Abstract
OBJECTIVE:
Assessing outcomes after pediatric critical illness is imperative to evaluate practice and improve recovery of patients and their families. We conducted a scoping review of the literature to identify domains and instruments previously used to evaluate these outcomes.
DESIGN:
Scoping Review.
SETTING:
We queried PubMed, EMBASE, PsycINFO, Cumulative Index of Nursing and Allied Health Literature, and the Cochrane Central Register of Controlled Trials Registry for studies evaluating pediatric critical care survivors or their families published between 1970-2017. We identified articles using key words related to pediatric critical illness and outcome domains. We excluded articles if the majority of patients were >18-years or <1-month-old, mortality was the sole outcome, or only instrument psychometrics or procedural outcomes were reported. We used dual review for article selection and data extraction and categorized outcomes by domain (overall health, emotional, physical, cognitive, health-related quality of life, social, family).
SUBJECTS:
Manuscripts evaluating outcomes after pediatric critical illness.
MEASUREMENTS AND MAIN RESULTS:
Of 60,349 citations, 407 articles met inclusion criteria; 87% were published after 2000. Study designs included observational (85%), interventional (7%), qualitative (5%), and mixed methods (3%). Populations most frequently evaluated were traumatic brain injury (n=96), general pediatric critical illness (n=87), and congenital heart disease (n=72). Family members were evaluated in 74 (18%) studies. Studies used a median of 2 [IQR 1-4] instruments and evaluated a median of 2 [IQR 2-3] domains. Social (n=223), cognitive (n=183) and overall health (n=161) domains were most frequently studied. Across studies, 366 unique instruments were used, most frequently the Wechsler and Glasgow Outcome scales. Individual domains were evaluated using a median of 77 [IQR 39-87] instruments.
CONCLUSIONS:
A comprehensive, generalizable understanding of outcomes after pediatric critical illness is limited by heterogeneity in methodology, populations, domains, and instruments. Developing assessment standards may improve understanding of post-discharge outcomes and support development of interventions after pediatric critical illness.
Keywords: pediatric, critical care outcomes, patient reported outcome measures, survivors, family, outcome assessment, health care
Introduction
As mortality of pediatric intensive care unit (PICU) patients declines, long-term morbidities facing survivors are increasingly recognized (1-4). These sequelae can affect cognitive, social, physical, and emotional health of survivors and their families and are collectively termed Post-Intensive Care Syndrome-pediatrics (PICS-p) (5). It is imperative that future trials evaluating the delivery and efficacy of pediatric critical care include both short-term outcomes, such as mortality or length of stay, and long-term, patient- and family-centered outcomes such as those described in the PICS-p framework. Design of such trials requires enhanced understanding of long-term outcomes after pediatric critical illness and the instruments used to evaluate them.
To improve our understanding of long-term outcomes after pediatric critical illness, the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network’s POST-PICU Investigators (Table S1, Supplemental Digital Content) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) collaborated to conduct a scoping review of pediatric critical care medicine (PCCM) outcomes research. This effort aimed to inform the development of a core outcome set (COS) for use in future research (6, 7). A COS delineates a minimum set of outcomes reported in studies within a specific field to improve the comparability of studies across time and populations and decrease bias from selective reporting (8, 9). To inform the COS and future studies, we aimed to identify the number of studies published, domains evaluated, and study designs and instruments used in PCCM outcomes research.
Materials and Methods
We conducted a scoping review to identify studies evaluating outcomes of survivors or families after pediatric critical illness published 1970-2017, inclusive. We searched PubMed, EMBASE, PsycINFO, Cumulative Index of Nursing and Allied Health Literature, and the Cochrane Central Register of Controlled Trials Registry using search strategies that included a combination of keywords and controlled vocabulary for the concept of “pediatric” and “critical care/illness” combined with comprehensive terms for the pre-specified domains of social, cognitive, emotional, physical, health-related quality of life (HRQL), and family functioning (Table S2, Supplemental Digital Content).
Articles were excluded if: 1) no post-discharge outcomes were assessed; 2) survival was the only outcome assessed; 3) only psychometric properties of an instrument were evaluated; 4) the outcome of a technical procedure/condition was evaluated without report of relationship to ICU care; 5) the majority of the study sample was >18 years old, preterm infants, neonates, or had not been definitively admitted to an ICU; 6) only one subject was included; or 7) the language was not English.
Citations produced from the above search were imported into Covidence® (Veritas Health Innovation. Melbourne, Australia) and independently screened in a two-stage process. First, two reviewers screened each abstract and excluded ineligible manuscripts. Second, two reviewers screened each full-text manuscript to determine final eligibility. For both stages, discrepancies were resolved by a third reviewer. For included articles, study characteristics and outcomes were independently extracted by two reviewers. Extracted data were compared and consensus was achieved through discussion. When consensus could not be achieved, a member of the steering committee was consulted for final determination. Study data were collected and managed using REDCap hosted at the University of Utah (10, 11). Study populations were classified as a targeted population (e.g. traumatic brain injury (TBI), trauma (other than TBI), congenital heart disease (CHD)), or as a general PICU population. Outcome domains were identified based on the World Health Organization’s International Classification of Functioning, Disability, and Health (ICF) framework for physical, emotional, cognitive and HRQL outcomes (12). In alignment with the PICS-p framework, social health and family domains were added (5). Instruments were classified by the domain(s) assessed. Based on evaluation of the instruments, an overall health domain was added. With the exception of the family domain, studies were categorized by the domain(s) evaluated. The family domain was identified based on whom the instrument evaluated. Data were summarized using frequency for categorical data and median and interquartile range [IQR] for continuous data.
Results
We identified 60,349 abstracts and excluded 55,683 (Figure S1, Supplemental Digital Content). The remaining 4,666 studies underwent dual full-text screening which further excluded 3,885 and identified 374 duplicates, resulting in 407 studies included (Table S3, Supplemental Digital Content).
The number of studies increased markedly after 2000, with 354 (87%) published after 2000 (Figure 1). Studies were from 45 countries, predominantly the United States (n=147), United Kingdom (n=56), and Canada (n=52) (Table 1). One-fifth of studies (n=77) included sites representing more than one country.
Figure 1. Number of manuscripts evaluating outcomes after pediatric critical illness and study designs.
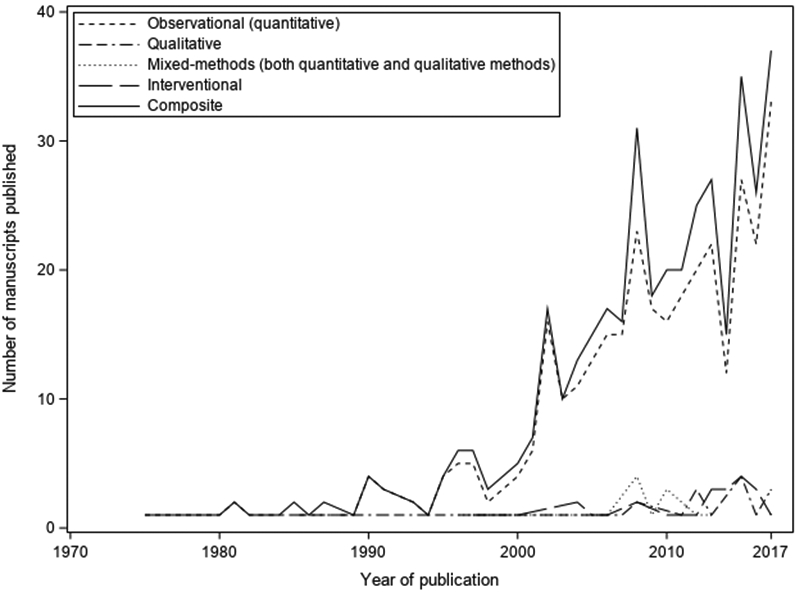
The study of outcomes after pediatric critical illness has increased substantially after 2000, with most of the articles using an observational study design.
Table 1.
Study Characteristics.
| Study Characteristic | All Studies N=407 |
Observational N=345 |
Interventional N=27 |
Qualitative N=21 |
Mixed Methods N=14 |
|---|---|---|---|---|---|
| Study Location, n (%) | |||||
| United States | 147 (36) | 121 (35) | 14 (52) | 10 (48) | 2 (14) |
| United Kingdom | 56 (14) | 36 (10) | 9 (33) | 6 (29) | 5 (36) |
| Canada | 52 (13) | 46 (13) | 6 (22) | 0 (0) | 0 (0) |
| Other Europeana | 40 (10) | 38 (11) | 0 (0) | 1 (5) | 1 (7) |
| Australia | 34 (8) | 27 (8) | 3 (11) | 2 (10) | 2 (14) |
| Netherlands | 32 (8) | 27 (8) | 0 (0) | 1 (5) | 4 (29) |
| Other | 108 (27) | 99 (29) | 8 (30) | 1 (5) | 0 (0) |
| Population | |||||
| Targeted population, n (%) | |||||
| General PICU | 87 (21) | 59 (17) | 7 (26) | 15 (71) | 6 (43) |
| Traumatic brain injury | 96 (24) | 81 (23) | 10 (37) | 3 (14) | 2 (14) |
| Congenital heart disease | 72 (18) | 69 (20) | 2 (7) | 0 (0) | 1 (7) |
| Cardiac arrest | 33 (8) | 30 (9) | 2 (7) | 0 (0) | 0 (0) |
| Sepsis | 27 (7) | 21 (6) | 2 (7) | 0 (0) | 4 (29) |
| Solid organ transplant | 15 (4) | 15 (4) | 0 (0) | 0 (0) | 0 (0) |
| Acute respiratory failure | 10 (2) | 10 (3) | 0 (0) | 0 (0) | 0 (0) |
| Trauma | 9 (2) | 8 (2) | 0 (0) | 1 (5) | 0 (0) |
| Bone marrow transplant | 2 (0.5) | 2 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Renal Failure/ESRD | 2 (0.5) | 2 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Oncology | 1 (0.3) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) |
| Other | 92 (23) | 86 (25) | 4 (15) | 1 (5) | 1 (7) |
| Family Members Evaluated | |||||
| Any | 74 (18) | 43 (12) | 5 (19) | 18 (86) | 8 (57) |
| Parent/Grandparent | 71 (17) | 41 (12) | 5 (19) | 17 (81) | 8 (57) |
| Sibling | 4 (1) | 2 (0.6) | 0 (0) | 2 (10) | 0 (0) |
| Targeted Enrollment Age | |||||
| Anchor: PICU Admission, n (%) | 287 (70) | 250 (72) | 24 (89) | 9 (43) | 4 (29) |
| Lowest (median [IQR]) | 0.02 [0.00-1.00] | 0.02 [0.00-1.00] | 0.25 [0.00-1.00] | 2.00 [0.00-5.00] | 0.58 [0.08-1.08] |
| Highest (median [IQR]) | 16.0 [10.0-18.0] | 16.0 [8.5-18.0] | 16.0 [9.5-17.0] | 16.0 [15.0-16.0] | 16.0 [8.5-18.0] |
| Anchor: post-discharge evaluation, n (%) | 63 (15) | 50 (14) | 1 (4) | 3 (14) | 9 (64) |
| Lowest (median [IQR]) | 5.50 [3.50-7.00] | 5.00 [3.50-7.00] | [-] | 3.00 [3.00-3.00] | 6.00 [6.00-7.00] |
| Highest (median [IQR]) | 16.0 [8.0-18.0] | 16.0 [8.0-18.0] | [-] | 9.50 [3.0-16.0] | 17.0 [17.0-17.0] |
| Targeted age not specified, n (%) | 56 (14) | 44 (13) | 2 (7) | 9 (43) | 1 (7) |
| Domain Assessments and Instruments | |||||
| Domains evaluated/study, median [IQR] | 2 [2-3] | 2 [1-3] | 2 [2-4] | 2 [1-2] | 3 [2-4] |
| Instruments/study, median [IQR] | 2 [1-4] | 2 [1-4] | 3 [1-6] | 1 [1-1] | 4 [2-5] |
Other European countries included Croatia, Czech Republic, Denmark, Finland, Ireland, Latvia, Lithuania, Norway, Poland, Portugal, Romania, Sweden, Switzerland, Turkey. PICU: Pediatric Intensive Care Unit; ESRD: end-stage renal disease; IQR: interquartile range.
Methodologic Characteristics
Among the 407 studies, 345 (85%) were observational, 27 (7%) interventional, 21 (5%) qualitative, and 14 (3%) mixed methods (Table 1). Interventional studies were more commonly conducted in the United States (52%) or United Kingdom (33%). Similarly, qualitative studies were primarily performed in the United States (48%) or United Kingdom (29%).
The TBI (n=96), general PICU (n=87), and CHD (n=72) populations were most frequently studied (Table 1). Certain study designs were more common in specific populations. For example, interventional studies were disproportionally conducted in the TBI and general PICU populations. Qualitative and mixed methods studies were most frequently conducted in the general PICU population.
Family members were evaluated in 74 (18%) studies (Table 1). Parents or grandparents were most frequently evaluated; only 4 (1%) studies evaluated siblings. A higher proportion of qualitative (86%) and mixed methods (57%) studies evaluated family members compared to other study designs.
Enrollment and Retention
Across all studies, the median enrollment rate was 86% [IQR 61-100] with observational studies having the highest rates (92%) followed by interventional (81%) and mixed methods (72%) (Table 2). Enrollment rate was not specified in 74 (18%) studies. Qualitative studies had the lowest enrollment rates (44% [IQR 20-90]) and highest proportion of articles (43%) that did not specify an enrollment rate. Sample size varied widely across studies with a median of 65 [IQR 32-132] subjects. When reported, the median follow-up rate (of patients eligible at hospital discharge) was 88% [IQR 68-100]; 84 (21%) studies did not report the extent of missing data at the final follow-up time point. In addition to other measurements, half of the studies reported post-discharge mortality, which was a median of 2.3% [IQR 0-12].
Table 2.
Enrollment, mortality, and retention rate.
| Study Characteristic | All Studies N=407 |
Observational N=345 |
Interventional N=27 |
Qualitative N=21 |
Mixed Methods N=14 |
|---|---|---|---|---|---|
| Enrollment rate | |||||
| Median % [IQR] | 86 [61-100] | 92 [63 -100] | 81 [56 -95] | 44 [20-90] | 72 [45-81] |
| Not specified, n studies (%) | 74 (18) | 56 (16) | 6 (22) | 9 (43) | 3 (21) |
| Sample size, median [IQR] | 65 [32-132] | 69 [35-140] | 75 [31-174] | 18 [11-35] | 75 [18-102] |
| Retention ratea | |||||
| Median % [IQR] | 88 [68-100] | 88 [68-100] | 89 [81-99] | 43 [25-60] | 69 [67-72] |
| Not reported, n studies (%) | 84 (21) | 72 (21) | 4 (15) | 6 (29) | 2 (14) |
| Post-discharge mortality | |||||
| Median % [IQR] | 2.3 [0.0-12.0] | 3.4 [0.0-12.2] | 0.0 [0.0-1.9] | 0.0 [0.0-0.0] | 1.1 [0.0-5.1] |
| Not measured, n studies (%) | 204 (50) | 160 (46) | 14 (52) | 18 (86) | 12 (86) |
Retention rate is defined as subjects for whom data was collected at the final follow-up time point. To calculate the retention rate, we excluded studies that evaluated family members (n=74) and studies missing either number of subjects eligible for follow-up at hospital discharge or number assessed at last follow-up time (n=92).
Data Collection
One post-discharge time point was typically evaluated (median 1 [IQR 1-1]) with a range of up to 11. The distribution of the final follow up time point was bimodal, occurring most commonly within the first year or after 3 years (Figure S2, Supplemental Digital Content).
Data were most frequently collected from the parents/guardians (54%) and/or patient (54%) (Table 3). In 97 (24%) studies, the source of data was not specified. The method of assessment was most frequently in-person (49%) followed by telephone (22%) (Table 3, Figure S3, Supplemental Digital Content). Nearly one-fifth of studies (n=68) used multiple methods of assessment. The method of assessment was not reported in 110 (27%) studies. Baseline (pre-PICU) data were collected in only 45 (11%) studies.
Table 3.
Study characteristics by domain assessed.
| Study Characteristic | All (n=407) |
Overall Health (n=161) |
Social (n=223) |
Cognitive (n=183) |
Emotional (n=111) |
Physical (n=102) |
HRQL (n=73) |
Family (n=74) |
|---|---|---|---|---|---|---|---|---|
| Unique instruments | 366 | 39 | 49 | 115 | 78 | 77 | 22 | 87 |
| Studies reporting > 1 instrument/domain, n (%) | 200 (49) | 19 (12) | 70 (31) | 99 (54) | 58 (52) | 44 (43) | 13 (18) | 39 (53) |
| Article to instrument ratioa | 1.11 | 4.13 | 4.55 | 1.59 | 1.42 | 1.32 | 3.32 | 0.85 |
| Source of datab, n (%) | ||||||||
| Parent/Guardian | 220 (54) | 70 (43) | 134 (60) | 64 (35) | 89 (80) | 32 (31) | 59 (81) | 70 (95) |
| Patient | 218 (54) | 54 (34) | 95 (43) | 121 (66) | 42 (38) | 70 (69) | 38 (52) | 8 (11) |
| Medical Record | 46 (11) | 25 (16) | 15 (7) | 12 (7) | 1 (0.9) | 10 (10) | 2 (3) | 0 (0) |
| Sibling | 2 (0.5) | 0 (0) | 1 (0.5) | 1 (0.6) | 1 (0.9) | 0 (0) | 0 (0) | 2 (3) |
| Other | 21 (5) | 1 (0.6) | 7 (3) | 5 (3) | 10 (9) | 3 (3) | 0 (0) | 3 (4) |
| Not reported | 97 (24) | 54 (34) | 51 (23) | 44 (24) | 8 (7) | 18 (18) | 6 (8) | 0 (0) |
| Method of assessment, n (%) | ||||||||
| In-person | 200 (49) | 47 (29) | 104 (47) | 116 (63) | 62 (56) | 60 (59) | 26 (36) | 36 (49) |
| Phone | 89 (22) | 43 (27) | 57 (26) | 28 (15) | 19 (17) | 15 (15) | 21 (29) | 13 (18) |
| Standard Mail | 65 (16) | 12 (7) | 35 (16) | 6 (3) | 38 (34) | 2 (2) | 23 (32) | 28 (38) |
| Chart Review | 55 (14) | 29 (18) | 17 (8) | 17 (9) | 2 (2) | 18 (18) | 1 (1) | 2 (3) |
| Email/web | 8 (2) | 2 (1) | 6 (3) | 0 (0) | 3 (3) | 0 (0) | 4 (5) | 3 (4) |
| Other | 22 (5) | 9 (6) | 8 (4) | 5 (3) | 3 (3) | 5 (5) | 0 (0) | 2 (3) |
| Not Specified | 110 (27) | 52 (32) | 56 (25) | 48 (26) | 25 (23) | 23 (23) | 15 (21) | 11 (15) |
| Studies using > 1 method of assessment, n (%) | 68 (17) | 25 (16) | 37 (17) | 18 (10) | 23 (21) | 14 (14) | 12 (16) | 15 (20) |
| Frequency instruments collected using > 1 method, n (%) | 124/1206 (10) | 27/182 (15) | 48/323 (15) | 23/466 (5) | 34/216 (16) | 18/187 (10) | 14/90 (16) | 34/159 (21) |
| Baseline data collected, n (%) | 45 (11) | 13 (8) | 18 (8) | 21 (11) | 8 (7) | 7 (7) | 8 (11) | 4 (5) |
Article to instrument ratio: the quotient number of articles to the number of unique instruments. A higher ratio indicates greater consolidation around a core set of instruments.
Some manuscripts described more than one data source. The manuscripts in the family domain with the patient as the source of data used instruments that collected data from both the patient and the family member pertaining to both the patient and the family member. HRQL: health-related quality of life; IQR: interquartile range.
Domain Assessments and Instruments
Studies evaluated a median of 2 domains [IQR 2-3] (Table 1). Across all studies, 366 unique outcome instruments were used, yielding an article to instrument ratio of 1.11, indicating significant heterogeneity of instruments (Table 3). Studies used a median of 2 [IQR 1-4] instruments (Table 1). Eighty-five (21%) articles used 5 or more instruments. The most frequently used instruments were the Wechsler and the Glasgow Outcomes scales used in 82 (20%) and 75 (18%) studies, respectively (Table S4, Supplemental Data File).
Overall Health
Overall health was evaluated in 161 (40%) studies using 39 instruments (Table 3). This domain was frequently studied in the TBI and trauma populations (Figure S4, Table S5, Supplemental Digital Content). The article to instrument ratio was the second highest among all domains at 4.13, suggesting better consolidation around a core set of instruments (Table 3). Studies most frequently used the Glasgow Outcome Scales (47%) followed by the Pediatric Overall Performance Category (17%) (Table S4, Supplemental Data File). Outcomes were measured most frequently from parents/guardians (43%) or patients (34%) through in-person (29%) or telephone (27%) contact. Source of data and method of assessment were not specified in 34% and 32% of studies, respectively. Baseline data were collected in 8% of studies.
Social
Social outcomes were evaluated in 223 (55%) studies using 49 instruments. This domain was frequently studied in the TBI, solid organ transplant, and CHD populations. The article to instrument ratio was 4.55, highest of any domain. Socially relevant instruments frequently overlapped with other domains. For example, the most frequent instrument used was the Glasgow Outcome Scales, used in 34% of manuscripts evaluating the social domain and in 18% of all studies. Additional commonly employed instruments capturing elements of social functioning were the Child Behavior Checklist (16%) and Bayley Scales of Infant Development (13%). Outcomes were most commonly evaluated from the parents/guardians (60%) or patients (43%) through in-person (47%) or telephone (26%) contact. Many articles did not specify the data source (23%) or method of assessment (25%). Baseline data were reported in 8% of studies.
Cognitive
Cognitive outcomes were reported in 183 (45%) studies using 115 instruments. Cognitive outcomes were frequently studied in the cardiac arrest and CHD populations. The article to instrument ratio was 1.59. Instruments most frequently used were the Wechsler Intelligence Scales (45%), neurologic evaluations including EEG and radiographic studies (21%), and the Pediatric Cerebral Performance Category (20%). Patients (66%) or parents/guardians (35%) were the most common sources of information through in-person (63%) or telephone (15%) contact. Many articles did not specify the data source (24%) or method of assessment (26%). Baseline cognitive function data were collected in 11% of studies.
Emotional
Emotional outcomes were measured in 111 (27%) studies using 78 instruments. These outcomes were frequently studied in the general PICU, sepsis, and CHD populations. The article to instrument ratio was 1.42. The most commonly used instruments were the Child Behavioral Checklist (32%) and the Impact of Events Scale (21%). Parents/guardians (80%) or patients (38%) reported outcomes most frequently in-person (56%) or by standard mail (34%). Data source was not specified in 7% of studies and 23% did not specify method of assessment. Only 7% of studies included baseline emotional assessments.
Physical
The physical domain was evaluated in 102 (25%) studies using 77 instruments. Physical outcomes were frequently studied in the acute respiratory distress syndrome and solid organ transplant populations. The article to instrument ratio was 1.32. Studies most commonly evaluated sensory evaluations such as hearing and vision (20%), cardiac functional measures (17%), growth parameters (13%), and pulmonary evaluations (13%). The Functional Independence Measures (including the pediatric version, WeeFIM), used in 10% of studies, was the most frequently used instrument. The source of data was most often the patients (69%) or parents/guardians (31%) through in-person (59%) contact. The data source was not reported in 18% of studies and method of assessment was not specified in 23%. Baseline physical data were reported in 7% of studies.
Health-related quality of life
Health-related quality of life was evaluated in 73 (18%) studies using 22 instruments. These outcomes were frequently studied in the general PICU, sepsis, and trauma populations. The article to instrument ratio was 3.32. The most commonly used instruments were the Health State Utility Index (23%), the Pediatric Quality of Life Inventory (23%), and the Child Health Questionnaire (22%). These studies relied significantly on proxy-reporting, with 81% of studies using parents/guardians as the data source while also collecting directly from the patient in 52% of studies most often by in-person (36%) contact or standard mail (32%). The source of data was not reported in 8% of studies and method of assessment was not specified in 21%. Baseline data was collected in 11% of studies.
Family
Family function was evaluated in 74 (18%) studies using 87 instruments. The general PICU population was most frequently studied. The article to instrument ratio was 0.85. The most commonly used instruments were the Impact of Events Scale (15%) and the General Health Questionnaire (14%). Parents/guardians (95%) most frequently provided these data. Most studies measured family effects by in-person contact (49%) or standard mail (38%). The source of data was reported in all studies and method of assessment was not specified in 15%. Baseline data was reported in 5% of studies.
Discussion
We identified 407 articles evaluating post-discharge outcomes after pediatric critical illness. TBI and general PICU populations were studied most frequently, followed by CHD patients. A comprehensive, generalizable understanding of long-term outcomes is limited by tremendous heterogeneity in study design and methodology and by incomplete reporting of missing data (13). Additionally, there is wide variability in the combinations of domains evaluated and instruments used without consolidation around a core set of instruments. The diverse scope of domains evaluated, instruments used, and variable follow-up periods reflect different priorities among PCCM researchers and the multi-dimensionality of outcomes research. The steep increase in publications after 2000 reflects increasing attention to this topic. These findings highlight the importance of understanding long-term outcomes while underscoring the need for development and implementation of a COS to guide future research.
The generalizability of study results was influenced by variability in several aspects of study design and methodology including populations evaluated, source and method of data collection, and follow-up intervals. Conclusions about the long-term effects of critical illness are challenged by the heterogeneous populations studied because patient-level (age, development, socioeconomic status) or disease-specific (natural progression, cure, treatment side effects) factors may affect outcomes after discharge. The source of data (patients, parents/guardians) and the method of assessment (direct observation, telephone, mail) were not standardized, introducing potential bias and affecting comparability of results.
Additional methodologic variability occurred in the choice of follow-up time points. Most studies utilized only a single time point without determining pre-hospitalization baseline functioning, a particularly important component due to the high rates of comorbidities in PICU patients but particularly challenging to collect given the unpredictable, infrequent nature of most critical illness (14-16). The limited number of follow-up evaluations hinders the ability to attribute long-term outcomes to the hospitalization itself or identify post-discharge trajectories or sensitive periods of recovery. While it is important to maintain flexibility in study methodology, developing standards for how and from whom to assess outcomes, when feasible, will reduce differential sources of bias between studies, improve the comparability of studies across populations, locations, and time points, and better describe the natural history of morbidity and recovery.
Missing data further limits the generalizability of individual studies (13). Enrollment and retention rates were inconsistently reported, making it difficult to understand specific barriers to participation. Patients not enrolled and those lost to follow-up may introduce important systematic biases. Missing data may represent patients more likely to be disadvantaged who may be most vulnerable to worse outcomes (17-19). Improved standards for reporting as described by the STROBE guidelines and standardization of study methodology will enhance the quality and reliability of outcomes research (20).
Generalizability and the ability to pool study results for comparison and meta-analyses are also limited by the heterogeneity of outcome domains and instruments used. Most studies evaluated more than one domain, yet the combination of assessed domains varied. There was poor consolidation around a core set of instruments without agreement about which instruments may be best suited to assess a specific domain.
The lack of standardization in operational definitions of long-term outcomes, assessment instruments, and follow-up intervals and durations among studies yields results that are not only difficult to pool for comparison and meta-analysis but also reflective of different research priorities (21). For example, fewer than 25% of studies evaluated physical, HRQL and family functioning outcomes. In particular, the paucity of attention to family functioning is concerning as poor family functioning is associated with worse outcomes after TBI and may affect recovery after all types of pediatric critical illness (22, 23).
Our findings mirror trends observed in the adult literature (24). The scoping review of adult ICU survivorship included studies from a similar timeframe (1970-2013) and utilized analogous methodology. While the absolute number of studies included in both reviews is challenging to compare directly (n=407 versus n=425), an unexpected finding was the similarity in the timing of growth in attention to ICU survivorship (4, 18, 25-28). The year 2000 reflected a turning point for both fields with a subsequent proliferation in the number of published studies (300 for adults and 354 for pediatrics). Similarly, the numbers of instruments used to assess outcomes were vast and, in both the adult and pediatric reviews, the consolidation around which instruments to use was low (article to instrument ratios of 1.7 versus 1.1, respectively). The proportion of interventional studies was similar (7%), representing an opportunity to enhance the use of long-term outcomes when measuring the effectiveness of ICU interventions (29).
Our scoping review has important limitations. While we employed an exhaustive search strategy, some relevant articles may have been missed. Additionally, data extraction was complicated by variability in reporting among studies. To ensure accuracy, we performed dual extraction with discrepancies resolved by discussion. Evaluation of non-standardized instruments also posed a challenge. Instruments and outcomes were classified into domain(s) based on the information reported in the manuscripts and the data extracted. Notably, there was significant overlap between domains, with many instruments evaluating more than one domain. Likewise, our domain list was not exhaustive, and some outcomes (e.g. health resource use) were not fully evaluated.
Conclusions
This scoping review highlights increasing efforts to describe the long-term outcomes of critically-ill children and their families. The heterogeneity of these studies and populations evaluated highlights the need to develop consensus around research methodologies, reporting standards, domains, instruments, and follow-up time points to standardize future research efforts specific to pediatric critical care. The combined efforts of PALISI’s POST-PICU investigators and CPCCRN have initiated the crucial next steps in developing a core outcome set and instrument recommendations. The recommendations will incorporate the quantitative assessment outlined in this manuscript, our group’s qualitative study interviewing PICU survivors and their parents, and additional assessment provided by our expert panel (6). This will improve comparability of studies over time and across populations but will need to be balanced with flexibility to ensure that important uncommon or newly identified outcomes are not overlooked.
Supplementary Material
Acknowledgements
We would like to acknowledge the outstanding work of our additional library scientists including Tisha Mentnech at North Carolina University, Mary McFarland at the University of Utah, Carolyn Biglow at the University of Pittsburgh Medical Center, and Richard James at the University of Pennsylvania and the support of Martha A.Q. Curley, PhD, RN, FAAN at the University of Pennsylvania for the methodological advising and library scientist support.
Copyright form disclosure: Dr. Maddux’s institution received funding from Francis Family Foundation, National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) K23HD096018. Drs. Maddux, Fink, Killien, Ringwood, Smith, Olson, Sorenson, Meert, Pollack, and Mourani received support for article research from the NIH. Drs. Fink, Ringwood, Meert, Pollack, and Mourani’s institutions received funding from the NIH. Drs. Killien, Smith, Olson, and Sorenson’s institutions received funding from the NICHD. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Conflicts of Interest and Sources of Funding: Supported, in part, by NICHD K23HD096018 (Maddux) and the Francis Family Foundation (Maddux). Additional funding was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. It was approved by the CPCCRN Steering Committee and funded by grant numbers U01-HD049934, UG1-HD049983 (Fink), UG1-HD083171 (Mourani), UG1-HD050096 (Meert), UG1-HD049981 (Pollack). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The remaining authors have disclosed that they do not have any conflicts of interest.
Footnotes
Site: Data management and statistical analyses completed at the University of Utah.
References
- 1.Hartman ME, Saeed MJ, Bennett T et al. : Readmission and Late Mortality After Critical Illness in Childhood. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2017; 18(3):e112–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herridge MS, Chu LM, Matte A et al. : The RECOVER Program: Disability Risk Groups and 1-Year Outcome after 7 or More Days of Mechanical Ventilation. American journal of respiratory and critical care medicine 2016; 194(7):831–844. [DOI] [PubMed] [Google Scholar]
- 3.Needham DM, Wozniak AW, Hough CL et al. : Risk factors for physical impairment after acute lung injury in a national, multicenter study. American journal of respiratory and critical care medicine 2014; 189(10):1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto NP, Rhinesmith EW, Kim TY et al. : Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2017; 18(3):e122–e130. [DOI] [PubMed] [Google Scholar]
- 5.Manning JC, Pinto NP, Rennick JE et al. : Conceptualizing Post Intensive Care Syndrome in Children-The PICS-p Framework. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2018; 19(4):298–300. [DOI] [PubMed] [Google Scholar]
- 6.Fink EL, Jarvis JM, Maddux AB et al. : Development of a Core outcome set for Pediatric critical care outcomes research. Contemp Clin Trials 2020:105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham MT, Rajic A, Greig JD et al. : A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Research synthesis methods 2014; 5(4):371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbull AE, Sepulveda KA, Dinglas VD et al. : Core Domains for Clinical Research in Acute Respiratory Failure Survivors: An International Modified Delphi Consensus Study. Critical care medicine 2017; 45(6):1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke M: Standardising outcomes for clinical trials and systematic reviews. Trials 2007; 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Minor BL et al. : The REDCap consortium: Building an international community of software platform partners. Journal of biomedical informatics 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009; 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwashyna TJ, Netzer G: The burdens of survivorship: an approach to thinking about long-term outcomes after critical illness. Seminars in respiratory and critical care medicine 2012; 33(4):327–338. [DOI] [PubMed] [Google Scholar]
- 13.Sterne JA, White IR, Carlin JB et al. : Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ (Clinical research ed) 2009; 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balamuth F, Weiss SL, Neuman MI et al. : Pediatric severe sepsis in U.S. children’s hospitals. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2014; 15(9):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson RS, Carcillo JA, Linde-Zwirble WT et al. : The epidemiology of severe sepsis in children in the United States. American journal of respiratory and critical care medicine 2003; 167(5):695–701. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman JJ, Banks R, Berg RA et al. : Trajectory of Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Critical care medicine 2020; 48(3):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddux AB, Zimmerman JJ: Awake or Sedate . . . Do We Know the Best State? American journal of respiratory and critical care medicine 2018; 197(11):1378–1380. [DOI] [PubMed] [Google Scholar]
- 18.Watson RS, Asaro LA, Hertzog JH et al. : Long-Term Outcomes after Protocolized Sedation versus Usual Care in Ventilated Pediatric Patients. American journal of respiratory and critical care medicine 2018; 197(11):1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aspesberro F, Fesinmeyer MD, Zhou C et al. : Construct Validity and Responsiveness of the Pediatric Quality of Life Inventory 4.0 Generic Core Scales and Infant Scales in the PICU. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2016; 17(6):e272–279. [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M et al. : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of clinical epidemiology 2008; 61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 21.Angus DC, Mira JP, Vincent JL: Improving clinical trials in the critically ill. Critical care medicine 2010; 38(2):527–532. [DOI] [PubMed] [Google Scholar]
- 22.Sameroff AJ: Environmental risk factors in infancy. Pediatrics 1998; 102(5 Suppl E):1287–1292. [PubMed] [Google Scholar]
- 23.Keenan HT, Runyan DK, Nocera M: Longitudinal follow-up of families and young children with traumatic brain injury. Pediatrics 2006; 117(4):1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnbull AE, Rabiee A, Davis WE et al. : Outcome Measurement in ICU Survivorship Research From 1970 to 2013: A Scoping Review of 425 Publications. Critical care medicine 2016; 44(7):1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollack MM, Holubkov R, Funai T et al. : Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Critical care medicine 2015; 43(8):1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollack MM, Holubkov R, Funai T et al. : Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2014; 15(9):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quasney MW, Lopez-Fernandez YM, Santschi M et al. : The outcomes of children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2015; 16(5 Suppl 1):S118–131. [DOI] [PubMed] [Google Scholar]
- 28.Watson RS, Choong K, Colville G et al. : Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. The Journal of pediatrics 2018; 198:16–24. [DOI] [PubMed] [Google Scholar]
- 29.Spragg RG, Bernard GR, Checkley W et al. : Beyond mortality: future clinical research in acute lung injury. American journal of respiratory and critical care medicine 2010; 181(10):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


