Abstract
Background:
Previous studies of prenatal phthalate exposure and childhood asthma are inconsistent. These studies typically model phthalates as individual, rather than co-occurring, exposures. We investigated whether prenatal phthalates are associated with childhood wheeze and asthma using a mixtures approach.
Methods:
We studied dyads from two prenatal cohorts in the ECHO-PATHWAYS consortium: CANDLE, recruited 2006–2011 and TIDES, recruited 2011–2013. Parents reported child respiratory outcomes at age 4–6 years: ever asthma, current wheeze (symptoms in past 12 months) and current asthma (two affirmative responses from ever asthma, recent asthma-specific medication use, and/or current wheeze). We quantified 11 phthalate metabolites in third trimester urine and estimated associations with child respiratory outcomes using weighted quantile sum (WQS) logistic regression, using separate models to estimate protective and adverse associations, adjusting for covariates. We examined effect modification by child sex and maternal asthma.
Results:
Of 1481 women, most identified as White (46.6%) or Black (44.6%); 17% reported an asthma history. Prevalence of ever asthma, current wheeze and current asthma in children was 12.3%, 15.8% and 12.3%, respectively. Overall, there was no adverse association with respiratory outcomes. In sex-stratified analyses, boys’ phthalate index was adversely associated with all outcomes (e.g., boys’ ever asthma: adjusted odds ratio per one quintile increase in WQS phthalate index (AOR): 1.42; 95% confidence interval (CI): 1.08, 1.85, with mono-ethyl phthalate (MEP) weighted highest). Adverse associations were also observed in dyads without maternal asthma history, driven by MEP and mono-butyl phthalate (MBP), but not in those with maternal asthma history. We observed protective associations between the phthalate index and respiratory outcomes in analysis of all participants (e.g., ever asthma: AOR; 95% CI: 0.81; 0.68, 0.96), with di(2-ethylhexyl)phthalate (DEHP) metabolites weighted highest.
Conclusions:
Results suggest effect modification by child sex and maternal asthma in associations between prenatal phthalate mixtures and child asthma and wheeze.
Keywords: Phthalate, Prenatal, Mixtures, Asthma, Respiratory, Pregnancy
1. Introduction
Asthma is a complex disease that is influenced by both genetic and environmental factors and is associated with substantial morbidity in childhood (Akinbami et al., 2009; Keet et al., 2015; Shaw et al., 2011; Smith et al., 2005). The prenatal period is a particularly important time during which maternal environmental exposures may have an adverse effect on the developing lung and immune system. Phthalates, for example, are a class of widely used chemicals that may be associated with perturbations in lung development (Chen et al., 2010; Liu et al., 2016; Rosicarelli and Stefanini, 2009). Used as plasticizers to increase flexibility, solvency, or durability, phthalates are commonly added to products such as polyvinyl chloride, medical supplies, food packaging, and personal care products (Wang et al., 2019; Zota et al., 2014). Phthalates leach out of products and enter the environment, where human exposure may occur via multiple routes, including ingestion, inhalation, dermal absorption, as well as placental transfer (Adibi et al., 2003; Duty et al., 2005; Jensen et al., 2012; Meeker et al., 2009; Rudel et al., 2003; Serrano et al., 2014). Humans are exposed to many different phthalate chemicals at the same time, so exposure to phthalate mixtures is common in the general population (Silva et al., 2004), as well as in pregnant women (Adibi et al., 2003; Adibi et al., 2008; Shu et al., 2018; Wenzel et al., 2018). However, phthalates have traditionally been studied as individual chemical exposures. Several recent studies have incorporated a mixtures approach to epidemiologic studies of phthalate exposure and adverse health outcomes (Chiu et al., 2018; Romano et al., 2018; Stroustrup et al., 2018; Woods et al., 2017), but associations between prenatal phthalate mixtures and childhood wheeze and asthma have not been described.
Prenatal exposures to certain phthalates have been associated with adverse health outcomes in children (Bornehag et al., 2018; Kobrosly et al., 2014; Sathyanarayana et al., 2016; Swan et al., 2015; Wittassek et al., 2011), including child respiratory and atopic disease (Berger et al., 2018; Gascon et al., 2015; Jahreis et al., 2018; Ku et al., 2015; Whyatt et al., 2014). For example, prenatal maternal urinary metabolites of di(2-ethylhexyl) phthalate (DEHP), butylbenzyl phthalate (BBzP), and di-n-butyl phthalate (DnBP) have previously been associated with childhood wheeze and asthma, although findings across studies are inconsistent (Gascon et al., 2015; Ku et al., 2015; Whyatt et al., 2014) and are limited by relatively small sample sizes. Studies support several plausible pathways linking prenatal exposure to individual phthalates and adverse childhood respiratory outcomes, including endocrine disruption (Yoon et al., 2014), proinflammatory immune responses (Berger et al., 2018; Chalubinski and Kowalski, 2006), allergic sensitization (Soomro et al., 2018), airway inflammation (Jahreis et al., 2018), and oxidative stress during pregnancy (Ferguson et al., 2014; Ferguson et al., 2015; Guo et al., 2014; Holland et al., 2016). However, little is understood regarding cumulative exposure to multiple phthalates simultaneously and child respiratory health, particularly in the epidemiologic literature. Therefore, we investigated the association between mixtures of third trimester phthalate metabolites and childhood wheeze and asthma outcomes in a large combined analysis of two geographically and demographically diverse mother–child cohorts.
2. Methods
This investigation included mother–child dyads enrolled in two prenatal cohorts participating in the NIH ECHO-PATHWAYS consortium: The Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study and The Infant Development and the Environment Study (TIDES). The CANDLE study was designed to reflect the demographics of pregnant women in Shelby County (Memphis), TN. Healthy women, between 16 and 40 years of age, with an uncomplicated singleton pregnancy and plans to deliver at a participating study hospital were recruited in their second trimester of pregnancy using community-based and clinic-based recruitment (Sontag-Padilla et al., 2015; Tylavsky et al., 2015). Between 2007 and 2011, 1457 children were born whose mothers were active in the study (Tylavsky et al., 2015). The study included visits in the second and third trimesters and at delivery during which trained research staff obtained sociodemographic, mental health, and medical histories and collected biospecimens (Sontag-Padilla et al., 2015; Volgyi et al., 2013). Postnatally, study participation included clinic visits for the dyads at approximately child age 1, 2, 3, and 4–6 years.
The TIDES study is a prospective prenatal cohort (Bornehag et al., 2018) that recruited women older than 18 years with a healthy pregnancy in their first trimester from four university-based prenatal clinics including the University of San Francisco, California (San Francisco, CA), the University of Rochester Medical Center (Rochester, NY), the University of Minnesota (Minneapolis, MN), and University of Washington/Seattle Children’s (Seattle, WA). In total, 749 children were born to TIDES mothers between 2011 and 2013. Study procedures included prenatal visits every trimester, a birth exam, birth record review, and a postnatal visit around age 4 years. Each visit included biospecimen collection and survey-based assessments of sociodemographics, medical history, health behaviors, maternal stress and depression, as well as other factors (Bornehag et al., 2018).
For the current study, we included CANDLE and TIDES dyads with third trimester urinary phthalate metabolite measurements and children who completed the 4 to 6-year follow-up visit between ages 3.7 to 6.5 years. We excluded children born very preterm (< 32 weeks gestational age) to decrease the likelihood that children had underlying lung abnormalities. All mothers provided consent for themselves and their children. All ECHO-PATHWAYS research activities were approved by the University of Washington (UW) IRB.
2.1. Maternal urinary phthalate metabolites
Our primary prenatal exposure of interest included phthalate mixtures characterized by phthalate metabolites measured in spot urine samples collected in the women’s third trimester. Both cohorts collected urine samples in polypropylene (i.e., phthalate-free) containers and subsequently processed and stored specimens using phthalate-free materials (Swan et al., 2015). For TIDES, urine samples were stored on dry ice and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control (CDC) and Prevention (Silva et al., 2007). Analytic details have been described previously (Serrano et al., 2014). In brief, solid-phase-extraction coupled with isotope dilution high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) was used to measure urinary metabolite concentrations. For women in CANDLE, samples were processed and stored at −80 °C in the study repository (University of Tennessee Health Science Center Department of Pathology) until shipment for analysis. Urine samples were analyzed for 21 metabolites using solid phase extraction and high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) at Wadsworth Laboratory, New York State Department of Health. Detailed methods have been previously described (Asimakopoulos et al., 2016; Guo et al., 2011; Rocha et al., 2017). The Wadsworth Laboratory participates in the CDC Proficiency Testing Program, which ensures comparability and reliability of analytic methods utilized at each lab in the program as well as harmonization of methods across labs. Process and instrument blanks were included for quality control at both labs. Specific gravity (SpG) of all samples was determined using a handheld refractometer.
2.2. Child wheeze and asthma outcomes
We assessed three respiratory outcomes in study children, including current wheeze, ever asthma, and current asthma. Outcomes were ascertained by parent-report to questions from the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire (Asher et al., 1995) and other questions related to asthma-specific medication and healthcare use. In both the CANDLE and TIDES cohorts, current wheeze was characterized by affirmative responses to both of the following questions: “has your child ever had wheezing or whistling in the chest at any time in the past?” and “has your child ever had wheezing or whistling in the chest in the last 12 months?”(Adams et al., 2019; Asher et al., 1995). Ever asthma was assessed using the ISAAC questionnaire in both cohorts (“Has your child ever had asthma?”). Lastly, we characterized current asthma as children with a history of asthma with current medication use or symptoms, as well as children with current symptoms and medication use. Accordingly, parent report of two of the following fulfilled the criteria for current asthma: 1) current wheeze, 2) ever asthma, or 3) asthma-specific medication use in the past 12 months (CANDLE) or asthma-specific medication or health care use in the previous two years (TIDES), which is how the data was captured during the respective cohort study visits.
2.3. Covariates
The CANDLE and TIDES studies collected sociodemographic, medical, and family history for the mother–child dyads at enrollment and the prospective study visits. Investigators harmonized variable definitions for analysis. Maternal characteristics obtained during pregnancy included age at delivery, self-reported race, self-reported ethnicity, education level (less than high-school, high-school completed (including some college), graduated college or technical school, at least some graduate work or graduate/professional degree), pre-pregnancy body mass index (BMI) calculated from self-reported pre-pregnancy weight and height (BMI, kg/m2), smoking during pregnancy (yes/no), parity (number of previous live births), and study site (CANDLE [TN] or TIDES [NY, MN, CA, WA]). Child sex and birth year were obtained at delivery (CANDLE [2007–2012], TIDES [2011–2013]). Maternal history of asthma (yes/no) was ascertained at the 4 to 6 year follow-up visit.
2.4. Statistical analyses
We determined the concentrations and examined the distributions of the individual urinary phthalate metabolites (ng/mL). We imputed values below the limit of detection (LOD) as LOD divided by the square root of two as performed in numerous environmental analyses (Hornung and Reed, 1990). In order to normalize their distributions, phthalate metabolite concentrations were log−transformed. In addition, the metabolite concentrations were adjusted for specific gravity using the following formula, where SpG_median refers to the median SpG for each respective cohort: [phth metab]_adj = [phth metab]_raw*[(−SpG_median − 1)/(SpG − 1)] (Boeniger et al., 1993; Bornehag et al., 2018). We used descriptive analyses to describe cohort characteristics using means and standard deviations, medians and interquartile ranges, percentiles, frequencies, and correlations as appropriate.
Our analyses included 11 metabolites, corresponding to low-molecular weight metabolites (monoethyl phthalate [MEP], mono-butyl phthalate [MBP], and monoisobutyl phthalate [MiBP]) and high-molecular weight metabolites (monobenzyl phthalate [MBzP], mono[3-carboxypropyl] phthalate [MCPP], monocarboxyoctyl phthalate [MCOP], monocarboxynonyl phthalate [MCNP], mono[2-ethylhexyl] phthalate [MEHP], mono[2-ethyl-5-hydroxyhexyl] phthalate [MEHHP], mono[2-ethyl-5oxo-hexyl] phthalate [MEOHP] and mono [20ethyl-5-carboxypentyl] phthalate [MECPP]). We selected metabolites for inclusion if they were detectable in at least 80% of the combined CANDLE and TIDES cohorts to allow for values < LOD to fit in the lowest quintile, although a small number of samples with values < LOD (3%) were not included in the lowest quintile after specific gravity correction. We also included MEHP (detected in 79% of the combined cohort), due to its importance as a primary metabolite of DEHP and previously observed toxicity (Jaakkola and Knight, 2008). The lowest two quintiles of MEHP were modeled as a single category to account for high proportion of less than LOD values.
For our primary analyses, we employed weighted quantile sum regression (WQS) to quantify the association between third trimester urinary phthalate metabolite mixtures and child study outcomes (Carrico et al., 2015). WQS is a statistical learning methodology that identifies a weighted sum of components in the mixture of urinary metabolite concentrations that is most strongly associated with a health outcome of interest, in this case binary measures of asthma and wheeze. The WQS index is a weighted sum of individual component concentrations, with each concentration categorized into quantiles. The weights are selected using bootstrap resampling methods to identify the mixture (including key drivers) that is most strongly associated with the outcome in a multivariable logistic regression model in a pre-specified direction of association. We repeated the analysis for positive and negative directions of association (i.e., adverse and protective associations). We used the R package (gWQS) to conduct WQS in logistic regression, using site-specific quintiles to categorize phthalate concentrations and 1000 bootstrap runs for each analysis. We calculated p-values and confidence intervals based on an analysis of the full sample using robust sandwich standard errors (Zeileis, 2004, 2006).
We a priori identified maternal and child characteristics as potential confounders to be included in multivariable models based on review of the literature (Buckley et al., 2018; Gascon et al., 2015; Vernet et al., 2017). Models were adjusted for maternal race (Black, other), ethnicity (Hispanic, Non-Hispanic), age at enrollment (years), education (less than high school, high school completion, college/technical graduate, or at least some graduate/professional), prenatal smoking (no, yes), prior live births (none, one or more), asthma history (no, yes), pre-pregnancy BMI, birth year (2007–2013), study site (TN, NY, MN, CA, WA), and child sex (female, male). We performed three sensitivity analyses. First, we included the molar sum of DEHP metabolites (Σ DEHP: MEHP, MEHHP, MEOHP and MECPP) in WQS models instead of the individual DEHP metabolite constituents. Next, we repeated the multivariable WQS model with additional adjustment for gestational age. Last, we repeated primary WQS models omitting one study site at a time to assess the robustness of our findings with respect to site-specific influence.
In separate models, we assessed effect modification in the association between the WQS prenatal phthalate index and child wheeze/asthma outcomes by child sex and maternal asthma history. In one approach to this analysis, we assessed interaction by including a cross-product of the variable of interest with each covariate and with the WQS phthalate index optimized in the model without interaction (Wu et al., 2018). The interaction model allows for determination of whether associations observed in the entire population are consistent or different across strata. We additionally fit stratified models to allow for different WQS weights within each stratum of child sex and maternal asthma history (Czarnota et al., 2015). The fully stratified approach allows for the identification of distinct phthalate mixtures that are associated with the outcome of interest in each stratum.
In secondary analyses, we investigated the association between individual phthalate metabolites and child wheeze and asthma outcomes, evaluating the 11 individual phthalate metabolites included in the WQS analysis, as well as the molar sum of the DEHP metabolites (Σ DEHP). We used multivariable logistic regression and robust sandwich standard errors to calculate adjusted odds ratios of child wheeze and asthma outcomes for each metabolite separately as well as Σ DEHP, adjusting for variables listed previously. All metabolite concentrations were log-transformed because we hypothesized a logistic-linear relationship between metabolite concentrations on the transformed scale and respiratory outcomes (i.e., odds ratios per 2-fold increase in concentration were similar across the range of exposure). We used generalized additive models (R package MGCV), adjusted for covariates, to examine dose–response relationships on both transformed (Supplemental Figs. 1–3) and untransformed scales (Supplemental Figs. 4–6), confirming appropriateness of log-transformation. All analyses were conducted in R 3.5. Two-sided, type 1 error rates of 0.05 were used for statistical significance.
3. Results
The combined CANDLE-TIDES analytic sample included 987 mother–child dyads from CANDLE and 494 dyads from TIDES for a total sample of 1481 dyads. On average, women in the combined cohort were 28.3 ± 5.9 years at delivery (Table 1). Most identified as either White (46.6%) or Black (44.6%) and were non-Hispanic (96.0%); few identified as Asian (2.7%), multiple race (2.3%), or other (3.7%). Over half had a college/technical school education or more (54.6%) and were parous at enrollment (55.6%). Few women reported smoking during pregnancy (7.4%) and 17.2% had a history of asthma. Compared to those enrolled in TIDES, women in CANDLE were more likely to have lower maternal educational attainment and to identify as Black (Table 1). Children were born at a mean gestational age of 39.1 ± 1.5 weeks, including 7.6% born moderate-to-late preterm (32− < 37 gestational weeks), and 51.2% were female (n = 758 female, n = 723 male) (Table 1).
Table 1.
Description of the CANDLE and TIDES Study Participants.
| CANDLEa | TIDESb | Total | |
|---|---|---|---|
| Total | 987 | 494 | 1481 |
| Maternal Characteristics | |||
| Age (years), mean ± SD | 26.7 ± 5.5 | 31.6 ± 5.3 | 28.3 ± 5.9 |
| Body mass index, mean ± SD | 27.9 ± 7.8 | 26.1 ± 6.2 | 27.3 ± 7.3 |
| Education, n (%) | |||
| < High School | 100 (10.1) | 31 (6.3) | 131 (8.9) |
| High School | 463 (47.0) | 76 (15.4) | 539 (36.5) |
| College or Technical School Graduate | 306 (31.0) | 154 (31.3) | 460 (31.1) |
| ≥ Some Graduate/Professional | 117 (11.9) | 231 (47.0) | 348 (23.5) |
| Race, n (%) | |||
| Black | 608 (61.6) | 51 (10.4) | 659 (44.6) |
| White or Other | 379 (38.4) | 438 (89.6) | 817 (55.4) |
| Ethnicity, n (%) | |||
| Hispanic | 16 (1.6) | 43 (8.8) | 59 (4.0) |
| Non-Hispanic | 965 (98.4) | 447 (91.2) | 1412 (96.0) |
| Prenatal Smoking, n (%) | |||
| Yes | 88 (8.9) | 21 (4.3) | 109 (7.4) |
| No | 898 (91.1) | 468 (95.7) | 1366 (92.6) |
| Prior Live Births, n (%) | |||
| 0 | 392 (39.7) | 263 (53.8) | 655 (44.4) |
| 1 + | 595 (60.3) | 226 (46.2) | 821 (55.6) |
| History of asthma, n (%) | |||
| Yes | 178 (18.2) | 74 (15.1) | 252 (17.2) |
| No | 798 (81.8) | 416 (84.9) | 1214 (82.8) |
| Child Characteristics | |||
| Sex, n (%) | |||
| Male | 493 (49.9) | 230 (46.6) | 723 (48.8) |
| Female | 494 (50.1) | 264 (53.4) | 758 (51.2) |
| Gestational Age (weeks), mean ± SD | 38.9 ± 1.3 | 39.3 ± 1.7 | 39.1 ± 1.5 |
| Moderate-to-late pretermc, n (%) | |||
| Yes | 66 (6.7) | 47 (9.5) | 113 (7.6) |
| No | 921 (93.3) | 447 (90.5) | 1368 (92.4) |
| Age at ISAAC (years), mean ± SD | 4.4 ± 0.5 | 4.5 ± 0.3 | 4.4 ± 0.4 |
| Child Outcomes | |||
| Current Wheeze, n (%) | |||
| Yes | 187 (19.0) | 47 (9.6) | 234 (15.8) |
| No | 798 (81.0) | 445 (90.4) | 1243 (84.2) |
| Current Asthma, n (%) | |||
| Yes | 155 (15.7) | 28 (5.7) | 183 (12.3) |
| No | 832 (84.3) | 466 (94.3) | 1298 (87.7) |
| Ever Asthma, n (%) | |||
| Yes | 144 (14.6) | 38 (7.7) | 182 (12.3) |
| No | 840 (85.4) | 456 (92.3) | 1296 (87.7) |
SD: standard deviation; ISAAC: International Study of Asthma and Allergies in Childhood; a missing data: maternal body mass index = 3; maternal education, n = 1; maternal ethnicity, n = 6; maternal prenatal smoking, n = 1; maternal history of asthma, n = 11; child current wheeze, n = 2; child ever asthma, n = 3b missing data: maternal body mass index = 3; maternal education, n = 2; maternal race, n = 5; maternal ethnicity, n = 4; maternal prenatal smoking, n = 5; maternal parity, n = 5; maternal history of asthma, n = 4; child current wheeze, n = 2c moderate-to-late preterm: estimated gestational age 32 to < 37 weeks
Children were, on average, 4.4 ± 0.4 years at ISAAC assessment. The prevalence for respiratory outcomes was 15.8% for current wheeze, 12.3% for current asthma and 12.3% for ever asthma (Table 1). Prevalence for all outcomes was higher in CANDLE than TIDES. Wheeze and asthma outcomes were more common in boys than girls (e.g., 15% in boys vs. 10% in girls for current asthma), and among children whose mothers had a history of asthma (e.g., 25% with maternal asthma history vs. 10% without maternal asthma history for current asthma).
Concentrations of third trimester urinary phthalate metabolites in the combined cohort appeared similar to concentrations reported for U.S. women in national biomonitoring survey estimates for years overlapping sample collection (2007–2008; 2011–2012) (Centers for Disease Control and Prevention, 2019), with the exception of MCPP, MCOP and MCNP which were lower than national estimates (Table 2). Concentrations were also similar between the cohorts, except for MEP and MBP, which were higher in CANDLE than TIDES, and MCOP and MCNP, which were higher in TIDES than CANDLE (Supplemental Tables 1 and 2). Each metabolite was at least moderately correlated (Spearman r ≥ 0.3) with another metabolite (Supplemental Table 3).
Table 2.
Distribution of concentration of urinary phthalate metabolites (ng/ml) in CANDLE and TIDES (n = 1481) and the National Health and Nutrition Examination Survey (NHANES).
| CANDLE and TIDES cohorts | NHANES | ||||||
|---|---|---|---|---|---|---|---|
| % < LOD | Geo. Mean(95% CI) | 50th | 75th | 95th | 2007–2008a | 2011–2012a | |
| MEP | 0 | 75.0 (68.9, 81.6) | 70.5 | 212.9 | 1330 | 88.2 (77.8, 99.9) | 37.7 (30.6, 46.4) |
| MBP | 1 | 11.8 (11.1, 12.4) | 12.7 | 23.1 | 55.5 | 19.3 (17.2, 22.0) | 7.1 (6.1, 8.4) |
| MiBP | 1 | 6.4 (6.1, 6.8) | 6.5 | 12.4 | 32.0 | 6.8 (6.2, 7.5) | 5.5 (4.9, 6.1) |
| MBzP | 3 | 6.8 (6.3, 7.3) | 7.7 | 17.3 | 57.1 | 6.7 (5.8, 7.8) | 4.3 (3.8, 4.8) |
| MCPP | 5 | 1.5 (1.4, 1.6) | 1.4 | 2.9 | 12.6 | 2.5 (2.3, 2.8) | 2.6 (2.2, 3.0) |
| MCOP | 0 | 4.1 (3.7, 4.4) | 3.4 | 10.9 | 67.9 | 6.5 (5.5, 7.7) | 17.1 (14.7, 19.9) |
| MCNP | 1 | 0.8 (0.7, 0.8) | 0.7 | 2.0 | 7.3 | 2.1 (1.8, 2.5) | 2.2 (2.0, 2.4) |
| ΣDEHPb | 0 | 0.09 (0.08, 0.09) | 0.09 | 0.16 | 0.55 | – | – |
| MEHP | 21 | 1.8 (1.7, 2.0) | 1.9 | 4.5 | 18.0 | 2.5 (2.2, 2.9) | 1.2 (1.1, 1.3) |
| MEHHP | 1 | 6.8 (6.4, 7.2) | 6.9 | 13.0 | 44.1 | 21.0 (17.8, 24.8) | 7.2 (6.8, 7.7) |
| MEOHP | 0 | 5.4 (5.1, 5.7) | 5.2 | 9.8 | 33.2 | 11.8 (10.0, 14.0) | 4.7 (4.4, 5.1) |
| MECPP | 0 | 11.1 (10.5, 11.7) | 10.8 | 19.7 | 68.4 | 32.3 (27.8, 37.5) | 11.8 (10.8, 12.9) |
Concentrations in table are not adjusted for specific gravity.
Geometric mean in women as reported by Centers of Disease Control and Prevention (Centers for Disease Control and Prevention 2019)
ΣDEHP = umol/L, (MEHP*(1/278.34)) + (MEHHP*(1/294.34)) + (MEOHP*(1/292.33)) + (MECPP*(1/308.33))
Using WQS regression to assess adverse associations between phthalate mixtures and respiratory outcomes, we observed no association between the phthalate index and current wheeze (adjusted OR: 1.03 (95% CI: 0.86, 1.23)), current asthma (adjusted OR: 1.09 (95% CI: 0.89, 1.34)), or ever asthma (OR: 1.10 (95% CI: 0.91, 1.34)) (Fig. 1a). However, interactions between phthalate index and child sex were significant for current asthma (p-interaction = 0.019) and borderline for current wheeze (p-interaction = 0.062) and ever asthma (p-interaction = 0.094), corresponding to elevated odds of respiratory outcomes per quintile increase in phthalate index among boys (e.g., current asthma: adjusted OR: 1.35 (95% CI: 1.03, 1.78)), but not girls (e.g., current asthma: adjusted OR: 0.83 (95% CI: 0.61, 1.13)) (Supplemental Table 4). In models stratified by sex, we observed odds ratios similar to interaction model results (Fig. 2a–c). The phthalate index weights corresponding to adverse associations in boys were highest for MEP (39%, 39% and 40% for current wheeze, current asthma, and ever asthma, respectively), followed by MiBP and MBP (Fig. 2a–c). Interactions between phthalate index and maternal asthma were significant for current wheeze (p-interaction = 0.026) and current asthma (p-interaction = 0.014), and borderline for ever asthma (p-interaction = 0.060). Odds of child respiratory outcomes were elevated among women without a history of asthma, but not among women with asthma (Supplemental Table 4). In stratified analyses, adverse associations among women without a history of asthma were driven by MEP (36%) and MCOP (30%) for current wheeze; MEP (29%) and MiBP (26%) for current asthma; and MBP (30%) and MiBP (23%) for ever asthma (Fig. 3a–c).
Fig. 1. Odds ratios (95% confidence intervals) and weights from weighted quantile sum regression for maternal urinary phthalate index and childhood asthma/wheeze, (a) adverse and (b) protective associations.
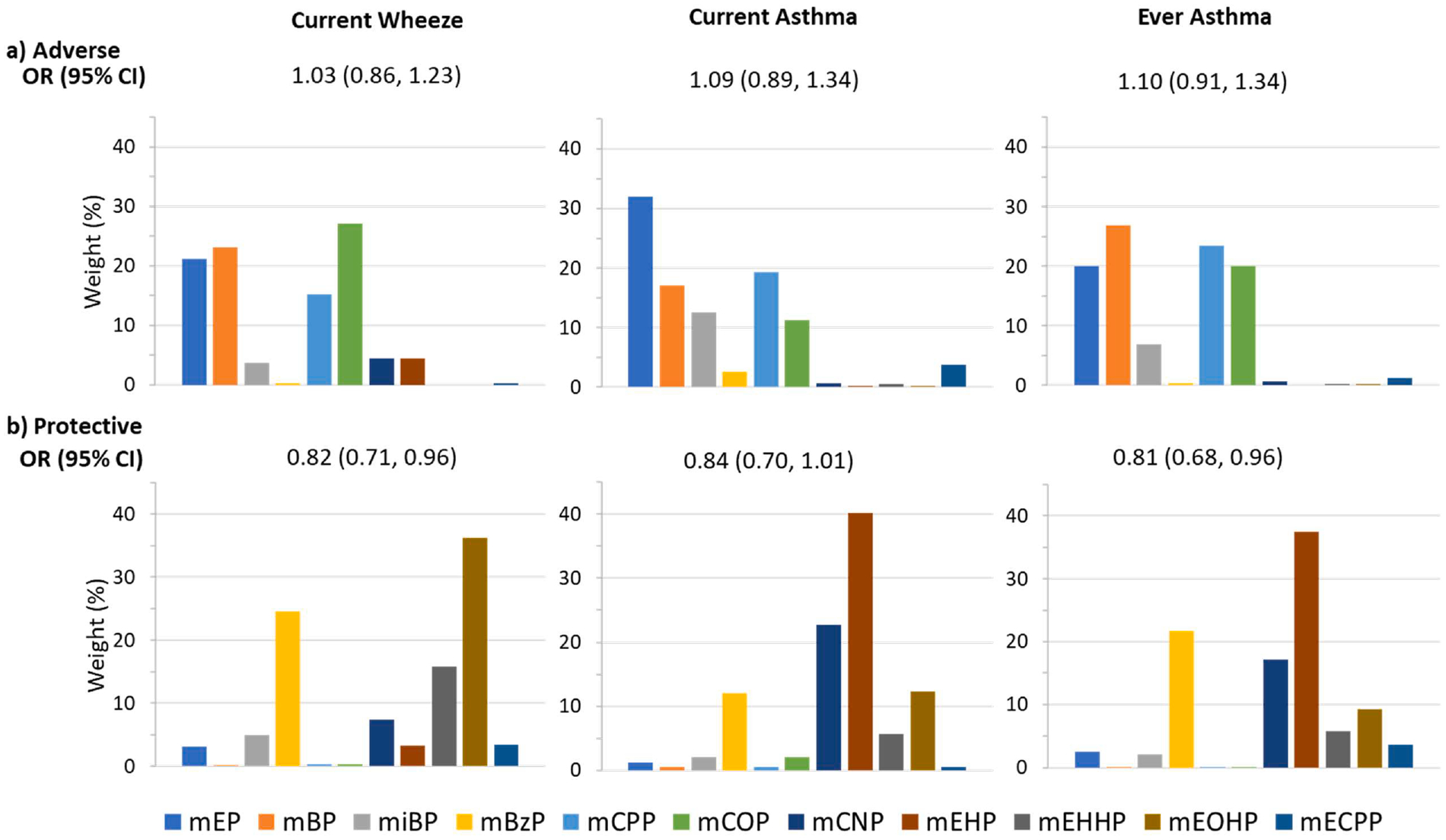
Models are adjusted for maternal age, race, ethnicity, education, prenatal smoking, pre-pregnancy BMI, asthma history, parity, birth year, study site and child sex.
Fig. 2. a-c. Stratified odds ratios (95% confidence intervals) and weights from weighted quantile sum regression for maternal urinary phthalate index and childhood a) current wheeze, b) current asthma, c) ever wheeze by child sex.
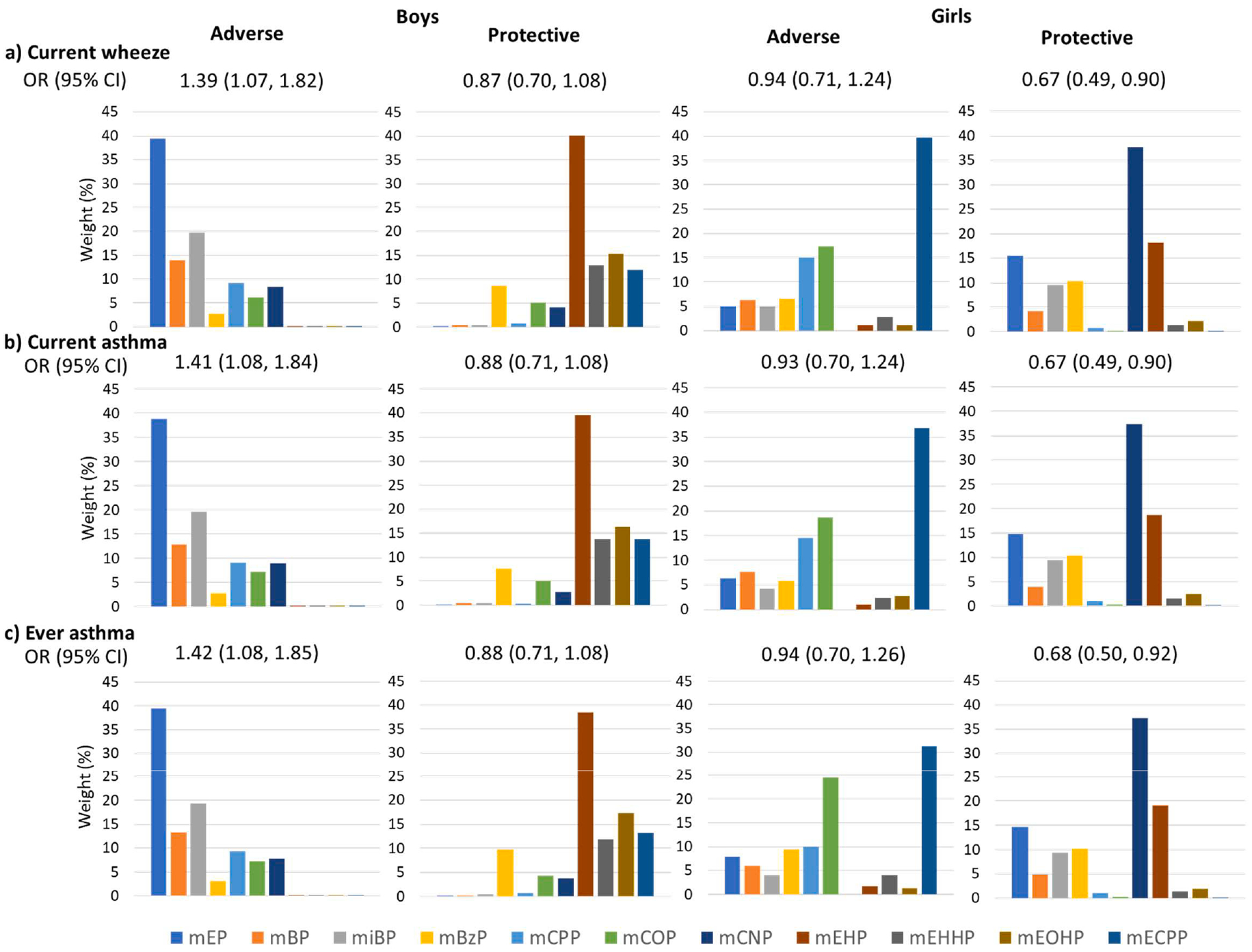
Adverse and protective models are shown. Models are adjusted for maternal race, ethnicity, age, education, prenatal smoking, pre-pregnancy BMI, parity, asthma history, birth year, and study site.
Fig. 3. a-c. Stratified odds ratios (95% confidence intervals) and weights from weighted quantile sum regression for maternal urinary phthalate index and childhood a) current wheeze, b) current asthma, c) ever wheeze by maternal asthma.
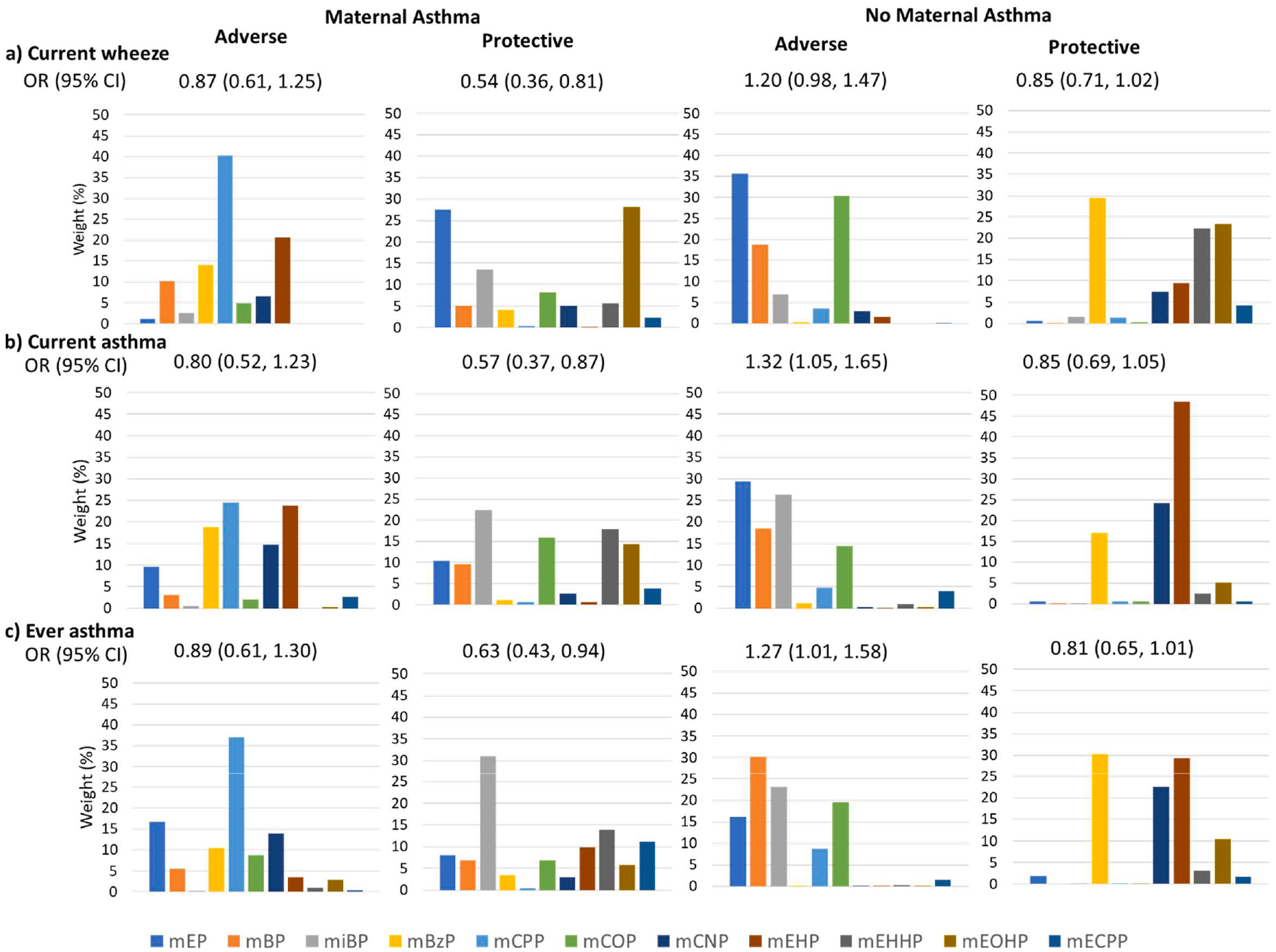
Adverse and protective models are shown. Models are adjusted for maternal race, ethnicity, age, education, prenatal smoking, pre-pregnancy BMI, parity, child sex, birth year, and study site.
Using WQS to assess protective associations, we observed lower relative odds per quintile increase in phthalate index for all outcomes (Fig. 1b). Several high-molecular weight phthalate metabolites, such as MEOHP (36%, current wheeze), MEHP (40%, current asthma; 37% ever asthma), MCNP (23% current asthma) and MBzP (25% current wheeze; 22% ever asthma) were the primary contributors to these associations. No significant interactions with child sex or maternal asthma were observed for protective associations (p-interaction for current wheeze, current asthma and ever asthma by child sex = 0.318, 0.295 and 0.492, respectively; p-interaction for current wheeze, current asthma and ever asthma by maternal asthma = 0.192, 0.542 and 0.595, respectively; Supplemental Table 4). However, in sex-stratified models, odds ratios were significantly protective for current wheeze, current asthma and ever asthma in girls, but not boys, and the distribution of weights for each metabolite differed between boys and girls (Fig. 2a–c). In girls, the highest weighted metabolite was MCNP (38% for current wheeze; 37% for current asthma; 37% for ever asthma), followed by MEHP. In boys, the highest weighted metabolite was MEHP. In WQS models stratified by maternal asthma (Fig. 3a–c), protective associations were larger among dyads with maternal asthma; associations were not statistically significant in dyads without maternal asthma. The phthalate index was weighted most heavily by MEOHP, MEP or MiBP in women with asthma (MEOHP = 28% and MEP = 27% for current wheeze; MiBP = 22% for current asthma; MiBP = 31% for ever asthma), and by MBzP (30% current wheeze; 30% ever asthma) and MEHP (48% current asthma; 29% ever asthma) among women without asthma.
3.1. Sensitivity analyses
In sensitivity analyses, we observed similar associations for both adverse and protective models if DEHP metabolites were modeled as a sum, with ΣDEHP weighted at 40% (current wheeze), 34% (current asthma) and 25% (ever asthma) in protective models and having negligible weight (< 1%) in adverse models. Additional adjustment for gestational age did not modify protective or adverse WQS results. Lastly, to further assess robustness, we repeated the primary WQS analysis excluding one study site at a time. Excluding the largest site (CANDLE [TN]) resulted in decreased precision and a loss of statistical significance for all models (Supplemental Fig. 7). For example, the OR for a protective association with ever asthma changed from 0.81 (95%CI 0.68, 0.96) using the full sample to 0.67 (95% CI 0.42, 1.05) after CANDLE exclusion. Exclusion of CANDLE also modified metabolite weights within the phthalate index (data not shown). Exclusion of any TIDES site did not substantially modify the estimated ORs or confidence intervals (Supplemental Fig. 7), and while there was some variability in metabolite weights, the qualitative patterns and top-ranked metabolites in the statistically significant protective associations were not substantially changed (data not shown). We conclude that our main findings are robust to site exclusion on the basis that the 95% confidence intervals of each sensitivity analysis included the point estimate derived from the full analytic sample.
3.2. Secondary analyses
Multivariable logistic regression models for individual metabolites revealed few associations between third trimester phthalates and respiratory outcomes (Table 3). DEHP metabolites tended to be inversely associated with respiratory outcomes; in particular, 2-fold higher MEHP was associated with reduced odds of current wheeze (OR: 0.91 (95% CI: 0.84, 0.99)), current asthma (OR: 0.88 (95% CI: 0.80, 0.97)), and ever asthma (OR: 0.90 (95% CI: 0.82, 0.98)). MEP and MBP were associated with increased odds of wheeze and asthma in unadjusted models, however estimates were substantially attenuated following adjustment for covariates. MBzP was not significantly associated with any outcome in these models, but results should be interpreted cautiously given a potential U-shaped dose–response relationship (Supplemental Figs. 1–3).
Table 3.
Association of prenatal phthalate metabolite with asthma and wheeze outcomes at 4–6 years of age in combined CANDLE/TIDES cohort.
| Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Unadjusted | Adjusted | |
| Current Wheeze | ||
| MEP | 1.08 (1.02, 1.15) | 1.02 (0.94, 1.10) |
| MBP | 1.16 (1.04, 1.30) | 1.00 (0.87, 1.15) |
| MiBP | 1.05 (0.93, 1.18) | 0.93 (0.81, 1.07) |
| MBzP | 1.05 (0.96, 1.14) | 0.92 (0.84, 1.01) |
| MCPP | 1.03 (0.94, 1.14) | 1.04 (0.93, 1.17) |
| MCOP | 0.93 (0.87, 1.00) | 1.01 (0.92, 1.11) |
| MCNP | 0.87 (0.80, 0.94) | 0.94 (0.84, 1.04) |
| ΣDEHP | 0.93 (0.83, 1.04) | 0.88 (0.77, 1.00) |
| MEHP | 0.93 (0.86, 1.01) | 0.91 (0.84, 0.99) |
| MEHHP | 0.95 (0.88, 1.03) | 0.93 (0.85, 1.02) |
| MEOHP | 0.93 (0.84, 1.02) | 0.87 (0.77, 0.98) |
| MECPP | 0.95 (0.85, 1.06) | 0.91 (0.80, 1.03) |
| Current Asthma | ||
| MEP | 1.13 (1.06, 1.20) | 1.06 (0.97, 1.15) |
| MBP | 1.22 (1.08, 1.38) | 1.05 (0.89, 1.23) |
| MiBP | 1.10 (0.97, 1.25) | 0.98 (0.83, 1.15) |
| MBzP | 1.09 (0.99, 1.21) | 0.95 (0.84, 1.06) |
| MCPP | 0.99 (0.90, 1.09) | 1.02 (0.90, 1.15) |
| MCOP | 0.89 (0.82, 0.96) | 0.98 (0.88, 1.09) |
| MCNP | 0.81 (0.74, 0.89) | 0.91 (0.81, 1.03) |
| ΣDEHP | 0.97 (0.86, 1.09) | 0.91 (0.79, 1.05) |
| MEHP | 0.90 (0.82, 0.99) | 0.88 (0.80, 0.97) |
| MEHHP | 0.97 (0.88, 1.07) | 0.95 (0.86, 1.05) |
| MEOHP | 0.97 (0.86, 1.08) | 0.91 (0.79, 1.05) |
| MECPP | 0.99 (0.88, 1.12) | 0.96 (0.84, 1.10) |
| Ever Asthma | ||
| MEP | 1.10 (1.03, 1.18) | 1.04 (0.95, 1.14) |
| MBP | 1.21 (1.07, 1.38) | 1.06 (0.91, 1.23) |
| MiBP | 1.08 (0.95, 1.23) | 0.96 (0.83, 1.12) |
| MBzP | 1.05 (0.95, 1.17) | 0.91 (0.81, 1.02) |
| MCPP | 1.03 (0.93, 1.14) | 1.03 (0.91, 1.16) |
| MCOP | 0.94 (0.87, 1.01) | 1.01 (0.91, 1.12) |
| MCNP | 0.86 (0.79, 0.94) | 0.93 (0.82, 1.06) |
| ΣDEHP | 0.94 (0.83, 1.05) | 0.89 (0.78, 1.02) |
| MEHP | 0.93 (0.85, 1.01) | 0.90 (0.82, 0.98) |
| MEHHP | 0.96 (0.89, 1.05) | 0.95 (0.87, 1.05) |
| MEOHP | 0.93 (0.83, 1.04) | 0.89 (0.78, 1.01) |
| MECPP | 0.96 (0.85, 1.08) | 0.92 (0.81, 1.05) |
Values below limit of detection (LOD) are imputed as LOD/√2. Phthalate concentrations are corrected for specific gravity and log-transformed. Robust standard errors were used. Adjusted estimates are adjusted for maternal age, maternal race, maternal ethnicity, maternal education, prenatal smoking, pre-pregnancy BMI, maternal history of asthma, child sex, year of child’s birth and study site. Odds ratios are reported per 2-fold increase in phthalate concentration.
For current asthma, a marginal interaction was detected between MEP and child sex (p-interaction = 0.053), suggesting increased relative odds in boys (OR: 1.13 (95% CI 1.01, 1.26), but not girls. Sexstratified estimates also indicated inverse associations for DEHP metabolites were generally stronger in boys than girls, although interactions were not significant, and that MCNP was inversely associated with current wheeze/asthma in girls, but not boys (Supplement Table 5). Maternal asthma significantly modified the association between MiBP and ever asthma (with maternal asthma: OR: 0.70 (95% CI: 0.52, 0.94); without maternal asthma: OR: 1.11 (95% CI: 0.92, 1.34), p-interaction = 0.008) and current asthma (p-interaction = 0.016), but no other interactions by maternal asthma were observed (Supplement Table 5).
4. Discussion
In this combined analysis of two large prospective cohort studies, we investigated the association between prenatal exposure to phthalate mixtures, characterized by third trimester urinary phthalate metabolites, and childhood wheeze and asthma at age 4 to 6 years of age. Characterizing adverse associations, we observed increased odds of wheeze and asthma in boys, but not girls, and among dyads where mothers did not have a history of asthma, but not among those with maternal asthma. These increased odds of wheeze and asthma were associated with a phthalate index predominantly weighted by low-molecular weight compounds (MEP, MiBP and MBP). MCOP was also weighted heavily in associations among women without asthma. In analyses designed to characterize protective associations, we observed reduced odds of wheeze and asthma with increasing exposure to phthalate index weighted heavily by DEHP metabolites such as MEHP and MEOHP overall, and by MCNP in girls. While regression analysis of individual metabolites identified some similar associations (e.g., reduced odds of asthma and wheeze with MEHP exposure), analysis of individual metabolites detected few adverse associations. By implementing a mixtures approach, we have characterized simultaneous exposure to multiple phthalate compounds and have identified associations between phthalate mixtures and wheeze/asthma not fully observable with traditional methods.
Protective associations for phthalate indices heavily weighted by MEHP and other DEHP metabolites were unexpected but are not entirely inconsistent with prior studies. While several prior epidemiologic studies have reported adverse associations between prenatal DEHP metabolites and other high molecular weight phthalates (e.g., MBzP and MCOP) and childhood asthma (Berger et al., 2019; Gascon et al., 2015; Whyatt et al., 2014) or wheeze (Gascon et al., 2015; Ku et al., 2015), several recent studies have reported null associations (Berger et al., 2019; Buckley et al., 2018; Jahreis et al., 2018; Ku et al., 2015; Vernet et al., 2017). Among these prior studies, Gascon et al is the only one to assess phthalate exposure in early pregnancy. Our study characterized third trimester exposures (first trimester samples were not collected in CANDLE), thus highlighting a potential importance of exposure timing in the prenatal phthalate-asthma relationship. Additionally, the samples sizes for previous studies have ranged from n = 136 (Ku et al., 2015) to n = 587 (Vernet et al., 2017). Several report non-significant protective effect estimates for ΣDEHP or DEHP metabolites and asthma that are comparable in magnitude to odds ratios reported in our study (Berger et al., 2019; Buckley et al., 2018; Ku et al., 2015). It is therefore possible that small sample size contributed to null findings in the prior literature and that our study, with a sample of n = 1481, is the first study with sufficient sample size to detect significantly protective effects. Nonetheless, it remains unclear whether we are detecting a true association. Residual confounding from un-measured sociodemographic or environmental factors (e.g., housing and indoor air quality) may have influenced our results. Further, multiple animal studies suggest maternal phthalate exposure is associated with increased airway inflammation in both juvenile and adult offspring (Jahreis et al., 2018; Wang et al., 2018). In a notable exception, Shin et al observed that murine maternal DEHP exposure significantly reduced inflammatory cell counts and proinflammatory cytokines in offspring following an ovalbumin challenge (Shin et al., 2014), consistent with the protective association we observed.
Prenatal exposure to low molecular weight phthalates has also been inconsistently associated respiratory outcomes in children. Many studies report null associations for prenatal MEP, MBP and MiBP exposures and childhood asthma or wheeze (Berger et al., 2018; Gascon et al., 2015; Ku et al., 2015; Vernet et al., 2017). Alternatively, Berger et al observed that prenatal MEP was associated with decreased lung function, and MBP was associated with an increased T helper (Th) 2 response (Berger et al., 2018), which plays an important role in asthma pathophysiology (Barnes, 2001). Jahreis et al. reported elevated odds of childhood asthma in association with prenatal MEP and MBP (Jahreis et al., 2018), and Whyatt et al reported that MBP, but not MEP, was associated with increased risk of asthma (Whyatt et al., 2014). Buckley et al. observed significant interactions with MEP and infant sex for both wheeze and asthma outcomes (Buckley et al., 2018). For both outcomes, MEP was associated with reduced odds in girls and non-significantly increased odds in boys (Buckley et al., 2018). Our study, in comparison, demonstrated significantly increased odds of wheeze and asthma in relation to a phthalate index heavily weighted by MEP in boys, but not girls.
The mechanism for a sex-specific association between MEP and childhood asthma is unclear, and in general, studies of MEP’s biologic activity are limited. Like other phthalates, MEP has been associated with increased oxidative stress during pregnancy (Ferguson et al., 2015). In vitro administration of diethyl phthalate, MEP’s parent compound, to peripheral blood mononuclear cells has been shown to influence cytokine production, including suppression of Th1 cell mediated interferon (IFN)-gamma, interleukin (IL)-2 and tumor necrosis factor (TNF)-alpha (Hansen et al., 2015; Romagnani, 2000). Innate immune response is known to differ by sex (Jaillon et al., 2019), suggesting a sex-specific immune response to phthalates is plausible. Phthalates are also recognized endocrine disruptors, and steroid hormones may play a role in the development in asthma (Fuseini and Newcomb, 2017). However, MEP has low antiandrogenic and estrogenic potency relative to other phthalates (Hong et al., 2005; Varshavsky et al., 2016). Additional research designed to assess sex-specific effects of low-molecular weight phthalates and asthma are needed to further explore plausible mechanisms.
We assessed maternal asthma status as a proxy for genetic risk for asthma, also considering that existing asthma may modify the maternal or fetal immunologic response to phthalate exposure. While we observed little evidence for statistical interaction between phthalates and maternal asthma, our findings suggest that phthalate mixtures heavily weighted by low molecular weight phthalates (MEP, MiBP) may be associated with childhood asthma in dyads where women do not have a history of asthma, but protective among women with asthma. In general, maternal asthma is a predominant risk factor for childhood asthma; therefore, it is plausible that the contribution of phthalate exposure to the increased asthma risk is weak relative to family history and only manifests in the absence of genetic risk (i.e., women without asthma). However, it will be important for these associations to be studied and confirmed in other large study populations. The protective association among women with asthma could plausibly be explained, in part, by product use patterns leading to low-molecular weight phthalate exposure. Women with severe or poorly controlled asthma, who are at increased risk of having a child with asthma relative to women with mild asthma (Liu et al., 2018), may have been inclined to actively avoid low-molecular weight phthalate-containing products (fragrances, cosmetics, personal care products) (Zota et al., 2014), while women with mild asthma were more tolerant and, thus, more highly exposed. However, our study was not designed to assess product use or maternal asthma severity during pregnancy, and thus this explanation is speculative.
Our study has many strengths. We employed WQS regression to estimate associations with a phthalate index comprised of multiple correlated metabolites. While findings were generally similar using WQS and traditional individual metabolite regression analyses, the WQS approach can be more sensitive and provides additional insights into associations by combining the individual metabolite concentrations into a single-index exposure measure and simultaneously quantifying the relative importance of each mixture component. Using WQS allows for the evaluation of real-life exposure to mixtures that humans experience daily, as opposed to the traditional approach that looks at individual metabolite/outcome relationships. In addition, our combined cohort design capitalizes on two well-characterized cohorts to generate the largest study of prenatal phthalates and childhood asthma to date. The CANDLE cohort includes a racially and socioeconomically diverse sample of dyads in the Mid-South region of the U.S., with 60% of women identifying as Black. Few previous studies of prenatal phthalates and child health outcomes have characterized exposures in predominantly Black cohorts or within this geographic region. Inclusion of TIDES introduces additional sociodemographic and geographic diversity, lending to wider generalizability of our findings.
Our study also has limitations to consider. First, our study characterized phthalate exposure using single spot urines collected in the third trimester of pregnancy. Because phthalates have short half-lives, a single urinary measure is representative of only recent exposures. Certain phthalate metabolites, such as MEP and MBP, have been shown to be relatively stable across much of pregnancy, while other metabolites are likely to vary over time (Braun et al., 2012). Therefore, potentially relevant exposures occurring prior to the third trimester may not be well represented in our data. In addition, while the ISAAC questionnaire has been validated and widely used to identify cases of asthma and wheeze (Asher et al., 1995; Lukrafka et al., 2010), outcomes are based on parent report. Finally, since WQS regression includes a supervised learning algorithm wherein weights are estimated from the data prior to fitting a regression model to estimate the effect size and corresponding confidence interval and p-value, there is a danger of overestimating statistical significance (e.g., Type 1 error may exceed the nominal 0.05). One solution to this problem is to perform WQS on two independent data sets—one to build the WQS regression index through bootstrap sampling, and the second to test the significance of the signal from the created index. Especially given the central role of stratified analyses in this study, we did not consider the sample size large enough to obtain stable results if we split the data into a discovery data set and a validation dataset. Therefore, we estimated weights using the same data used to test for significance.
In conclusion, our findings suggest that increasing exposure to phthalate mixtures heavily weighted by high molecular weight compounds, including MEHP, MEOHP and MBzP, are associated with decreased odds of childhood asthma and wheeze. Exposure to mixtures heavily weighted by low molecular weight compounds (MEP, MBP, and MiBP) are associated with increased odds of childhood asthma and wheeze in boys and in children born to women without a history of asthma. By combining two well-characterized cohorts, our study represents the largest study of prenatal phthalate exposure and child respiratory health to date. Modification of the association between prenatal MEP concentrations and childhood asthma by sex has been observed in one previous investigation but has not been widely studied overall. Further research is needed to replicate these findings, as well as explore potential mechanisms related to phthalate activity in the male fetal environment and in non-asthmatic women.
Supplementary Material
Acknowledgments
ECHO PATHWAYS is funded by NIH (1UG3OD023271-01, 4UH3OD023271-03). The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute and NIH (R01 HL109977). The Infant Development and the Environment Study (TIDES) was funded by NIH (R01ES016863, 1 R01 ES25169). We are grateful for the participation of families enrolled in the CANDLE and TIDES cohorts, as well as the dedication of CANDLE and TIDES research staff and investigators.
Footnotes
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.105970.
References
- Adams SN, Adgent MA, Gebretsadik T, Hartman TJ, Vereen S, Ortiz C, et al. , 2019. Prenatal vitamin D levels and child wheeze and asthma. J. Matern Fetal Neonatal. Med 1–9. 10.1080/14767058.2019.1607286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Perera FP, Jedrychowski W, Camann DE, Barr D, Jacek R, et al. , 2003. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environmental Health Perspectives 111 (14), 1719–1722. 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. , 2008. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environmental Health Perspectives 116 (4), 467–473. 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ, 2009. Status of Childhood Asthma in the United States, 1980–2007. Pediatrics 123 (Supplement 3), S131–S145. 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. , 1995. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. EurRespirJ 8 (3), 483–491. 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, Xue J, De Carvalho BP, Iyer A, Abualnaja KO, Yaghmoor SS, et al. , 2016. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environmental Research 150, 573–581. 10.1016/j.envres.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, 2001. Th2 cytokines and asthma: an introduction. Respir. Res 2 (2), 64–65. 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, Eskenazi B, Balmes J, Holland N, Calafat AM, Harley KG, 2018. Associations between prenatal maternal urinary concentrations of personal care product chemical biomarkers and childhood respiratory and allergic outcomes in the CHAMACOS study. Environment International 121 (Pt 1), 538–549. 10.1016/j.envint.2018.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, Eskenazi B, Balmes J, Kogut K, Holland N, Calafat AM, et al. , 2019. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatric Allergy and Immunology 30 (1), 36–46. 10.1111/pai.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J, 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. American Industrial Hygiene Association Journal 54 (10), 615–627. 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Lindh C, Reichenberg A, Wikstrom S, Unenge Hallerback M, Evans SF, et al. , 2018. Association of Prenatal Phthalate Exposure With Language Development in. Early Childhood. JAMA Pediatr 172 (12), 1169–1176. 10.1001/jamapediatrics.2018.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. , 2012. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental Health Perspectives 120 (5), 739–745. 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Quiros-Alcala L, Teitelbaum SL, Calafat AM, Wolff MS, Engel SM, 2018. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7years. Environment International 115, 79–88. 10.1016/j.envint.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P, 2015. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 20 (1), 100–120. 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. Available: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf [accessed October 10, 2019 Volume One].
- Chalubinski M, Kowalski ML, 2006. Endocrine disrupters–potential modulators of the immune system and allergic response. Allergy 61 (11), 1326–1335. 10.1111/j.1398-9995.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- Chen SQ, Chen JN, Cai XH, Chen GR, Gao Y, Ge RS, et al. , 2010. Perinatal exposure to di-(2-ethylhexyl) phthalate leads to restricted growth and delayed lung maturation in newborn rats. J. Perinatal Med 38 (5), 515–521. 10.1515/JPM.2010.083. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Bellavia A, James-Todd T, Correia KF, Valeri L, Messerlian C, et al. , 2018. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: A comparison of three statistical approaches. Environment International 113, 231–239. 10.1016/j.envint.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK, et al. , 2015. Analysis of Environmental Chemical Mixtures and Non-Hodgkin Lymphoma Risk in the NCI-SEER NHL Study. Environmental Health Perspectives 123 (10), 965–970. 10.1289/ehp.1408630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R, 2005. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental Health Perspectives 113 (11), 1530–1535. 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Cantonwine DE, Rivera-Gonzalez LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, et al. , 2014. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environmental Sci. Technol 48 (12), 7018–7025. 10.1021/es502076j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD, 2015. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ. Health Perspectives 123 (3), 210–216. 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuseini H, Newcomb DC, 2017. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma Rep 17 (3), 19. 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Casas M, Morales E, Valvi D, Ballesteros-Gomez A, Luque N, et al. , 2015. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol 135 (2), 370–378. 10.1016/j.jaci.2014.09.030. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wu Q, Kannan K, 2011. Phthalate metabolites in urine from China, and implications for human exposures. Environment Int. 37 (5), 893–898. 10.1016/j.envint.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Guo Y, Weck J, Sundaram R, Goldstone AE, Louis GB, Kannan K, 2014. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2’-deoxyguanosine, a biomarker of oxidative stress: longitudinal investigation of fertility and the environment study. Environ. Sci. Technol 48 (16), 9804–9811. 10.1021/es5024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JF, Nielsen CH, Brorson MM, Frederiksen H, Hartoft-Nielsen ML, Rasmussen AK, et al. , 2015. Influence of phthalates on in vitro innate and adaptive immune responses. PLoS ONE 10 (6), e0131168. 10.1371/journal.pone.0131168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N, Huen K, Tran V, Street K, Nguyen B, Bradman A, et al. , 2016. Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy. Toxics 4 (1). 10.3390/toxics4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, Ji YK, Choi KC, Manabe N, Jeung EB, 2005. Conflict of estrogenic activity by various phthalates between in vitro and in vivo models related to the expression of Calbindin-D9k. J. Reprod. Dev 51 (2), 253–263. 10.1262/jrd.16075. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occupational Environ. Hygiene 5 (1), 46–51. 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Jaakkola JJ, Knight TL, 2008. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environ. Health Perspect 116 (7), 845–853. 10.1289/ehp.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahreis S, Trump S, Bauer M, Bauer T, Thurmann L, Feltens R, et al. , 2018. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J. Allergy Clin. Immunol 141 (2), 741–753. 10.1016/j.jaci.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Jaillon S, Berthenet K, Garlanda C, 2019. Sexual Dimorphism in Innate Immunity. Clinical Rev. Allergy Immunol 56 (3), 308–321. 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Norgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, et al. , 2012. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environmental Health Perspectives 120 (6), 897–903. 10.1289/ehp.1104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC, 2015. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. J. Allergy Clin. Immunology 135 (3), 655–662. 10.1016/j.jaci.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, et al. , 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environmental Health Perspectives 122 (5), 521–528. 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku HY, Su PH, Wen HJ, Sun HL, Wang CJ, Chen HY, et al. , 2015. Prenatal and postnatal exposure to phthalate esters and asthma: a 9-year follow-up study of a taiwanese birth cohort. PLoS ONE 10 (4), e0123309. 10.1371/journal.pone.0123309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Agerbo E, Schlunssen V, Wright RJ, Li J, Munk-Olsen T, 2018. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J. Allergy Clinical Immunology 141 (3), 886–892. 10.1016/j.jaci.2017.05.016.e883. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Li EH, Xu DL, Sun WL, Hong Y, Zhao W, et al. , 2016. Genetic research and structural dysplasia assessment of anorectal malformations in neonatal male rats induced by di(n-butyl) phthalate. Environmental Toxicology 31 (3), 261–268. 10.1002/tox.22040. [DOI] [PubMed] [Google Scholar]
- Lukrafka JL, Fuchs SC, Moreira LB, Picon RV, Fischer GB, Fuchs FD, 2010. Performance of the ISAAC questionnaire to establish the prevalence of asthma in adolescents: a population-based study. J. Asthma 47 (2), 166–169. 10.3109/02770900903483766. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH, 2009. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philosophical Transactions Royal Society of London. Series B, Biological Sci. 364 (1526), 2097–2113. 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Asimakopoulos AG, Barbosa F Jr., Kannan K, 2017. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environment 586, 152–162. 10.1016/j.scitotenv.2017.01.193. [DOI] [PubMed] [Google Scholar]
- Romagnani S, 2000. T-cell subsets (Th1 versus Th2). Annals Allergy, Asthma Immunology 85 (1), 9–21. 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- Romano ME, Eliot MN, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR, et al. , 2018. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Int. J. Hygiene Environ. Health 221 (4), 623–631. 10.1016/j.ijheh.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosicarelli B, Stefanini S, 2009. DEHP effects on histology and cell proliferation in lung of newborn rats. Histochemistry Cell Biology 131 (4), 491–500. 10.1007/s00418-008-0550-4. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG, 2003. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technology 37 (20), 4543–4553. 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Grady R, Barrett ES, Redmon B, Nguyen RHN, Barthold JS, et al. , 2016. First trimester phthalate exposure and male newborn genital anomalies. Environmental Research 151777–782. 10.1016/j.envres.2016.07.043. [DOI] [PubMed] [Google Scholar]
- Serrano SE, Karr CJ, Seixas NS, Nguyen RH, Barrett ES, Janssen S, et al. , 2014. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int. J. Environ. Res. Public Health 11 (6), 6193–6215. 10.3390/ijerph110606193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TE, Currie GP, Koudelka CW, Simpson EL, 2011. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J. Investigat. Dermatology 131 (1), 67–73. 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin IS, Lee MY, Cho ES, Choi EY, Son HY, Lee KY, 2014. Effects of maternal exposure to di(2-ethylhexyl)phthalate (DEHP) during pregnancy on susceptibility to neonatal asthma. Toxicology Appl. Pharmacology 274 (3), 402–407. 10.1016/j.taap.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Shu H, Jonsson BA, Gennings C, Svensson A, Nanberg E, Lindh CH, et al. , 2018. Temporal trends of phthalate exposures during 2007–2010 in Swedish pregnant women. J. Expo Sci. Environ. Epidemiol 28 (5), 437–447. 10.1038/s41370-018-0020-6. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. , 2004. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental Health Perspectives 112 (3), 331–338. 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, Calafat AM, 2007. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 860 (1), 106–112. 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Smith LA, Hatcher-Ross JL, Wertheimer R, Kahn RS, 2005. Rethinking race/ethnicity, income, and childhood asthma: racial/ethnic disparities concentrated among the very poor. Public Health Reports 120 (2), 109–116. 10.1177/003335490512000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, et al. 2015. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description Available: https://www.rand.org/pubs/research_reports/RR1336.html. [accessed August 14 2017].
- Soomro MH, Baiz N, Philippat C, Vernet C, Siroux V, Nichole Maesano C, et al. , 2018. Prenatal Exposure to Phthalates and the Development of Eczema Phenotypes in Male Children: Results from the EDEN Mother-Child Cohort Study. Environmental Health Perspectives 126 (2), 027002. 10.1289/EHP1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup A, Bragg JB, Andra SS, Curtin PC, Spear EA, Sison DB, et al. , 2018. Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS ONE 13 (3), e0193835. 10.1371/journal.pone.0193835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. , 2015. First trimester phthalate exposure and anogenital distance in newborns. Human Reproduction 30 (4), 963–972. 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Volgyi E, et al. , 2015. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive. Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients 7 (12), 9918–9930. 10.3390/nu7125499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky JR, Zota AR, Woodruff TJ, 2016. A Novel Method for Calculating Potency-Weighted Cumulative Phthalates Exposure with Implications for Identifying Racial/Ethnic Disparities among U.S. Reproductive-Aged Women in NHANES 2001–2012. Environmental Science & Technology 50 (19), 10616–10624. 10.1021/acs.est.6b00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, Pin I, Giorgis-Allemand L, Philippat C, Benmerad M, Quentin J, Calafat AM, Ye X, Annesi-Maesano I, Siroux V, Slama R, 2017. In Utero Exposure to Select Phenols and Phthalates and Respiratory Health in Five-Year-Old Boys: A Prospective Study. Environmental Health Perspectives 125 (9), 097006. 10.1289/EHP1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi E, Carroll KN, Hare ME, Ringwald-Smith K, Piyathilake C, Yoo W, et al. , 2013. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients 5 (5), 1511–1530. 10.3390/nu5051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Liu F, Dong J, You M, Fu Y, Li C, et al. , 2018. Maternal exposure to environmental DEHP exacerbated OVA-induced asthmatic responses in rat offspring. Science of the Total Environment 615, 253–261. 10.1016/j.scitotenv.2017.09.276. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu H, Kannan K, 2019. A Review of Biomonitoring of Phthalate Exposures. Toxics 7 (2). 10.3390/toxics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, et al. , 2018. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere 193, 394–402. 10.1016/j.chemosphere.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, et al. , 2014. Asthma in inner-city children at 5–11 years of age and prenatal exposure to phthalates: the Columbia Center for Children’s Environmental Health Cohort. Environmental Health Perspectives 122 (10), 1141–1146. 10.1289/ehp.1307670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Koch HM, Angerer J, Bruning T, 2011. Assessing exposure to phthalates - the human biomonitoring approach. Molecular Nutrition & Food Research 55 (1), 7–31. 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- Woods MM, Lanphear BP, Braun JM, McCandless LC, 2017. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environ Health 16 (1), 115. 10.1186/s12940-017-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Gennings C, Wright RJ, Wilson A, Burris HH, Just AC, et al. , 2018. Prenatal Stress, Methylation in Inflammation-Related Genes, and Adiposity Measures in Early Childhood: the Programming Research in Obesity, Growth Environment and Social Stress Cohort Study. Psychosomatic Medicine 80 (1), 34–41. 10.1097/psy.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Kwack SJ, Kim HS, Lee BM, 2014. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. J Toxicol Environ Health B Crit Rev 17 (3), 127–174. 10.1080/10937404.2014.882194. [DOI] [PubMed] [Google Scholar]
- Zeileis A, 2004. Econometric Computing with HC and HAC Covariance Matrix Estimators. Journal of Statistical Software 11 (10), 1–17. 10.18637/jss.v011.i10. [DOI] [Google Scholar]
- Zeileis A, 2006. Object-oriented computation of sandwich estimators. Journal of Statistical Software 16 (9), 1–16. 10.18637/jss.v016.i09. [DOI] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ, 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental Health Perspectives 122 (3), 235–241. 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


