Abstract
Background:
Current data regarding the risk of malignancy in a large thyroid nodule with benign fine-needle aspiration biopsy(FNAB) is conflicting. We investigated the impact of patient age on the risk of malignancy in nodules≥4cm with benign cytology.
Methods:
We performed a single-institution retrospective review of patients who underwent surgery from 07/2008-08/2019 for a cytologically benign thyroid nodule ≥4cm. The relationship between malignant histopathology and patient and ultrasound features was assessed with multivariable logistic regression.
Results:
Of 474 nodules identified, 25(5.3%) were malignant on final pathology. In patients <55 years old, 21/273(7.7%) nodules were malignant, compared to 4/201(2.0%) in patients ≥55. Patient age ≥55 was independently associated with significantly lower risk of malignancy(OR:0.2,95%CI:0.1-0.7,p=0.011). Increasing nodule size >4cm and high-risk ultrasound features were not associated with risk of malignancy(OR:1.0,95%CI:0.7-1.4,p=0.980, and OR:9.6,95%CI:0.9-107.8,p=0.066, respectively).
Conclusions:
Patients <55 years old are 3.7-fold more likely to have a falsely benign FNA biopsy in a nodule≥4cm.
Keywords: Thyroid Nodules, Thyroid Surgery, Thyroid Cancer, Thyroid Ultrasound, Thyroid Cytology
Introduction:
Thyroid nodules are common, with an estimated prevalence of up to 67% (1, 2), but only approximately 5-15% prove to be malignant (3). Although thyroid fine-needle aspiration biopsy (FNAB) is the gold standard for diagnostic workup of a thyroid nodule, the literature is conflicting regarding increased risk of falsely benign FNAB, or the false-negative rate, in larger nodules (3–9). While some studies did not find a significant association between false-negative rates and nodule size, others reported rates as high as 50% for thyroid nodules ≥4cm (10). A recent, large meta-analysis of 35 studies found that the risk of malignancy in nodules ≥4cm, including all cytology results, was not increased(5). However, in cytologically benign nodules, a higher false-negative rate of 6.7% was observed across the studies for nodules ≥4cm (5). Therefore, it is standard practice at our institution to recommend surgical resection for cytologically benign nodules ≥4cm.
However, these studies were conducted prior to the introduction of the noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFT-P) classification, and therefore a proportion of the reported malignant nodules would no longer be classified as malignant, further reducing false-negative rates(11–14). Furthermore, over the past decade, the development of scoring systems for the interpretation of ultrasound characteristics continues to improve diagnostic reliability of FNAB (15–17), even though significant inter-observer disagreement exists within these imaging classifications(18–20). Finally, these studies did not incorporate patient factors that could impact the risk of malignancy.
The prevalence of thyroid nodules increases with advancing age, while the risk of malignancy within each nodule decreases with age (1, 21). Age is an important prognostic factor for differentiated thyroid cancer, with the most recent guidelines utilizing a threshold age of 55 for staging and management recommendations (22). Additionally, increasing age has been associated with more aggressive disease including higher risk histology(1). However, it remains unclear if age is associated with the risk of malignancy in a cytologically benign, large thyroid nodule. Therefore, the primary objective of our study was to determine if age modifies the risk of malignancy in a cytologically benign thyroid nodule ≥4cm after accounting for NIFT-P as benign pathology.
Methods:
Patient Population and Data Collection
Under IRB approval, a single institution retrospective review was performed of all adult patients who underwent thyroidectomy for a cytologically benign thyroid nodule ≥4cm from 07/2008 to 08/2019. Any patient with missing cytology reports, surgical pathology reports, or measurements of nodules was excluded. For each patient, demographic data including age, gender, race, smoking history, and BMI was collected. Clinic and operative notes were reviewed to ascertain presence of compressive symptoms or thyroid cancer risk factors including family history of thyroid cancer or prior exposure to ionizing radiation. For each nodule, sonographic size (centimeters by greatest dimension), cytopathology, and corresponding histopathology were gathered from the medical record and correlation confirmed. The ultrasound reports were further reviewed to categorize each nodule as high suspicion, intermediate suspicion, low suspicion, very low suspicion, or benign using the risk stratification system in the 2015 ATA guidelines(3). Each nodule was classified as benign or malignant based on final surgical pathology of the biopsied nodule, and therefore incidentally discovered microcarcinomas were not classified as malignant. NIFT-P was considered benign. Because NIFT-P was not defined during most of the study period, surgical pathology reported as “encapsulated follicular variant of papillary thyroid carcinoma”, a diagnosis previously considered malignant, prior to 2017 was re-reviewed by a single head and neck pathologist (LMR) to determine if it should be reclassified to NIFT-P. All data was entered into a Qualtrics XM (SAP, WA, USA) and then exported into excel for analysis.
Statistical Analysis
All statistical analyses were performed using STATA version 15 (StataCorp., TX, USA). Nodules in each patient were analyzed individually. Age was categorized into two groups with a threshold at 55 years, corresponding with the AJCC 8th edition thyroid cancer staging system. The association between risk of malignancy and age, gender, race, family history, radiation exposure, ATA ultrasound classification, nodule size, and number of nodules ≥4 cm was assessed using Pearson Chi-square (χ2) for categorical variables and t-test for continuous variables. Univariable logistic regression assessed the association between each co-variable and risk of malignancy. A multivariable logistic regression was then performed to evaluate factors associated with risk of malignancy.
Results:
Characteristics of the Thyroid Nodules
Of the 443 patients having at least one nodule ≥4cm who underwent thyroid surgery, 474 nodules ≥4cm with benign cytology were identified, and 25 (5.3%) of those nodules were malignant on final pathology. However, if NIFT-P had been considered malignant, 33 (7.0%) of the nodules in this cohort would have been classified as malignant. The average nodule size for the entire cohort was 5.2cm (range 4.0-11.3cm). The average nodule size of only the 25 malignant nodules was also 5.2cm (range 4.0-8.2cm).
Upon review of the ultrasound reports, 5 (1.1%) nodules had high-risk features including microcalcifications, irregular margins, taller-than-wide shape, and/or peripheral rim calcifications, corresponding to the high suspicion classification by the ATA guidelines(3). Only 1 (4.0%) of the 25 nodules with malignant final pathology as opposed to 4 (0.9%) of 449 nodules with benign final pathology had high-risk ultrasound features (p= 0.147). The majority of the nodules (98.9%) had ultrasound findings consistent with benign, very low, low, or intermediate suspicion by the ATA guidelines(3). Therefore, ultrasound findings were non-specific in the diagnosis of malignancy.
Characteristics of the Patient Cohort
Of the 443 patients with cytologically benign nodules ≥4cm, 256 (57.8%) were adults <55 years old and 187 (42.2%) were ≥55 years old (Table 1). The majority of patients were female (81.9%) and white (46.1%). Twenty-three (5.2%) patients had a family history of thyroid cancer, 8 (1.8%) had prior exposure to ionizing radiation, 189 (42.7%) presented with substernal extension, and 230 (51.9%) complained of compressive symptoms including dysphagia, dysphonia, or neck pressure. Most had only one nodule ≥4cm biopsied (414 patients, 93.5%), 27 (6.1%) had two nodules biopsied, and 2 (0.5%) had three nodules biopsied. Thyroid lobectomy was performed in 181 (40.9%) patients, total thyroidectomy in 261 (58.9%) patients, and isthmusectomy in 1 (0.2%) patient. Final pathology was malignant in 24 (5.4%) patients, benign in 419 (94.6%) patients, of which 8 had NIFT-P. Incidental microcarcinomas were identified in 28 (6.3%) patients in the cohort.
Table 1.
Demographic and clinical characteristics of patients with cytologically benign nodules ≥4cm stratified by final pathology.
| Benign Final Pathology n = 419 No. (%) |
Malignant Final Pathology n = 24 No. (%) |
Total Cohort n = 443 No. (%) |
p-value | |
|---|---|---|---|---|
| Age* | 0.009 | |||
| 18-54 Years | 236 (56.3) | 20 (83.3) | 256 (57.8) | |
| ≥55 Years | 183 (43.7) | 4 (16.7) | 187 (42.2) | |
| Number of Nodules ≥4cm* | 0.008 | |||
| 1 | 394 (94.0) | 20 (83.3) | 414 (93.5) | |
| 2 | 24 (5.8) | 3 (12.5) | 27 (6.1) | |
| 3 | 1 (0.2) | 1 (4.2) | 2 (0.5) | |
| Gender | 0.146 | |||
| Female | 346 (82.6) | 17 (70.8) | 363 (81.9) | |
| Male | 73 (17.4) | 7 (29.2) | 80 (18.1) | |
| Prior History of Radiation Exposure | 8 (1.9) | 0 (0) | 8 (1.8) | 0.784 |
| Family History of Thyroid Cancer | 22 (5.3) | 1 (4.2) | 23 (5.2) | 0.526 |
| High Suspicion Ultrasound | 4 (1.0) | 1 (4.2) | 5 (1.1) | 0.147 |
| Race | 0.511 | |||
| White | 193 (46.1) | 11 (45.8) | 204 (46.1) | |
| Black or African American | 173 (41.3) | 9 (37.5) | 182 (41.1) | |
| Asian or Pacific Islander | 21 (5.0) | 3 (12.5) | 24 (5.4) | |
| Hispanic or Latino Origin | 15 (3.6) | 0 (0.0) | 15 (3.4) | |
| Other | 17 (4.1) | 1 (4.2) | 18 (4.1) | |
| Substernal Extension | 180 (43.0) | 9 (37.5) | 189 (42.7) | 0.599 |
| Presence of Compressive Symptomsa | 219 (52.3) | 11 (45.8) | 230 (51.9) | 0.540 |
| Smoking History | 0.119 | |||
| Current | 28 (6.7) | 2 (8.3) | 30 (6.8) | |
| Former | 91 (21.7) | 1 (4.2) | 92 (20.8) | |
| Never | 300 (71.6) | 21 (87.5) | 321 (73.5) | |
| Presence of Incidental Microcarcinoma* | 23 (5.5) | 5 (20.8) | 28 (6.3) | 0.003 |
| Surgical Intervention | ||||
| Thyroid Lobectomy | 174 (41.5) | 7 (29.2) | 181 (40.8) | |
| Total Thyroidectomy | 244 (58.2) | 17 (70.8) | 261 (58.9) | |
| Isthmusectomy | 1 (0.2) | 0 (0.0) | 1 (0.2) |
p-values reflect chi2 test across columns, significance <0.05
Presence of Compressive Symptoms includes patient-reported dysphagia, dysphonia, or neck pressure
Characteristics of the cohort stratified by age
Patient demographics including gender, race, and BMI were similar across the two age groups: adults <55 and adults ≥55 years old. Of 256 patients <55 years old, 20 (7.8%) had a malignancy, whereas of 187 patients ≥55 years old, only 4 (2.1%) had malignancy (p=0.009). NIFT-P was diagnosed in 6 (2.2%) and 2 (1.0%) patients <55 and ≥55 years old, respectively. Family history of thyroid cancer was present in 16 (6.3%) patients aged <55 compared to only 7 (3.4%) of the adults aged ≥55. In both age groups, 4 (1.6% aged <55 and 2.1% aged ≥55) patients reported exposure to ionizing radiation. The thyroid nodule was substernal in 99 (38.7%) and 90 (48.1%) patients aged <55 and ≥55 years, respectively. Compressive symptoms were described by 136 (53.1%) and 94 (50.3%) patients aged <55 and ≥55 years, respectively. Ultrasound findings classified as high suspicion by the ATA guidelines were present in 2 (0.8%) patients <55 years old and 3 (1.6%) patients ≥55. There were 64 (25.0%) and 35 (18.7%) patients aged <55 and ≥55 years old, respectively, who were asymptomatic without high-risk ultrasound features and underwent surgery for size indication alone.
Characteristics of Patients with Malignant Histopathology
There were 25 malignant nodules identified in 24 patients. One patient had two separate, cytologically benign nodules ≥4cm that were both found to be malignant on surgical pathology, while the other 23 patients with malignancy had a single malignant nodule. Of the 24 patients with malignant final pathology, 18 (75%) had papillary thyroid cancer (PTC), 5 (20.8%) had follicular thyroid cancer (FTC), and 1 (4.2%) had a Hurthle cell carcinoma (HCC) (Figure 1A). Of patients with PTC, 5 (20.8%) were multifocal, 1 (5.6%) had angioinvasion, 2 (11.1%) had lymphatic invasion, and 0 had extrathyroidal extension. Of patients <55, 15 (75.0%) had PTC and 5 (25.0%) had FTC, and similarly, of patients ≥55, 3 (75.0%) had PTC and 1 (25.0%) had HCC (Figures 1B and 1C). Total thyroidectomy was performed on 17 (70.8%) and thyroid lobectomy on 7 (29.2%) patients who had a malignant nodule. Of the 7 patients who underwent a thyroid lobectomy, only 1 (14.3%) underwent a completion thyroidectomy. Only 11 (45.8%) patients with malignancy complained of compressive symptoms of dysphagia, dysphonia, or neck pain pre-operatively, compared to 52.3% of patients with benign final pathology. Of those with malignancy, 9 (37.5%) presented with substernal extension compared to 48.1% of patients with benign pathology. One (4.2%) patient with malignancy had a nodule classified as high suspicion on ultrasound, compared to 4 (1.0%) patients with benign final pathology. Of the 24 patients with malignancy, 8 (33.3%) underwent surgery for a size indication alone and did not have family history of thyroid cancer, prior exposure to ionizing radiation, substernal extension, compressive symptoms, or high-risk ultrasound features, compared to 21.7% of patients with benign final pathology. Incidental microcarcinomas were identified in 5 (20.8%) patients with malignant final pathology, and 23 (5.5%) patients with benign final pathology.
Figure 1.
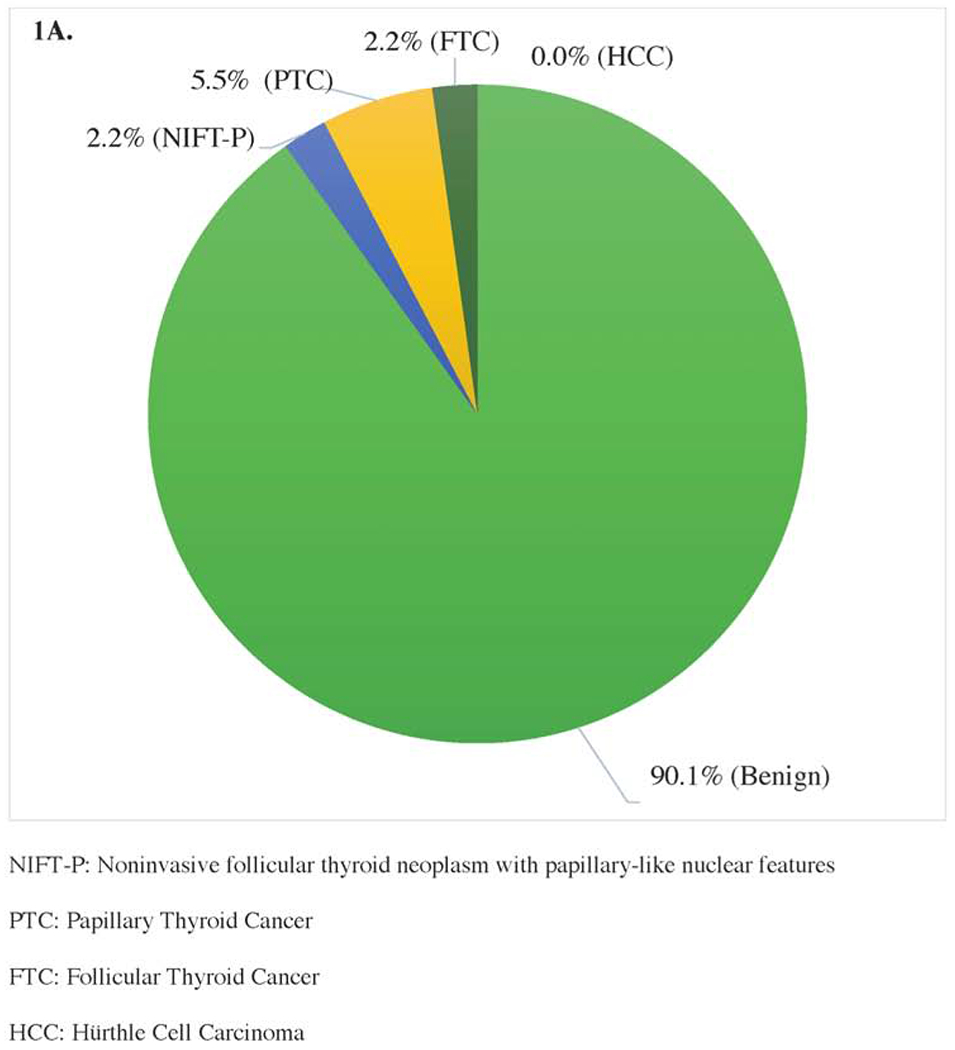

Surgical Pathology of Cytologically Benign Thyroid Nodules ≥4cm of (A) Adults <55 Years (n=273) and (B) Adults ≥55 Years (n=201).
Univariable and multivariable logistic regression for risk of malignancy
Univariable logistic regression for risk of malignancy demonstrated that adults ≥ 55 years old had a significantly lower risk of malignancy compared to adults <55 (OR: 0.3, 95% CI: 0.1-0.8, p=0.015). Univariable models also showed that having ≥3 nodules measuring ≥4 cm on imaging (OR: 26.9, 95% CI: 1.3-543.2, p=0.032) was associated with an increased risk of malignancy. Neither increasing nodule size greater than 4cm (OR: 1.0, 95% CI: 0.7-1.4, p=0.980), nor gender (OR: 2.2, 95% CI: 0.9-5.5, p=0.101), family history of thyroid cancer (OR: 0.7, 95% CI: 0.1-5.1, p=0.685), presence of substernal thyroid extension (OR: 0.9, 95% CI: 0.4-2.1, p=0.776), compressive symptoms (OR: 0.7, 95% CI: 0.3-1.7, p=0.490), high suspicion ultrasound classification (OR: 6.1, 95% CI: 0.6-62.8, p=0.129), or exposure to ionizing radiation (0 patients with exposure had malignancy) was associated with risk of malignancy in this study (Table 2).
Table 2.
Univariable and multivariable logistic regression for risk of malignancy
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age group | ||||||
| 18-54 | Reference | Reference | ||||
| ≥55 | 0.3* | 0.1-0.8 | 0.015 | 0.2* | 0.1-0.7 | 0.011 |
| Number of nodules ≥4cm | ||||||
| 1 | Reference | Reference | ||||
| 2 | 2.6 | 0.7-9.5 | 0.152 | 2.5 | 0.7-9.2 | 0.180 |
| 3 | 26.9* | 1.3-543.2 | 0.032 | 21.4* | 1.2-389.9 | 0.038 |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 2.2 | 0.9-5.5 | 0.101 | 1.9 | 0.8-5.1 | 0.178 |
| History of Radiation Exposure | ||||||
| No | Reference | Reference | ||||
| Yes | 1 | 1 | ||||
| Family History of Thyroid Cancer | ||||||
| No | Reference | Reference | ||||
| Yes | 0.7 | 0.1-5.1 | 0.685 | 0.6 | 0.1-5.2 | 0.678 |
| ATA Ultrasound Classification | ||||||
| Not High Suspicion | Reference | Reference | ||||
| High Suspicion | 6.1 | 0.6-62.8 | 0.129 | 9.6 | 0.9-107.8 | 0.066 |
| Race | ||||||
| White | Reference | |||||
| Black | 0.9 | 0.4-2.2 | 0.789 | |||
| Other | 1.1 | 0.4-3.8 | 0.820 | |||
| Increasing Size (cm) | 1.0 | 0.7-1.4 | 0.980 | |||
| Substernal Extension | ||||||
| No | Reference | |||||
| Yes | 0.9 | 0.4-2.1 | 0.776 | |||
| Presence of Compressive Symptomsa | ||||||
| No | Reference | |||||
| Yes | 0.7 | 0.3-2.7 | 0.490 | |||
| Smoking History | ||||||
| Never Smoker | Reference | |||||
| Current Smoker | 1.1 | 0.3-4.8 | 0.943 | |||
| Former Smoker | 0.2 | 0.0-1.6 | 0.129 | |||
HR: Hazard Ratio
CI: Confidence Interval
p-value across benign vs malignant groups, significance <0.05
Presence of Compressive Symptoms includes patient-reported dysphagia, dysphonia, or neck pressure
In the multivariable model, patient age ≥55 was still associated with significantly lower risk of malignancy (OR: 0.2, 95% CI: 0.1-0.7, p=0.011), while ≥3 nodules measuring ≥4 cm on imaging (OR: 21.4, 95% CI: 1.2-389.9, p=0.038) was independently associated with a higher risk of malignancy (Table 2).
Discussion:
This is the first and largest study to incorporate the reclassification of NIFT-P and assess the association between age and risk of malignancy in a cytologically benign thyroid nodule ≥4cm. The false-negative rate of a benign FNAB was 5.3% for the entire cohort, 7.8% for those <55 years of age, and 2.1% for those ≥55 years. In addition to patient age <55 years old, the presence of ≥3 nodules measuring ≥4cm was also independently associated with increased risk of malignancy. This not only suggests that surgical decision-making for large, cytologically benign nodules should incorporate patient age, but also highlights the low risk of malignancy in these nodules since the introduction of NIFT-P.
Similar to our study, many studies did not identify a linear relationship between increasing nodule size ≥4cm and risk of malignancy, with some studies reporting a lower rate of malignancy in nodules ≥4cm compared to nodules <4cm (5, 6, 8, 23). The false-negative rate in larger nodules is also debated; a recent study by Kizilgul and colleagues found no significant difference between nodules <4cm versus >4cm, while a study by Bestepe, et al. cited greater than a 2-fold increase in false-negative FNAB for larger nodules (6, 9). While the overall false-negative rate in our study was consistent with or lower than the rates cited by many large studies and meta-analyses, these other studies did not incorporate patient age (4, 5, 9). The findings of our study attribute most of the false-negative FNAB to patients <55 years old, and facilitate discussion with the patient regarding this risk of false-negative biopsy and consideration of surgical resection. Additionally, the low risk of malignancy in patients ≥55 years old encourages discussion of continued surveillance or consideration of ablative techniques after a period of surveillance.
Patient age and life expectancy are important considerations for surgical decision-making of thyroid disease as most thyroid cancers are slow-growing(24). Prior studies have shown that increasing patient age is associated with a higher prevalence of thyroid nodules, but a lower risk of malignancy in patients over the age of 70 (1, 21, 25). This study demonstrates that adults ≥55 years old also have a much lower risk of false-negative FNAB within large thyroid nodules. The false-negative rate for this population in our study was only 2.1%, and half of these patients had other indications for surgery including compressive symptoms or substernal goiter. Therefore, only two (1.1%) malignant nodules were discovered in patients ≥55 who underwent surgery on the basis of nodule size ≥4cm alone. Furthermore, unlike prior investigations, our study did not find a higher prevalence of more aggressive histological subtypes in the older population with malignancy(1). This work supports the safety of active surveillance for adults ≥55 with cytologically benign nodules ≥4cm. Furthermore, this data suggests that non-surgical therapies, such as radiofrequency and microwave ablation, are appropriate treatment options for this cohort as studies continue to demonstrate their efficacy and safety(26, 27).
However, for patients <55 years old, the rate of false-negative FNAB was 3.7-fold higher than the older cohort. Lee et al. investigated the cost-effectiveness of thyroid lobectomy versus active surveillance for cytologically benign nodules ≥4cm and found that quality-adjusted life-years and life expectancy were significantly increased for those who underwent thyroid lobectomy, despite increased overall lifetime cost for the surgical strategy(28). The incremental lifetime costs were lower for patients <55 years old, supporting thyroid lobectomy as an appropriate and in some situations better treatment option for this age group. The findings in our study further corroborate thyroid lobectomy as a diagnostic and therapeutic treatment strategy for this age cohort due to a higher risk of harboring malignancy in large nodules.
There are several limitations in this study in addition to those inherent in retrospective, single-institution studies including missing values or reports. The data included a relatively limited number of malignant nodules, and therefore all variables affecting this risk of malignancy may not have statistical significance, including known thyroid cancer risk factors such as exposure to ionizing radiation and family history of thyroid cancer. The general time frame between biopsy and surgery could also not be ascertained for all patients. This study also only included patients who underwent surgery, and therefore there may have been referral and selection bias, as all nodules ≥4cm may not have been referred to the surgical clinic, and some patients may have chosen to not undergo surgery even after referral. Additionally, most ultrasound examinations in our study took place before the introduction of ACR-TIRADS and there is significant variation in ultrasound reports, limiting the ability to apply this classification. However, multiple studies have found that the ATA guidelines have a higher sensitivity, greater negative predictive value, and similar overall diagnostic performance when compared to the ACR-TIRADS (29, 30). When applying the ATA risk stratification methods in our study, only one patient with malignant final pathology had characteristics consistent with the high suspicion classification, and this classification was not significantly associated with malignancy on regression analysis. Therefore, ultrasound interpretation did not contribute to the reliability of FNAB in this study, highlighting the benefit of incorporating an objective modifier such as age (19, 20).
In conclusion, adults ≥55 years old have a lower risk of malignancy in thyroid nodules 4cm that were benign on cytopathology as compared to adults <55 years. This work suggests that patient age ≥55 years old independently decreases the risk of false-negative results in nodules ≥4cm, and should be incorporated into management decisions. With the growing data supporting active surveillance and emerging alternative therapies such as ablation, it is increasingly important to understand patient factors that impact risk of malignancy.
Highlights:
The overall risk of malignancy in cytologically benign thyroid nodules ≥4cm was 5.3%.
Patients <55 years old have a 3.7-fold higher risk of malignancy than those ≥55.
Patients with ≥3 nodules measuring ≥4cm had higher risk of malignancy.
Increasing nodule size and high-risk ultrasound were not associated with malignancy.
Treatment decisions should incorporate age for nodules ≥4cm with benign cytology.
Acknowledgements:
Funding: This work was supported by the National Institutes of Health, NIH K23 AG053429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors have no conflicts of interests to disclose.
References:
- 1.Kwong N, Medici M, Angell TE, et al. The Influence of Patient Age on Thyroid Nodule Formation, Multinodularity, and Thyroid Cancer Risk. J Clin Endocrinol Metab. 2015. December;100(12):4434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008. December;22(6):901–11. [DOI] [PubMed] [Google Scholar]
- 3.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016. January;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin JJ, Caragacianu D, Randolph GW. Impact of thyroid nodule size on prevalence and post-test probability of malignancy: a systematic review. Laryngoscope. 2015. January;125(1):263–72. [DOI] [PubMed] [Google Scholar]
- 5.Cipriani NA, White MG, Angelos P, Grogan RH. Large Cytologically Benign Thyroid Nodules Do Not Have High Rates of Malignancy or False-Negative Rates and Clinical Observation Should be Considered: A Meta-Analysis. Thyroid. 2018. Oct 30. [DOI] [PubMed] [Google Scholar]
- 6.Kizilgul M, Shrestha R, Radulescu A, et al. Thyroid nodules over 4 cm do not have higher malignancy or benign cytology false-negative rates. Endocrine. 2019. May 29. [DOI] [PubMed] [Google Scholar]
- 7.Chiofalo MG, Signoriello S, Fulciniti F, et al. Predictivity of clinical, laboratory and imaging findings in diagnostic definition of palpable thyroid nodules. A multicenter prospective study. Endocrine. 2018. July;61(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Bobanga I, McHenry CR. Are large thyroid nodules classified as benign on fine needle aspiration more likely to harbor cancer? Am J Surg. 2017. March;213(3):464–6. [DOI] [PubMed] [Google Scholar]
- 9.Bestepe N, Ozdemir D, Tam AA, et al. Malignancy risk and false-negative rate of fine needle aspiration cytology in thyroid nodules >/=4.0 cm. Surgery. 2016. August;160(2):405–12. [DOI] [PubMed] [Google Scholar]
- 10.Koo DH, Song K, Kwon H, et al. Does Tumor Size Influence the Diagnostic Accuracy of Ultrasound-Guided Fine-Needle Aspiration Cytology for Thyroid Nodules? Int J Endocrinol. 2016;2016:3803647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoy KL, Jabbour N, Ogilvie JB, et al. The incidence of cancer and rate of false-negative cytology in thyroid nodules greater than or equal to 4 cm in size. Surgery. 2007. December;142(6):837–44; [DOI] [PubMed] [Google Scholar]
- 12.Meko JB, Norton JA. Large cystic/solid thyroid nodules: a potential false-negative fine-needle aspiration. Surgery. 1995. December;118(6):996–1003; [DOI] [PubMed] [Google Scholar]
- 13.Mehanna R, Murphy M, McCarthy J, et al. False negatives in thyroid cytology: impact of large nodule size and follicular variant of papillary carcinoma. Laryngoscope. 2013. May;123(5):1305–9. [DOI] [PubMed] [Google Scholar]
- 14.Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016. August 1;2(8):1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbosa TLM, Junior COM, Graf H, et al. ACR TI-RADS and ATA US scores are helpful for the management of thyroid nodules with indeterminate cytology. BMC Endocr Disord. 2019. October 29;19(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram SB, Rasmussen JH, Feldt-Rasmussen U, et al. Risk of Thyroid Cancer in 1,504 Patients Referred for Thyroid Surgery with Assumed Benign Histology. Eur Thyroid J. 2019. October;8(5):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JH, Lee HS, Kim EK, et al. Follow-Up Strategies for Thyroid Nodules with Benign Cytology on Ultrasound-Guided Fine Needle Aspiration: Malignancy Rates of Management Guidelines Using Ultrasound Before and After the Era of the Bethesda System. Thyroid. 2019. September;29(9):1227–36. [DOI] [PubMed] [Google Scholar]
- 18.Pandya A, Caoili EM, Jawad-Makki F, et al. Limitations of the 2015 ATA Guidelines for Prediction of Thyroid Cancer: A Review of 1947 Consecutive Aspirations. J Clin Endocrinol Metab. 2018. September 1;103(9):3496–502. [DOI] [PubMed] [Google Scholar]
- 19.Phuttharak W, Boonrod A, Klungboonkrong V, Witsawapaisan T. Interrater Reliability of Various Thyroid Imaging Reporting and Data System (TIRADS) Classifications for Differentiating Benign from Malignant Thyroid Nodules. Asian Pac J Cancer Prev. 2019. April 29;20(4):1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seifert P, Gorges R, Zimny M, et al. Interobserver agreement and efficacy of consensus reading in Kwak-, EU-, and ACR-thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine. 2019. November 18. [DOI] [PubMed] [Google Scholar]
- 21.Bessey LJ, Lai NB, Coorough NE, et al. The incidence of thyroid cancer by fine needle aspiration varies by age and gender. J Surg Res. 2013. October;184(2):761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael R Tuttle LFM, Haugen Bryan R., Shah Jatin P., Sosa Julie A., Rohren Eric, Subramaniam Rathan M., Hunt Jennifer L., Perrier Nancy D.. 73.1. Thyroid: Differentiated. AJCC Cancer Staging Manual, 8th Edition New York City: Springer International Publishing; 2016. [Google Scholar]
- 23.Bestepe N, Ozdemir D, Baser H, et al. Is thyroid nodule volume predictive for malignancy? Arch Endocrinol Metab. 2019. March 21;63(4):337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim H, Devesa SS, Sosa JA, et al. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA. 2017. April 4;317(13):1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rago T, Fiore E, Scutari M, et al. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. 2010. April;162(4):763–70. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2019. December 4. [DOI] [PubMed] [Google Scholar]
- 27.Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2019. July 20. [DOI] [PubMed] [Google Scholar]
- 28.Lee L, Mitmaker EJ, Chabot JA, et al. Cost-Effectiveness of Diagnostic Lobectomy Versus Observation for Thyroid Nodules >4 cm. Thyroid. 2016. February;26(2):271–9. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Xi X, Jiang Y, et al. Comparison among TIRADS (ACR TI-RADS and KWAK-TI-RADS) and 2015 ATA Guidelines in the diagnostic efficiency of thyroid nodules. Endocrine. 2019. April;64(1):90–6. [DOI] [PubMed] [Google Scholar]
- 30.Wu XL, Du JR, Wang H, et al. Comparison and preliminary discussion of the reasons for the differences in diagnostic performance and unnecessary FNA biopsies between the ACR TIRADS and 2015 ATA guidelines. Endocrine. 2019. July;65(1):121–31. [DOI] [PubMed] [Google Scholar]


