Abstract
Study Design:
Longitudinal Comparative Cohort
Objective:
The purpose of this study is to report on the cost-effectiveness of surgical versus nonsurgical treatment for Adult Symptomatic Lumbar Scoliosis (ASLS) using the as-treated data and provide a comparison to previously reported intent-to-treat (ITT) analysis.
Summary of Background Data:
Adult spinal deformity is a relatively prevalent condition for which surgical treatment has become increasingly common but concerns surrounding complications, revision rates and cost-effectiveness remain unresolved. Of these issues, cost-effectiveness is perhaps the most difficult to quantify as the requisite data is difficult to obtain. The purpose of this study is to report on the cost-effectiveness of surgical versus nonsurgical treatment for Adult Symptomatic Lumbar Scoliosis (ASLS) using the as-treated data and provide a comparison to previously reported intent-to-treat (ITT) analysis.
Methods:
Patients with at least five-year follow-up data within the same treatment arm were included. Data collected every three months included use of nonoperative modalities, medications and employment status. Costs for surgeries and non-operative modalities were determined using Medicare Allowable rates. Medication costs were determined using the RedBook and indirect costs were calculated based on reported employment status and income. Quality Adjusted Life Years (QALY) was determined using the SF-6D.
Results:
Of 226 patients, 195 patients (73 Non-op, 122 Op) met inclusion criteria. At five years, 29 (24%) patients in the Op group had a revision surgery of whom two had two revisions and one had three revisions. The cumulative cost for the Op group was $111,451 with a cumulative QALY gain of 2.3. The cumulative cost for the Non-Op group was $29,124 with a cumulative QALY gain of 0.4. This results in an ICER of $44,033 in favor of Op treatment.
Conclusion:
This as-treated cost effectiveness analysis demonstrates that surgical treatment for adult lumbar scoliosis becomes favorable at year-three, one year earlier than suggested by a previous intent-to-treat analysis.
Level of Evidence:
II
Keywords: Lumbar Scoliosis, Operative vs Non-Operative, Cost-effectiveness, As treated Analysis
Introduction
Adult spinal deformity is a relatively prevalent condition for which surgical treatment has become increasingly common [1]. Multiple studies have shown the potential for favorable outcomes in well-selected patients [2,3], but concerns surrounding complications, revision rates and cost-effectiveness remain unresolved [4–6]. Of these issues, cost-effectiveness is perhaps the most difficult to quantify as the requisite data is difficult to obtain [7,8].
Cost-effectiveness analysis (CEA) requires long-term data on well-matched patients undergoing both surgical and nonsurgical treatment [9–11]. In particular, comprehensive data on nonsurgical treatment, whether as an alternative to surgery or as an adjunct after surgery, is rarely available. Efforts to circumvent the difficulty and expense of long-term data collection using modeled data have often proven inaccurate [5, 8, 12, 13]. Cost-effectiveness is most often reported as cost/QALY gained (Quality Adjusted Life Years), a valuable but simplified metric that can be generated from high-quality datasets [14]. As the equation implies, achieving a threshold of cost-effectiveness becomes progressively difficult as the cost of the procedure increases. A more complete assessment of cost-effectiveness is the Incremental Cost Effectiveness Ratio (ICER), which reflects the comparative cost-effectiveness of an intervention versus the most likely alternative treatment [15]. ICER evaluation relies upon comparable surgical and nonsurgical cohorts, best generated in a randomized controlled trial (RCT). However, previous RCTs evaluating surgical versus nonsurgical treatments for spine pathologies have had high rates of cross-over. This analysis better reflects relative value, as opposed to cost/QALY for one intervention in isolation.
The purpose of this study is to report on the cost-effectiveness of surgical versus nonsurgical treatment for adult symptomatic lumbar scoliosis. The assessment is based upon an ICER evaluation from the NIH supported Adult Symptomatic Lumbar Scoliosis (ASLS) trial. This study analyzes the as-treated data and also provides a comparison to previously reported intent-to-treat (ITT) analysis [16].
Methods
This is a secondary analysis of prospective data collected from subjects enrolled in nine centers in North America. The primary study evaluated operative and nonoperative treatments in patients with ASLS and included randomized and observational arms [3]. The current study included patients between 40 and 80 years old with lumbar scoliosis with coronal Cobb ≥ 30°, and either an Oswestry Disability Index (ODI) [17] score of 20 or greater, or a Scoliosis Research Society-22 (SRS-22) [18] Pain, Function or Appearance score less than 4.0, and no prior fusion surgery. Exclusion criteria were the presence of medical comorbidities that precluded surgery, high-grade (≥3) spondylolisthesis, prior thoracic or lumbar fusion, prior multilevel (≥3) thoracolumbar decompression, severe osteoporosis (femoral neck t-score ≥−3.0), neuromuscular scoliosis and presence of congenital lumbar spine anomalies. Subjects were enrolled from 2010 to 2014. Funding was provided by the National Institutes of Health through an RO1 grant: A Multi-Center Prospective Study of Quality of Life in Adult Scoliosis (R01AR055176–01A2). Institutional review board approval was obtained at each participating center prior to subject enrollment. Institutional review board approval was also obtained for this secondary analysis.
The study database was queried for patients undergoing Operative (Op) or nonoperative (NonOp) treatment. Surgical approach, technique and levels fused in the Op cohort were under the discretion of the treating surgeon. Data collected every three months included frequency of use of nonoperative modalities (physical therapy, chiropractor, pain management visits and epidural steroid injections), medication use (NSAIDs, opioids, muscle relaxants) and employment status. Health-related quality-of-life scores (HRQOLs) collected included the ODI [17], the SRS-22R [18] and the SF-12 [19].
The cumulative incremental cost-effectiveness ratio (ICER) at each year of follow-up was determined. ICER is the difference in cost between two possible interventions, in this case Non-operative versus Operative treatment for ASLS, divided by the difference in their effect. Total costs included all surgical and nonsurgical costs in both cohorts. Surgical costs for the index and revision surgeries within five years and direct costs for non-operative care were determined using Medicare Allowable rates [20]. Medication costs were determined using the lowest price quoted [21] and indirect costs were determined based on reported employment status and income. Treatment effectiveness in terms of Quality Adjusted Life Years (QALY) was determined using the Short Form – 6 Dimensions (SF6D) [22] derived from the SF-12. Although determination of a specific dollar value threshold for appropriate medical treatment is controversial, interventions with a cost per QALY gained (cost/QALY) between $50,000 and $100,000, or less, are generally considered cost effective [23–26]. A treatment is dominant if it costs less and is more effective compared to the alternative treatment [15].
As part of the primary RCT study design, patients in the randomized and observational group were allowed to cross over six months after enrollment [3]. For this secondary, as-treated analysis, only patients who had five-year follow-up data while remaining in the same treatment arm were included. For a patient who crossed over, all costs and benefits prior to the cross-over were censored and not included in the analysis.
Baseline characteristics of the Op and NonOp cohorts were compared using unpaired independent t-tests for continuous variables and Fisher’s exact test for categorical variables. A p-value threshold of 0.05 was used in order for the difference to be statistically significant.
TreeAge Pro was used to perform the cost-effectiveness analysis to take into account the incidence of index and revision surgeries in both groups, as patients were allowed to cross-over to either arm during the study. A decision tree was used instead of using mean costs and mean QALY gains for each cohort. Using group means does not take into account the difference in the number of patients undergoing primary as well as revision surgeries in each cohort. Subjects undergoing revision surgery would incur higher costs with less QALY gain. This higher cost and less QALY gain cannot be applied equally to each subject in the cohort. The decision tree model allows for apportioning of these higher costs and lower QALY gains only to the subjects that have revision surgery [14, 27].
Funding was received from the National Institutes of Health, the Scoliosis Research Society and International Spine Study Group Foundation.
Results
286 were enrolled in the overall study, 195 met inclusion criteria with five year follow-up data within the same treatment arm at the time of analysis. Twenty-two subjects withdrew from the study and 69 subjects had not completed five year follow-up in the same treatment arm. There were 73 patients who remained in the NonOp group and 122 patients in the Op group for five years with available data. The proportion of patients from the Observational arm was similar between the Op (60, 82%) and NonOp groups (100, 82%, p=0.968). Four (31%) of the 13 subjects in the Randomized NonOp group had crossed-over from the Op group and 8 (36%) of the 22 subjects in the Randomized Op group had crossed-over from the Non-Op group. The Op and NonOp groups were similar in age, sex distribution and body mass index (Table 1). There were 3 (4%) smokers in the NonOp group and 2(1%) in the Op group (p=0.567). The baseline HRQOL scores including the ODI (=0.001), SF-6D (p=0.007), SRS-22R for Function (p<0.000), Self-Image (p<0.000) and Subscore (p<0.000), were worse in patients in the Op group compared to NonOp group. The major Cobb angle was significantly smaller in the NonOp group (50.2°), compared to the Op group (55.5°, p=0.007).All patients in the Op group had a posterior decompression and instrumented fusion, 3 patients had an additional anterior fusion, 52 (42%) had a transforaminal interbody fusion, one patient had a Vertebral Column Resection, 3 had a Pedicle Subtraction Osteotomy and 82 had fixation to the pelvis (67%).
Table 1.
Summary of Baseline Characteristics of Subjects in the NonOperative and Operative Cohorts
| NonOperative | Operative | ||
|---|---|---|---|
| N | 73 | 122 | |
| Arm, N (%) | 0.968 | ||
| Observational | 60 (82%) | 100 (82%) | |
| Randomized | 13 (18%) | 22 (18%) | |
| Crossed-over | 4 (31%) | 8 (36%) | |
| Age, Mean (SD) | 59.5 (9.80) | 58.7 (8.5) | 0.567 |
| Females, N (%) | 67 (92%) | 111 (91%) | 0.160 |
| Smoking Status, N (%) | 0.049 | ||
| Never Smoker | 46 (63%) | 80 (66%) | |
| Former Smoker | 24 (33%) | 40 (33%) | |
| Smoker | 3 (4%) | 2 (1%) | |
| Short Form-6D, Mean (SD) | 0.5 (0.2) | 0.4 (0.2) | 0.007 |
| Oswestry Disability Index | 28.5 (14.1) | 36.1 (14.9) | 0.001 |
| Scoliosis Research Society 22R | |||
| Pain | 3.0 (0.6) | 2.85 (0.8) | 0.078 |
| Function | 3.5 (0.6) | 3.17 (0.7) | <0.000 |
| Appearance | 3.3 (0.7) | 2.75 (0.7) | <0.000 |
| Mental Health | 3.8 (0.7) | 3.72 (0.8) | 0.333 |
| Subscore | 3.4 (0.5) | 3.13 (0.5) | <0.000 |
| Coronal Cobb Magnitude, °, Mean (SD) | 50.2 (11.6) | 55.48 (15.1) | 0.007 |
| Coronal Balance, mm, Mean (SD) | 16.3 (10.4) | 22.26 (19.8) | 0.006 |
| Sagittal Balance, mm, Mean (SD) | 18.55 (36.8) | 26.00 (35.5) | 0.167 |
p-value threshold of 0.05 considered statistically significant
Of the 122 patients in the Op group, 9 patients had a revision within the first year after the index surgery. In addition to these revision surgeries, one patient had a removal of a spinous process, one had a screw repositioning and one had an iliac screw removal. The cumulative cost for the Op group was $74,050 with a QALY gain of 0.1. The cumulative cost in the first year for the Non-Op group was $6,353 with a QALY gain of 0.0. The ICER was $ 704,897 per QALY gained (Figure 1) in favor of Non-Op treatment but did not reach the Willingness-to-Pay (WTP) threshold of $100,000.
Figure 1.
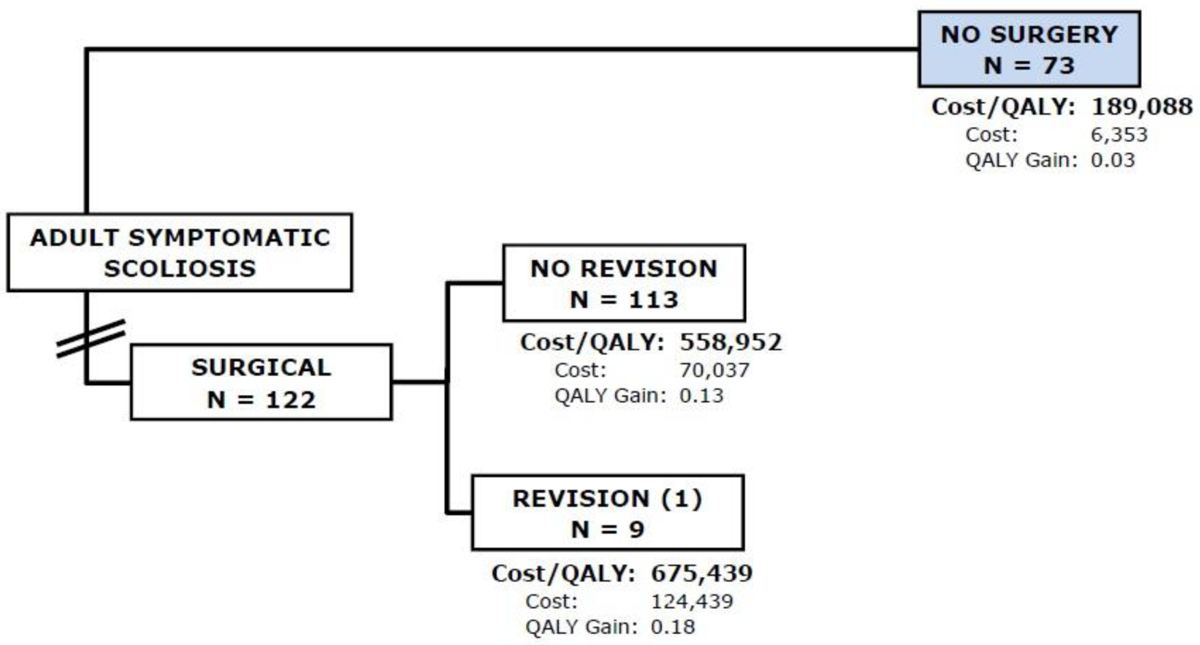
Flowchart of subjects in the first year of follow-up. Of the 122 subjects in the Op group, 9 (7%) subjects had a revision surgery within the first year after their index surgery. The cumulative cost for the Op group was $74,050 with a QALY gain of 0.1. The cumulative cost in the first year for the Non-Op group was $6,353 with a QALY gain of 0.0. The ICER was $704,897 per QALY gained.
By the second year, 16 (13%) of patients in the Op group had a revision surgery. The cumulative cost over two years for the Non-Op group was $15,101 with a QALY gain of 0.1. The cumulative cost over two years for the Op group was $79,325 with a QALY gain of 0.4. The ICER was $196,721 per QALY gained (Figure 2) in favor of Non-Op treatment but still not reaching the WTP threshold of $100,000.
Figure 2.
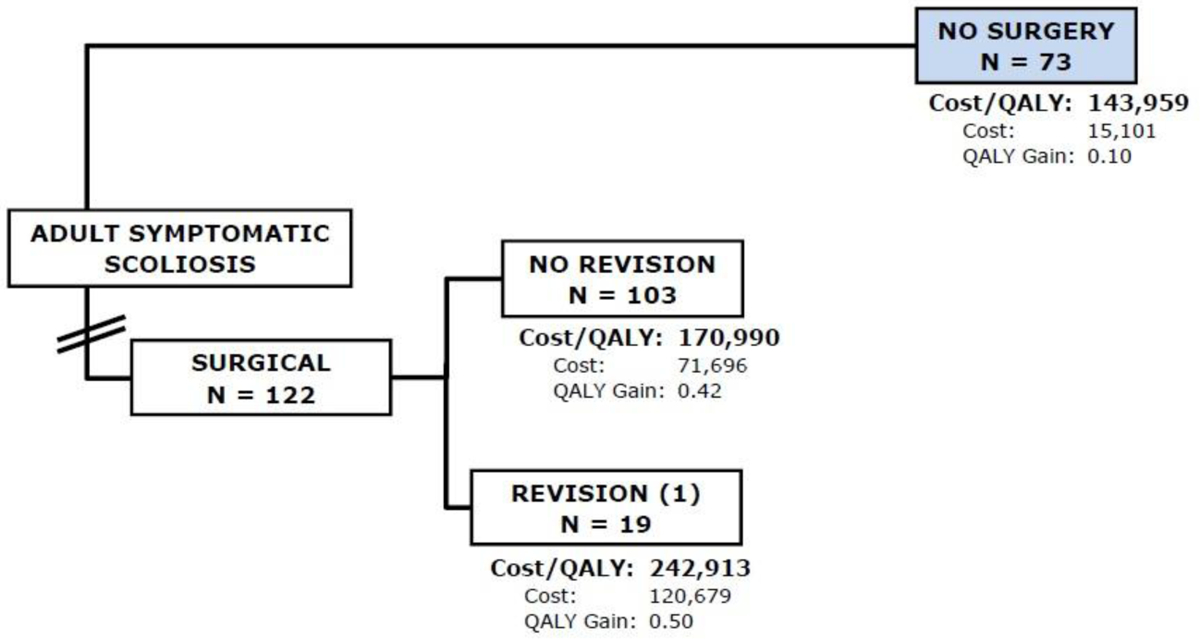
Flowchart of subjects in the second year of follow-up. Sixteen patients (13%) patients in the Op group had a revision surgery. The cumulative cost over two years for the Op group was $79,325 with a QALY gain of 0.4. The cumulative cost over two years for the Non-Op group was $15,101 with a QALY gain of 0.1. The ICER was $196,721 per QALY gained
By the third year, 22 patients (18%) in the Op group had a revision surgery with one patient having had two revisions, with a cumulative cost of $85,299 and QALY gain of 0.9. In the Non-Op group, the cumulative cost of $19,195 and QALY gain of 0.2. This results in an ICER of $93,405 per QALY gained (Figure 3) in favor of Op treatment, reaching the WTP threshold of $100,000.
Figure 3.
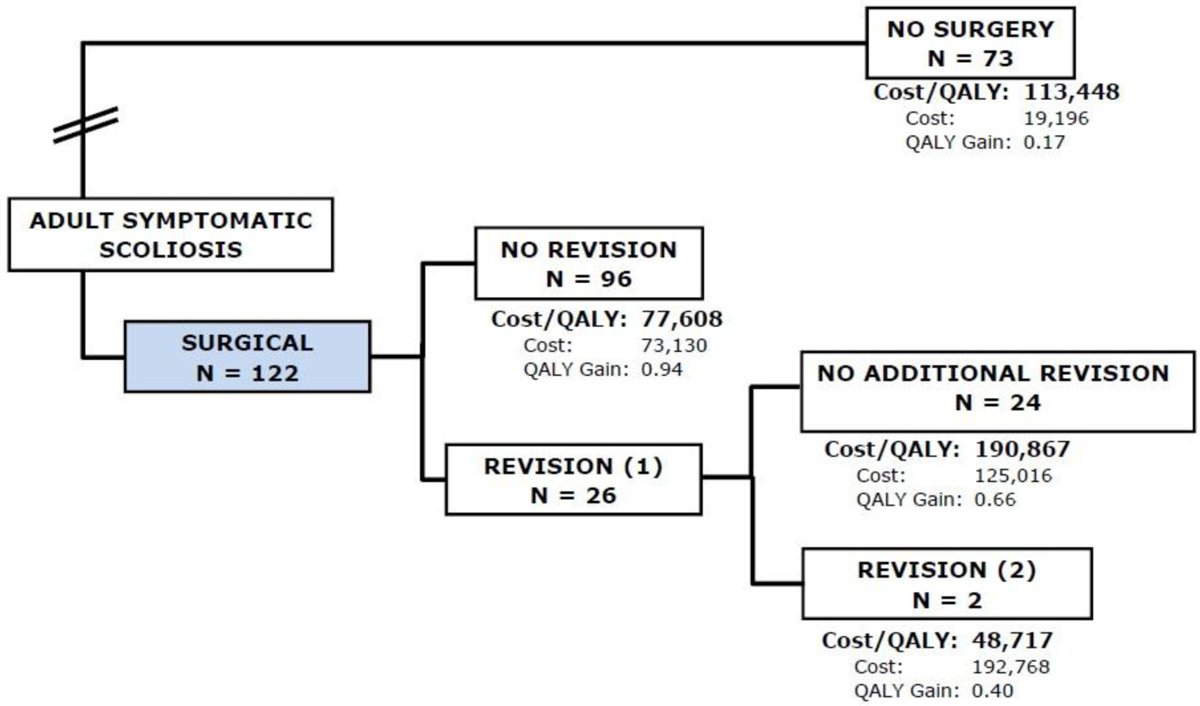
Flowchart of subjects in the third year of follow-up. 22 patients (18%) in the Op group had a revision surgery with one patient having had two revisions, with a cumulative cost of $85,299 and QALY gain of 0.9. In the Non-Op group, the cumulative cost was $19,195 and QALY gain of 0.2. This results in an ICER of $93,405 per QALY gained
By the fourth year, 25 (22%) patients had one revision and two patients had two revisions, with a cumulative cost of $95,572 and QALY gain of 1.5. The cumulative cost for the NonOp group was $22,953 with a QALY gain of 0.3. This results in an ICER of $57,309 per QALY gained in favor of Op treatment (Figure 4).
Figure 4.
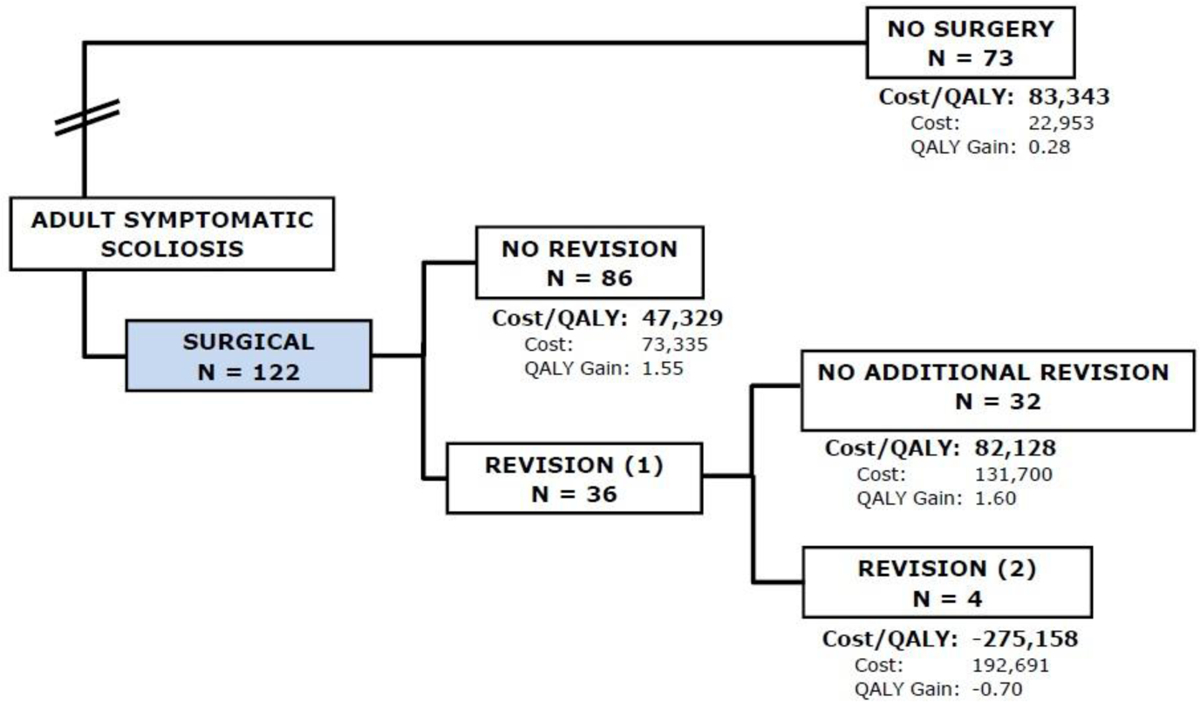
Flowchart of subjects in the fourth year of follow-up, 25 (22%) patients had one revision and two patients had two revisions, with a cumulative cost of $95,572 and QALY gain of 1.5. The cumulative cost for the NonOp group was $22,953 with a QALY gain of 0.3. This results in an ICER of $57,309 per QALY gained.
At five years, 29 (24%) of patients in the Op group had a revision surgery of whom two had two revisions and one had three revisions. The cumulative cost, including direct and indirect costs for the Op group at five years was $111,451 with a cumulative QALY gain of 2.3. The cumulative cost for the Non-Op group was $29,124 with a cumulative QALY gain of 0.4. This results in an ICER of $44,033 per QALY gained (Figure 5) in favor of Op treatment.
Figure 5.
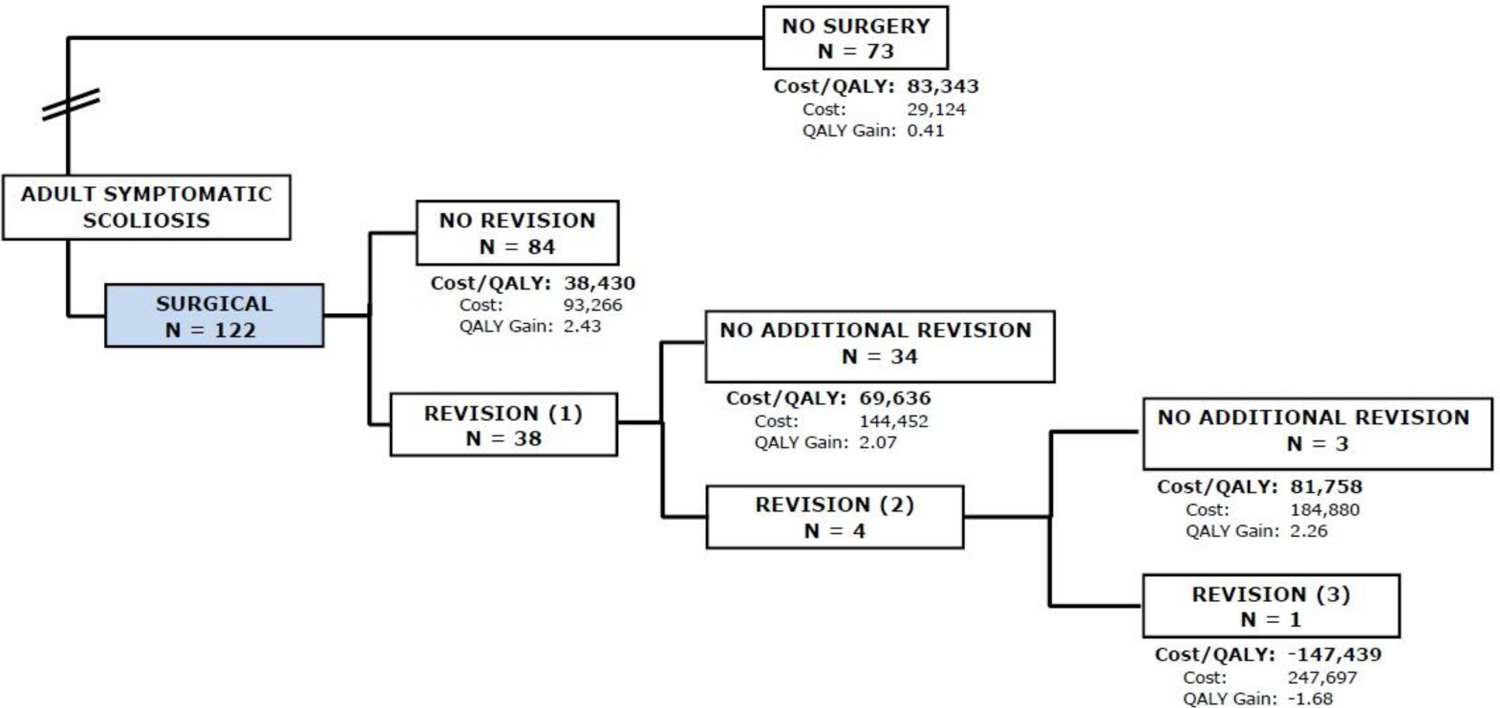
Flowchart of subjects in the fifth year of follow-up, 29 (24%) of patients in the Op group had a revision surgery of whom two had two revisions and one had three revisions. The cumulative cost for the Op group at five years was $111,451 with a cumulative QALY gain of 2.3. The cumulative cost for the Non-Op group was $29,124 with a cumulative QALY gain of 0.4. This results in an ICER of $44,033 per QALY gained.
Discussion
Surgical treatment of adult scoliosis is complex and may utilize substantial resources [28]. It is therefore important to determine whether surgical treatment of adult scoliosis can be both efficacious and cost-effective. Prior studies have shown that single-level lumbar fusion may be cost-effective, but only over a 4–5 year time horizon [11, 29], raising questions as to the economic viability of more costly adult deformity procedures. This analysis of as-treated data from the NIH supported Adult Symptomatic Lumbar Scoliosis (ASLS) trial suggests cost-effectiveness favoring surgical versus nonsurgical treatment in well-selected patients beginning at year-3 after surgery. This timeline is more favorable than was reported in a previous intent-to-treat analysis [16] study. The intent-to treat analysis favored surgical over nonsurgical treatment four years after patient enrollment and had smaller ICERs. While there is no uniformly accepted willingness-to-pay threshold , previously published parameters range from $50,000 [24–26] - $150,000 ICER/QALY gained. ICERs generated in this study fell within that range (ICER/QALY gain = $93,000; Year-3/$57,000 Year-4/$44,000 Year-5).
As opposed to previous cost-effectiveness studies for adult scoliosis [5,8,12], the ASLS trial did demonstrate some QALY improvement with nonsurgical treatment [3, 16]. However, the benefits of nonsurgical treatment were not sufficient to generate a favorable ICER, even at the substantially lower associated cost. Revision procedures were, as expected, a key determinant of cost-effectiveness. Perhaps surprisingly, even patients undergoing one revision surgery remained cost-effective as compared to nonsurgical treatment, although surgical patients requiring revision only became favorable at year-4 post-op, and the relative benefit was smaller. Four patients required more than one revision surgery and their cost/QALY was prohibitive in the year following the revision procedure.
Prior cost-effectiveness studies for adult spinal deformity have focused on deriving cost/QALY estimates in surgically treated patients [5, 7, 8]. Terran et.al. based their analysis on 541 patients with 2-year surgical and clinical outcomes data [8]. Cost data was derived from Medicare reimbursement rates and cost/QALY was then modeled to year-five based on the 2-year data. They calculated that at five years post-op 40.7% of patients would fall within a willingness to pay threshold of $100,000/QALY gained. McCarthy et.al. report on a single-center experience in 120 patients with 3-year follow-up [5]. The strength of this study was the availability of actual hospital costs, as well as HRQOL measures in the surgical cohort. The authors then modeled HRQOL outcomes for nonsurgical patients and estimated ICERs to a 10-year horizon. This analysis projected an ICER of $80,387/QALY gained at ten years post-op, which they compare favorably to a $140,000 per QALY gained World Health Organization proposed cost-effectiveness threshold [30]. McCarthy’s analysis also assumes deterioration in HRQOL for non-surgically treated adult deformity patients, which is contrary to the findings of the ASLS trial.
Given the particular concern with revision rate after adult deformity surgery, Raman et.al. compared cost/QALY gained for surgical treatment in primary versus revision adult spinal deformity cases [7]. The authors report on 2-year cost and clinical outcome data for 56 primary cases and 63 revision cases. They document a Cost/QALY gained of $197,809 for primary surgery and $129,950 for revision surgery cases. They conclude that revision adult deformity surgery, while technically challenging, was cost effective based on a threshold of three times the U.S. gross domestic product per capita ($154,458/QALY gained).
The present cost-effectiveness analysis is based upon high quality prospectively collected data, which is beneficial because the data is very complete for both the surgical and nonsurgical patients. While these benefits might accrue in the nonsurgical arm as well, the relative cost-effectiveness demonstrated in this study must be reproduced in standard clinical practice in order to demonstrate widespread applicability.
An additional consideration with regard to the ASLS trial is that the high crossover rate may confound the methodology of an intent-to-treat (ITT) analysis. This has been a consistent problem with RCT studies for spinal surgery, where crossover rates are often high and bi-directional [11]. Carreon et.al. performed an ITT based cost-effectiveness analysis for the ASLS trial, demonstrating cumulative cost for the surgical cohort of $96,000 with a QALY gain of 2.4 versus cumulative cost of $49,546 with a QALY gain of 0.75 for the non-surgical cohort at five-year follow-up [16]. The surgical cohort became more cost-effective than then non-surgical cohort at year-four with an ICER of $40,595, which was maintained with an ICER of $27,480 per QALY gained at year-five.
In contrast, the present as-treated analysis demonstrates that the surgically treated cohort becomes more cost-effective in year-three with an ICER of $93,000 /QALY gained, maintained at year-four with an ICER of $57,000/QALY gained and year-5 with and ICER of $44,000/QALY gained. Given the advantage of better HRQOL improvement, but the disadvantage of higher cost in surgically treated patients, it should not be surprising that the as-treated surgical cohort becomes cost-effective earlier, but at a higher overall cost, as compared to the ITT analysis.
The most significant limitations of this study result from the difficulty with enrollment, and substantial crossover in the randomized arm. Although there were patients who were randomized to the surgical and nonsurgical arms, most of the patients were followed in the observational cohort, and operative patients were worse at baseline as compared to nonoperative patients. Also, due to the variable interval at which crossovers occurred, a significant minority of the cases (91/286) did not have sufficient follow-up for inclusion in this analysis. Despite these limitations, the underlying dataset was much more complete than most of the adult spinal deformity literature [31].
Additionally, assessment of cost-effectiveness is limited by the lack of a consensus threshold for willingness-to pay. We report a range from $50,000 to $140,000, and generally use the $100,000 value most frequently cited in the spine literature, but cost-effectiveness as defined by this threshold is obviously impacted by the specific threshold selected. Finally, there may be limitations on the generalizability of the results, especially in the surgical cohort, as the enrolling physicians are experienced spine surgeons operating in specialized centers.
In summary, this study confirms the prior ITT analysis demonstrating cost-effectiveness for adult scoliosis surgery in the ASLS trial [16]. The as-treated analysis demonstrates that surgical treatment becomes favorable at year-three, one year earlier than suggested by the ITT analysis. As compared to most of the prior literature, this study is based upon prospective data with complete follow-up for both surgical and nonsurgical patients. This should elevate the level of confidence that well-selected and executed surgical treatment for symptomatic adult scoliosis can be cost-effective.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Schwab F, Dubey A, Gamez L, El Fegoun AB, Hwang K, Pagala M, Farcy JP. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976). 2005. May 1;30(9):1082–5. [DOI] [PubMed] [Google Scholar]
- 2.Bridwell KH, Glassman S, Horton W, Shaffrey C, Schwab F, Zebala LP, Lenke LG, Hilton JF, Shainline M, Baldus C, Wootten D. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine (Phila Pa 1976). 2009. September 15;34(20):2171–8. [DOI] [PubMed] [Google Scholar]
- 3.Kelly MP, Lurie JD, Yanik EL, Shaffrey CI, Baldus CR, Boachie-Adjei O, Buchowski JM, Carreon LY, Crawford CH 3rd, Edwards C 2nd, Errico TJ, Glassman SD, Gupta MC, Lenke LG, Lewis SJ, Kim HJ, Koski T, Parent S, Schwab FJ, Smith JS, Zebala LP, Bridwell KH. Operative Versus Nonoperative Treatment for Adult Symptomatic Lumbar Scoliosis. J Bone Joint Surg Am. 2019. February 20;101(4):338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glassman SD, Dimar JR 2nd, Carreon LY. Revision Rate After Adult Deformity Surgery. Spine Deform. 2015. March;3(2):199–203 [DOI] [PubMed] [Google Scholar]
- 5.McCarthy I, O’Brien M, Ames C, Robinson C, Errico T, Polly DW Jr, Hostin R; International Spine Study Group. Incremental cost-effectiveness of adult spinal deformity surgery: observed quality-adjusted life years with surgery compared with predicted quality-adjusted life years without surgery. Neurosurg Focus. 2014. May;36(5):E3. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Shaffrey CI, Kelly MP, Yanik EL, Lurie JD, Baldus CR, Edwards C, Glassman SD, Lenke LG, Boachie-Adjei O, Buchowski JM, Carreon LY, Crawford CH 3rd, Errico TJ, Lewis SJ, Koski T, Parent S, Kim HJ, Ames CP, Bess S, Schwab FJ, Bridwell KH. Effect of Serious Adverse Events on Health-related Quality of Life Measures Following Surgery for Adult Symptomatic Lumbar Scoliosis. Spine (Phila Pa 1976). 2019. September 1;44(17):1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raman T, Nayar SK, Liu S, Skolasky RL, Kebaish KM. Cost-Effectiveness of Primary and Revision Surgery for Adult Spinal Deformity. Spine (Phila Pa 1976). 2018. June 1;43(11):791–797. [DOI] [PubMed] [Google Scholar]
- 8.Terran J, McHugh BJ, Fischer CR, Lonner B, Warren D, Glassman S, Bridwell K, Schwab F, Lafage V. Surgical treatment for adult spinal deformity: projected cost effectiveness at 5-year follow-up. Ochsner J. 2014. Spring;(1):14–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Carreon LY, Anderson PA, Traynelis VC, Mummaneni PV, Glassman SD. Cost-effectiveness of single-level anterior cervical discectomy and fusion five years after surgery. Spine (Phila Pa 1976). 2013. March 15;38(6):471–5. [DOI] [PubMed] [Google Scholar]
- 10.Carreon LY, Glassman SD, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, Dimar JR 2nd. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: a cost-utility study. Spine (Phila Pa 1976). 2009. February 1;34(3):238–43. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006. November 22;296(20):2451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer CR, Terran J, Lonner B, McHugh B, Warren D, Glassman S, Bridwell K, Schwab F, Lafage V. Factors Predicting Cost-effectiveness of Adult Spinal Deformity Surgery at 2 Years. Spine Deform. 2014. September;2(5):415–422. [DOI] [PubMed] [Google Scholar]
- 13.Kuntz KM, Snider RK, Weinstein JN, Pope MH, Katz JN. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine (Phila Pa 1976). 2000. May 1;25(9):1132–9. [DOI] [PubMed] [Google Scholar]
- 14.Detsky AS, Naglie G, Krahn MD, Naimark D, Redelmeier DA. Primer on medical decision analysis: Part 1--Getting started. Med Decis Making. 1997. Apr-Jun;17(2):123–5. [DOI] [PubMed] [Google Scholar]
- 15.Gold MR, Siegel JE, Russel LB and Weinstein MC, Cost-Effectiveness in Health and Medicine. Oxford University Press, 1996. [Google Scholar]
- 16.Carreon LY, Glassman SD, Lurie J, Shaffrey CI, Kelly MP, Baldus CR, Bratcher KR, Crawford CH, Yanik EL, Bridwell KH. Cost-effectiveness of Operative versus Nonoperative Treatment of Adult Symptomatic Lumbar Scoliosis an Intent-to-treat Analysis at 5-year Follow-up. Spine (Phila Pa 1976). 2019. November 1;44(21):1499–1506. [DOI] [PubMed] [Google Scholar]
- 17.Fairbank J Revised Oswestry Disability questionnaire. Spine. 2000. October 1;25(19):2552. [DOI] [PubMed] [Google Scholar]
- 18.Asher MA, Lai SM, Glattes RC, Burton DC, Alanay A, Bago J. Refinement of the SRS-22 Health-Related Quality of Life questionnaire Function domain. Spine (Phila Pa 1976). 2006. March 1;31(5):593–7. [DOI] [PubMed] [Google Scholar]
- 19.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996. March;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 20.Center for Medicare and Medicaid Services. Medicare Fee Schedule. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-ServicePayment/FeeScheduleGenInfo/index.html. Accessed August 04, 2017
- 21.Drug Price Information available at https://www.drugs.com/price-guide. Accessed August 05, 2017.
- 22.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004. September;42(9):851–9 [DOI] [PubMed] [Google Scholar]
- 23.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000. Jul-Sep;20(3):332–42. [DOI] [PubMed] [Google Scholar]
- 24.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992. February 15;146(4):473–81. [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold. What it is and what it means. Pharmacoeconomics 2008:226:733–44. [DOI] [PubMed] [Google Scholar]
- 26.Winkelymayer WC, Weinstein MC, Mittelman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making 2002;22:417–30 [DOI] [PubMed] [Google Scholar]
- 27.Waldrop R, Cheng J, Devin C, McGirt M, Fehlings M, Berven S. The Burden of Spinal Disorders in the Elderly. Neurosurgery. 2015. October;77 Suppl 4:S46–50. [DOI] [PubMed] [Google Scholar]
- 28.Glassman SD, Polly DW, Dimar JR, Carreon LY. The cost effectiveness of single-level instrumented posterolateral lumbar fusion at 5 years after surgery. Spine (Phila Pa 1976). 2012. April 20;37(9):769–74. [DOI] [PubMed] [Google Scholar]
- 29.Fujishiro T, Boissière L, Cawley DT, Larrieu D, Gille O, Vital JM, Pellisé F, Pérez-Grueso FJS, Kleinstück F, Acaroglu E, Alanay A, Obeid I; European Spine Study Group, ESSG. Adult spinal deformity surgical decision-making score. Part 2:development and validation of a scoring system to guide the selection of treatment modalities for patients above 40 years with adult spinal deformity. EurSpine J. 2019. July 17. [Google Scholar]
- 30.World Health Organization. Macroeconomics and health: Investing in health for economic development: report of the commision on macroeconomics and health, 2001.
- 31.Passias PG, Bortz CA, Lafage V, Lafage R, Smith JS, Line B, Eastlack R, Gupta MC, Hostin RA, Horn SR, Segreto FA, Egers M, Sciubba DM, Gum JL, Kebaish KM, Klineberg EO, Burton DC, Schwab FJ, Shaffrey CI, Ames CP, Bess S. Durability of Satisfactory Functional Outcomes Following Surgical Adult Spinal Deformity Correction: A 3-Year Survivorship Analysis. Oper Neurosurg (Hagerstown). 2019. May [DOI] [PubMed] [Google Scholar]


