrad, and it is noticed that Adam optimizer performance is better than others.
The total number of epochs to train the DL classifier is 50. The model can overfit this dataset to prevent overfitting regularization techniques such as Dropouts and Batch Normalization49, Early stopping has been employed while training. As the number of neurons increases, the probability of noise generation will be higher among dense middle layers of the DL classifier, so 20% of dropouts are introduced after the middle-dense layer (512 units). Binary cross-entropy loss fits best for binary classification set up when trained on Deep learning techniques. Computing the gradient over the entire dataset is expensive, and hence batch size of 128 samples has been trained per epoch to get a reasonable approximation of the gradient. A glimpse of custom deep learning architecture is depicted in Fig. 3.
Figure 3.
Deep learning architecture, along with the training parameters explained.
Ensemble learning
Ensemble methods are the most famous learning algorithms in the ML domain because of its excellent performance. The whole crux of these methods is that it combines many ML algorithms to make an accurate decision. In this study, the following algorithms are trained.
Random Forest
AdaBoost
Voting classifier—soft/hard
Voting classifier
It is a wrapper of many classification models where the final decision is made by voting from individual models' predictions. For instance, if five binary classification models are trained and queried with an unseen sample, then the individual model's predictions are input for the voting system, declaring final prediction. Supplemental Fig. 1. illustrates a voting classifier.
The voting system works on two different strategies: Hard Voting, Soft Voting. Hard voting is also called the Majority voting in which the class that gets the highest number of votes from a set of individual models is selected42. If Nc is the number of votes for a class and y1, y2, y3, …., yn, are predictions of n different classifiers, then the hard-voting formula is given by Eq. (1).
| 1 |
Soft voting takes input as probability scores vector from individual class and sums it with all other classifiers later averages it42. The final output class will be the one that gets the maximum probability score. If p1, p2, …, pn are the probability scores of n different classifiers, then the formula for soft voting is given by Eq. (2).
| 2 |
The classifiers used for this voting classifier are Logistic Regression, Decision Tree, Linear Discriminant Analysis, Random Forest, and K Nearest Neighbours. In the next section, the model has been validated with experimental datasets.
Results and discussion
In this study, the TensorFlow library with Keras backend to train deep learning classifier and scikit-learn for the ML classifiers are utilized. The metrics compared in this study are F1-score, precision, recall, ROC AUC scores, and curves between various models. In this section, we demonstrate the results of trained models With and Without feature selection. In comparison tables, broader categories are displayed, such as ML-based, DL-based, Ensemble-based. Table 2 details the comparison between trained models Without feature selection.
Table 2.
Comparison between classification metrics for without feature selection models.
| Model | Precision (%) | Recall (%) | F1-score (%) | ROC AUC score (%) | |
|---|---|---|---|---|---|
| Machine learning models | Multi-layer Perceptron | 74 | 72 | 72.98 | 77.90 |
| K Nearest Neighbours | 71 | 71 | 71.00 | 77.60 | |
| Decision Tree | 76 | 76 | 76.00 | 83.30 | |
| Deep learning model | DL Classifier | 73 | 72 | 72.49 | 78.00 |
| Ensemble Learning models | Voting—hard classifier | 75 | 73 | 73.98 | 73.10 |
| Voting—soft classifier | 77 | 75 | 75.98 | 83.20 | |
| Random forest | 77 | 76 | 76.49 | 84.60 | |
| AdaBoost | 74 | 72 | 72.98 | 77.40 |
Results for without feature selection setting
Table 2 explains that the ensemble learning models category results in better classification performance in the recall, F1-score, and ROC AUC scores. Random Forest scores the highest F1-score of 76.49%. The recall value achieved by random forest is noticeable among other trained models, i.e., 76%. Figure 4a Illustrates the ROC AUC curves of models trained without feature selection. The Random Forest has the highest AUC score of 84.6%.
Figure 4.
(a) ROC Curve Analysis of models trained without feature selection. (b) ROC Curve Analysis of different models in With Feature Selection setting, i.e., Linear SVC + Select From Model method. (c) ROC Curve Analysis of models trained with feature selection method, i.e., Linear SVC + Extra Trees classifier.
Results for with feature selection setting
Method: linear SVC + SelectFromModel
Table 3 describes that the ensemble learning models category has a better classification. The multi-layer perceptron and AdaBoost has the highest F1-score of 72.98%. AdaBoost, i.e., 77.60%, achieve the maximum ROC AUC score.
Table 3.
Comparison between classification metrics of different models in With Feature Selection setting, i.e., Linear SVC + Select From Model.
| Model | Precision (%) | Recall (%) | F1-score (%) | ROC AUC score (%) | |
|---|---|---|---|---|---|
| Machine Learning models | Multi-layer Perceptron | 74 | 72 | 72.98 | 77.50 |
| K Nearest Neighbours | 67 | 66 | 66.49 | 72.20 | |
| Decision Tree | 67 | 67 | 67.00 | 70.10 | |
| Deep learning model | DL Classifier | 74 | 72 | 72.98 | 77.40 |
| Ensemble Learning models | Voting—Hard classifier | 73 | 71 | 71.98 | 71.70 |
| Voting—Soft classifier | 71 | 70 | 70.49 | 75.70 | |
| Random Forest | 69 | 68 | 68.49 | 74.00 | |
| AdaBoost | 74 | 72 | 72.98 | 77.60 |
Figure 4b exhibits the ROC AUC curves of models trained in With Feature Selection setting, i.e., Linear SVC + Select From Model. In this method, AdaBoost has the highest AUC score of 77.60%. When these results are compared with previous results in Table 2, there is an impact of the Feature selection method, which decreased the overall performance in metrics such as ROC AUC scores, F1-scores, and recall.
Method: linear SVC + Tree-based feature selection
This method has used a Tree-Based feature extractor as Extra Trees Classifier, which is slightly different from random forests. Extra Trees classifier is different because it selects the random split to divide a parent node into two random child nodes.
Table 4 represents that again the Machine Learning based classification is better. 73.46% is the highest F1 score achieved by this feature selection method, which is less than the previous method. The maximum recall value achieved here is 72%, which is the same as the previous method. However, ROC AUC scores have been increased from the previous method except for deep learning classifiers and AdaBoost. Figure 4c. Portrays the ROC AUC curves of models trained in With Feature Selection setting, i.e., Linear SVC + Extra Tree classifier. AdaBoost, MLP, DL classifiers have the highest AUC score.
Table 4.
Comparison between classification metrics of different models in With Feature Selection setting, i.e., Linear SVC + Extra Trees classifier.
| Model | Precision (%) | Recall (%) | F1-score (%) | ROC AUC score (%) | |
|---|---|---|---|---|---|
| Machine Learning models | Multi-layer Perceptron | 75 | 72 | 73.46 | 77.30 |
| K Nearest Neighbours | 66 | 66 | 66.00 | 72.60 | |
| Decision Tree | 70 | 69 | 69.49 | 73.80 | |
| Deep learning model | DL Classifier | 75 | 71 | 72.94 | 77.30 |
| Ensemble learning models | Voting—hard classifier | 73 | 71 | 71.98 | 71.00 |
| Voting—soft classifier | 72 | 70 | 70.98 | 76.20 | |
| Random Forest | 71 | 70 | 70.49 | 74.90 | |
| AdaBoost | 74 | 71 | 72.46 | 77.30 |
When results from the above three methods are compared, it is clear and advisable that regular features without using any feature selection method, i.e., especially Random Forests (Ensemble Learning method), have better accuracy of 76.49% and an AUC score of 84.6%. Therefore, it is preferable to use this model in production for real-time results.
Conclusion
In clinics, medical practitioners can provide counseling about live-birth based on their experience or their success rate of the fertility center, which can be inappropriate in some cases. This study will help both patients and medical practitioners make a concrete decision that depends on the tool predicting successful or unsuccessful IVF treatment based on a patient's natural measurable predictors. This tool will provide counseling to couples about their chances of getting live-birth to emotionally get prepared before going through costly and cumbersome IVF treatment. The Random Forest model without feature selection has shown the best result that achieved an AUC score of 84.60% and 76.49% F1-score compared with other models. However, it is not suggested to depend solely on this tool currently for decision making, as the data is received from a single source, so it is not generalized to all populations. The models were trained on limited factors, while several important factors, such as consumption of alcohol, smoking, caffeine consumption, hypertension, and other lifestyle factors that have a significant impact on predicting pregnancy, have not been considered due to the dataset's limitation.
The scope of the future works are that the data can be collected from various IVF clinics in different geographical locations to contain information on many races across the globe. Few parameters on individuals' lifestyle should be taken into consideration as these details indirectly affect fertility. AI performances can be improved if diverse data from various races and age groups is collected. A study can also be made regarding successful fertility that emphasizes each feature's importance in IVF. Different feature selection and dimensionality reduction methods can be used to improve model performances.
Supplementary information
Acknowledgements
We would like to thank Dr. Bharti Bansal (MS - Obstetrics and Gynaecology, - JawaharLal Nehru Medical College, Aligarh—2006) for guiding us in selecting and understanding the important features in IVF to make accurate predictions of live-birth occurence for the AI classifiers.
Author contributions
A.G.: Data collection, Computational Methodology, Creating model, validation, generating the results, writing the initial draft. M.K.: Conducting research and investigation process, AI computing, Deep learning model creation, writing, and editing the draft. K.P.R.A.: Conceptualization, Editing and Writing the final manuscript, and overall supervision.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-76928-z.
References
- 1.Gurunath S, Pandian Z, Anderson AR, Bhattacharya S. Defining infertility—a systematic review of prevalence studies. Hum. Reprod. Update. 2011;17:575–588. doi: 10.1093/humupd/dmr015. [DOI] [PubMed] [Google Scholar]
- 2.Loendersloot VL, Repping S, Bossuyt PMM, Veen FVD, Wely MV. Prediction models in vitro fertilization; where are we? A mini review. J. Adv. Res. 2014;5:295–301. doi: 10.1016/j.jare.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarinara A, et al. Models predicting success of infertility treatment: a systematic review. J. Reprod. Infertil. 2016;17:68–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper SG. An analysis of the cost of infertility treatment. Am. J. Public Health. 1986;76:1018–1019. doi: 10.2105/ajph.76.8.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do patients discontinue fertility treatments? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum. Reprod. Update. 2012;18:652–669. doi: 10.1093/humupd/dms031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranjbar F, Moghadam ZB, Borimnejad L, Saeed RG, Akhondi MM. Experiences of infertile women seeking assisted pregnancy in iran: a qualitative study. J. Reprod. Infertil. 2015;16:221–228. [PMC free article] [PubMed] [Google Scholar]
- 7.Smola A, Vishwanathan SVN. Introduction to Machine Learning. Cambridge: Cambridge University Press; 2008. pp. 3–9. [Google Scholar]
- 8.Goodfellow I, et al. Deep Learning. Cambridge: The MIT Press; 2016. pp. 151–161. [Google Scholar]
- 9.Deo RC. Machine learning in medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handelman GS, et al. Machine learning and the future of medicine. J. Intern Med. 2018;284:603–619. doi: 10.1111/joim.12822. [DOI] [PubMed] [Google Scholar]
- 11.Rahimian F, et al. Predicting the risk of emergency admission with machine learning: development and validation using linked electronic health records. PLoS Med. 2018;11:e1002695. doi: 10.1371/journal.pmed.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Fut. Healthc. J. 2019;6(2):94–98. doi: 10.7861/futurehosp.6-2-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blank C, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine learning perspective. Fertil. Steril. 2019;111:318–326. doi: 10.1016/j.fertnstert.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Barash O, Ivani K, Weckstein L, Hinckley M. High accuracy Machine Learning predictive model for embryo selection in IVF PGT cycles with single embryo transfer. Fertil. Steril. 2018;110:e372. doi: 10.1016/j.fertnstert.2018.07.1038. [DOI] [Google Scholar]
- 15.Vaughan D, et al. The application of machine learning methods to evaluate predictors of live-birth in programmed thaw cycles. Fertil. Steril. 2019;112:e273. doi: 10.1016/j.fertnstert.2019.07.808. [DOI] [Google Scholar]
- 16.John R. An application of machine learning in IVF: comparing the accuracy of classification algorithms for the prediction of twins. Gynecol. Obstet. 2019;9:497. doi: 10.4172/2161-0932.1000497. [DOI] [Google Scholar]
- 17.Tran D, Cooke S, Illingworth PJ, Gardner DK. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Hum. Reprod. 2019;34:1011–1018. doi: 10.1093/humrep/dez064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosravi P, et al. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. NPJ Digit Med. 2019;2:21. doi: 10.1038/s41746-019-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLernon DJ, Steyerberg E, Velde ERT, Lee AJ, Bhattacharya S. Predicting the chances of a live-birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data of 113873 women. BMJ. 2016;355:i5735. doi: 10.1136/bmj.i5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLernon DJ, Steyerberg E, Velde ERT, Lee AJ, Bhattacharya S. An improvement in the method used to assess discriminatory ability when predicting the chances of a live-birth after one or more complete cycles of in vitro fertilisation. BMJ. 2018;363:k3598. doi: 10.1136/bmj.k3598. [DOI] [PubMed] [Google Scholar]
- 21.Hassan R, Al-Insaif S, Hossain MI, Kamruzzaman J. A Machine Learning approach for prediction of pregnancy outcome following IVF treatment. Springer. 2020;32:2283–2297. doi: 10.1007/s00521-018-3693-9. [DOI] [Google Scholar]
- 22.Guvenir AH, et al. Estimating the chances of success in IVF treatment using a ranking algorithm. Springer. 2015;53:911–920. doi: 10.1007/s11517-015-1299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann SJ, et al. The application of neural networks in predicting the outcome of in-vitro fertilization. Hum. Reprod. 1997;12(7):1454–1457. doi: 10.1093/humrep/12.7.1454. [DOI] [PubMed] [Google Scholar]
- 24.Babitha. A survey on the Machine Learning techniques used in IVF treatment to improve the success rate. 7 (2019).
- 25.Qiu J, Li P, Dong M, et al. Personalized prediction of live-birth prior to the first in vitro fertilization treatment: a machine learning method. J. Transl. Med. 2019;17:317. doi: 10.1186/s12967-019-2062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olmedo SB, Chillik C, Kopelman S. Definition and causes of infertility. Reprod. Biomed. Online. 2001;2:41–53. doi: 10.1016/s1472-6483(10)62187-6. [DOI] [PubMed] [Google Scholar]
- 27.Cedars M, Jaffe RB. Infertility and women. J. Clin. Endocrinol. Metab. 2005;90:4. doi: 10.1210/jcem.90.4.9997. [DOI] [Google Scholar]
- 28.Masoumi ZS, et al. An epidemiologic survey on the causes of infertility in patients referred to infertility center in Fatemieh Hospital in Hamadan. Iran J. Reprod. Med. 2015;13:513–516. [PMC free article] [PubMed] [Google Scholar]
- 29.Templeton A, Morris KJ, Parslow W. Factors that affect outcome of in-vitro fertilisation. Lancet. 1996;348:1402–1406. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- 30.Bhattarcharya S, et al. Female infertility. Clin. Evid. 2010;11:819. [Google Scholar]
- 31.Lackner J, et al. Constant decline in sperm concentration in infertile males in an urban population: experience over 18 years. Fertil. Steril. 2005;84:1657–1661. doi: 10.1016/j.fertnstert.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 32.Cooper TG, et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 33.Human Fertilisation & Embryology Authority. https://www.hfea.gov.uk/media/2667/ar-2015-2016-xlsb.xlsb (2016).
- 34.Dreiseitl S, Machado LO. Logistic regression and artificial neural network classification models: a methodology review. J. Biomed. Inform. 2002;35:5–6. doi: 10.1016/S1532-0464(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 35.T. Cover & P. Hart. Nearest neighbor pattern classification. IEEE Trans. Inform. Theory, 13, 21–27. Doi: 10.1109/TIT.1967.1053964 (1967)
- 36.Almeida LB. Multilayer Perceptrons. Handbook of Neural Computation. Oxford: Oxford University Press; 1997. [Google Scholar]
- 37.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and Regression Trees. Boca Raton: CRC Press; 1984. [Google Scholar]
- 38.Anderson JA, et al. Talking Nets: An Oral History of Neural Networks. Cambridge: The MIT Press; 2000. [Google Scholar]
- 39.Dietterich, T. G. Ensemble methods in Machine Learning. In International Workshop on Multiple Classifier Systems (Springer, Berlin, 2000).
- 40.Breiman L. Random forests. Springer. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 41.Freund, Y. & Schapire, R. E. Experiments with a new boosting algorithm. In International Conference on International Conference on Machine Learning (ICML'96) 148–156 (1996)
- 42.Raschka S. Python Machine Learning. Birmingham: Packt Publishing; 2015. [Google Scholar]
- 43.Moon M, Nakai K. Stable feature selection based on the ensemble L 1-norm support vector machine for biomarker discovery. BMC Genom. 2016;17:1026. doi: 10.1186/s12864-016-3320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uddin, M. T. & Uddiny, M. A. Human activity recognition from wearable sensors using extremely randomized trees. In International Conference on Electrical Engineering and Information Communication Technology (ICEEICT), Dhaka, 1–6. 10.1109/ICEEICT.2015.7307384 (2015)
- 45.Ahemmed B, et al. Outcomes and recommendations of an Indian expert panel for improved practice in controlled ovarian stimulation for assisted reproductive technology. Int. J. Reprod. Med. 2017;2017:9451235. doi: 10.1155/2017/9451235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakesh R, Richa S. Chi-square test and its application in hypothesis testing. J. Pract. Cardiovasc. Sci. 2015;1:69–71. doi: 10.4103/2395-5414.157577. [DOI] [Google Scholar]
- 47.Vinod N., & Geoffrey, E. Hinton. Rectified linear units improve restricted boltzmann machines. In Proceedings of the 27th International Conference on International Conference on Machine Learning (ICML). Omnipress, Madison, WI, USA, 807–814 (2017)
- 48.Kingma, DP, & Ba, J. Adam: A Method for Stochastic Optimization. CoRR, https://arxiv.org/abs/1412.6980 (2015)
- 49.Ioffe, S., & Szegedy, C. Batch Normalization: Accelerating Deep Network Training by Reducing Internal Covariate Shift. https://arxiv.org/abs/1502.03167 (2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.