Abstract
Background:
Women can be exposed to a multitude of hardships prior to and during pregnancy that may affect fetal growth, but previous approaches have not analyzed them jointly as social exposure mixtures.
Methods:
We evaluated the independent, mutually adjusted, and pairwise joint associations between self-reported hardships and birthweight for gestational age z-scores in the Chemicals in Our Bodies-2 prospective birth cohort (N=510) using G-computation. We examined financial hardship, food insecurity, job strain, poor neighborhood environment, low community standing, caregiving, high burden of stressful life events, and unplanned pregnancy collected via questionnaire administered in the second trimester of pregnancy. We used propensity scores to ensure our analyses had sufficient data support and estimated absolute differences in outcomes.
Results:
Food insecurity was most strongly associated with reduced birthweight for gestational age z-scores individually, with an absolute difference of −0.16, 95% CI −0.45, 0.14. We observed an unexpected increase in z-scores associated with poor perceived neighborhood environment (0.18, 95% CI −0.04, 0.41). Accounting for co-exposures resulted in similar findings. The pairwise joint effects were strongest for food insecurity in combination with unplanned pregnancy (−0.45, 95% CI-0.93, 0.02) and stressful life events (−0.42, 95% CI −0.90, 0.05). Poor neighborhood environment in combination with caregiving was associated with an increase in z-scores (0.47, 95% CI −0.01, 0.95).
Conclusions:
Our results are consistent with the hypothesis that experiencing food insecurity during pregnancy, alone and in combination with stressful life events and unplanned pregnancy, may affect fetal growth.
Keywords: stress, hardship, pregnancy, fetal growth, birthweight
Introduction
Poor fetal growth is associated with infant morbidity and mortality (1, 2), and there is evidence that restricted growth in-utero is associated with increased disease risk later in life (3). Causes of poor fetal growth, also known as intrauterine growth restriction or fetal growth restriction, are multifactorial (4). Placental insufficiency is recognized as the best-known predictor of fetal growth restriction (5, 6), but severe malformations and chromosomal disorders may also cause retarded growth, and mothers with severe preeclampsia and/or chronic hypertension with superimposed preeclampsia tend to have higher risks of delivering growth restricted infants (7).
Previous evidence suggests exposure to stressors prior to or during pregnancy may also increase the likelihood of fetal growth restriction (8-13). In particular, experiencing economic and/or social hardships or other stressful experiences during pregnancy can affect fetal growth via behavioral or physiologic mechanisms (9, 14-16). For example, women who experience stressful events or hardships are more likely to use substances, including cigarettes, alcohol, and illicit substances, while pregnant (17, 18). Use of such substances during pregnancy has been linked to increased risk of low birthweight and poor fetal growth (19-21). Furthermore, stressful experiences during pregnancy can dysregulate the maternal immune system (22, 23), increasing risk for infection, preeclampsia, and other pregnancy complications that have been tied to fetal growth restriction (24).
There are a number of adverse experiences that could have implications for both maternal and fetal health during pregnancy, and women are often exposed to a multitude of these stressors during their pregnancies (25). However, prior studies have tended to focus on individual hardships as an attempt to isolate their hypothesized individualistic effects. Exposure to one stressful experience or hardship is likely to be correlated with exposure to others, especially for those that are associated with lower maternal socioeconomic status. Studies that have examined multiple adverse experiences, like stressful life events checklists, exclude other hardships that may also play a role in shaping maternal stress during pregnancy (26). While some previous studies have examined multiple stressors in relation to low birthweight (27, 28), birthweight (29, 30), or small-for-gestational-age (31), and have mutually adjusted for other stressors, we are not aware of any that have examined the joint effects of multiple stressors, nor any that have focused on continuous measures of fetal growth. There is currently a lack of understanding about how multiple hardships jointly affect pregnancy outcomes; addressing this gap requires methods that can evaluate multiple stressors and their cumulative impacts.
Environmental epidemiologists have developed methodologies for studying the health effects of chemical mixtures (32, 33), in which the tendency for clustering or co-occurrence of chemicals is acknowledged and investigated. Due to the fact that chemicals tend to originate from a point source of pollution or to co-occur in industrial or commercial applications, novel approaches were necessary to evaluate the individual and joint relationships between these multiple correlated exposures and health outcomes (34). Mixtures methodology comprises both statistical methods and a conceptual approach that could be applied to studies of social factors that tend to co-occur in accordance with socioeconomic patterning. Socioeconomic position can be conceived as a point source of inequality and material hardship that has downstream effects on health.
In particular, given what is known about how fundamental causes of poor health, like poverty and low educational attainment, operate through access to resources and adverse life experiences (35-37), further attention toward examining sets of hardships jointly as social exposure mixtures is warranted (38, 39). This study aims to address this gap by examining multiple hardships that women may experience either prior to or during pregnancy, using an approach inspired by analysis of chemical mixtures in environmental epidemiology (40). We evaluate the joint and independent relationships of fetal growth with financial hardship, food insecurity, job strain, stressful life events, caregiving, low perceived social status, poor perceived neighborhood, and unplanned pregnancy.
Methods
Study population
Our study utilized the University of California, San Francisco Chemicals in Our Bodies-2 cohort, which recruited pregnant women in their second trimester from the Moffitt Long, Mission Bay, and Zuckerberg San Francisco General Hospitals during 2014-2018. Information about eligibility is available in the supplemental material. Mothers filled out questionnaires during the second trimester, which we linked to medical records from their pregnancy and delivery. The Institutional Review Boards of the University of California, San Francisco and Berkeley approved this study (Protocol # 13-12160).
Measurement of hardships
At the second trimester visit, we asked participants to answer questions from validated questionnaires about various sources of stress in their lives prior to and during pregnancy that have previously been linked to reduced fetal growth, including financial hardship (41), food insecurity (8), job strain (42), neighborhood environment (43, 44), stressful life events (26, 45), and unplanned pregnancy (46). We also investigated perceived low community standing and caregiving. Low community standing, or low perceived social status, has previously been associated with worse maternal health (47, 48), although to our knowledge no prior studies have evaluated subjective social status with infant birthweight or other measures of fetal growth. Caregiving has also been associated with stress and emotional burden, although most work has evaluated the effects on older adults (49). To our knowledge, no prior studies have evaluated the relationship between caregiving and fetal growth.
Caregiving
Women were classified as experiencing caregiving burden if they were often responsible for the care and well-being of a parent or older relative, or a child who needs or uses more medical care, mental health, or educational services than is usual.
Financial hardship
We categorized women as experiencing financial hardship if they had a household income below the 2017 poverty level for their family size, or if they found it difficult to pay for basics like food, housing, medical care, and heating.
Food insecurity
Women were considered to be food insecure if they responded that they had skipped or cut the size of meals, if they ate less than they felt they should, or if they were hungry but didn’t eat because there wasn’t enough money. They were also considered food insecure if the food they bought didn’t last and they didn’t have money to get more, or if they couldn’t afford to eat balanced and nutritious meals.
Job strain
We categorized women as experiencing job strain if they reported having a job that was high demand and low control. Women were classified as having high demand jobs if they were expected to do excessive amount of work, their pay was not fair given their accomplishments, or they felt too tired after work. Women were classified as having low control jobs if they were not able to make decisions or did not have opportunities to develop abilities in the workplace.
Low perceived social status
Women were classified as having low perceived social status if, when asked to rate themselves from 1-10 according to status in their community (50), gave themselves a 4 or below.
Poor perceived neighborhood environment
We asked women to rate their neighborhoods for collective efficacy, neighborhood safety, neighborhood satisfaction, and neighborhood physical disorder (51, 52). Women were classified as having poor neighborhood quality if they experienced low collective efficacy; did not feel safe in their neighborhood; reported loud noise, vacant lots, or heavy traffic in the neighborhood; did not agree that their neighborhood was a good place to live; or responded they would move out of their neighborhood if they could.
Stressful life events
Women were classified as experiencing a high burden of stressful life events if they experienced two or more of the following: close family member was sick and went to the hospital, separated from partner, moved to a new address, partner lost a job, she lost a job, partner didn’t want her to be pregnant, had a lot of bills she couldn’t pay, was in a physical fight, partner had legal problems, someone close to her had a substance use problem, someone close to her died, she or a close family member had immigration problems.
Unplanned pregnancy
We considered women to have an unplanned pregnancy if she didn’t want to be pregnant or wanted to be pregnant later.
We created binary indicators for each hardship, indicating the presence or absence of each exposure prior to or during pregnancy. Detailed explanations of the questions we asked participants and the coding used to characterize each measure are provided in the eAppendix.
Covariates
We identified covariates a priori based on factors we hypothesized would be related to socioeconomic status and fetal growth, which included maternal age, educational attainment, race/ethnicity, parity, marital status, and pre-pregnancy body mass index (BMI). Maternal age at delivery and pre-pregnancy BMI were abstracted from the medical record. Educational attainment, race/ethnicity, and parity were self-reported using the second-trimester questionnaire. We used multiple imputation with 30 imputed data sets to account for data missingness. The number of missing values that were imputed for each covariate and hardship are shown in eTable 1. A correlation matrix for the covariates and hardships is visualized in eFigure 1.
Fetal growth measurement
We assessed fetal growth using birthweight for gestational age z-scores, which were calculated from a population reference (53) to disentangle potential effects on gestational age versus fetal growth (54). Birthweight and gestational age at birth were both abstracted from the mother’s medical record which was linked to the child’s medical record at birth. We include birthweight in the eAppendix as a stand-alone measure for comparability with other studies and to enhance interpretability of the results. We did not control for gestational age in the birthweight models as it may operate as a mediator of the hardship-birthweight relationship (55).
Statistical analysis
To estimate the associations between hardships and fetal growth, we used linear regression. Since we modeled continuous outcomes and binary exposures, linear regression coefficients were equivalent to estimates of the average treatment effect of reported hardships on fetal growth using G-computation, which has been used to estimate population-level parameters (56), hypothetical interventions (57), and exposure mixtures (40, 58). We elected to use this method rather than weighted quantile sum regression (59), another approach to estimate the effects of exposure mixtures, which was used previously to study combinations of stressors and depressive or post-traumatic-stress-disorder symptoms on oxidative stress (60). We chose G-computation over weighted quantile sum regression because the latter does not allow for exposures to act in opposite directions, does not allow exposures to interact, and is best suited for continuous exposures due to the creation of quantiles for each of the exposures. Because our exposures were binary rather than continuous, we used G-computation rather than quantile G-computation.
We estimated the absolute difference in fetal growth associated with: 1) each individual hardship, 2) the mutually adjusted effects, for which we estimated the association of individual hardships while controlling for the remaining hardships, and 3) pairwise joint effects, in which exposure to a pair of hardships was compared to lack of exposure for both. For the estimation of pairwise joint effects, we included an interaction between the exposure pair, and controlled for the remaining hardships and covariates. Since we examined eight individual hardships, pairwise joint combinations resulted in 28 hardship pairs. We elected to focus on pairwise combinations rather than all hardships together because only one woman in our study experienced all of the hardships (eTable 3).
We calculated variances for the individual and mutually adjusted effects from the standard error of the hardship exposure coefficient for each imputed data set, and those for pairwise joint effects were calculated using the delta method. We used Rubin’s combining rules to calculate the final variance for each parameter (61), which we then used to calculate Wald-type 95% confidence intervals.
To ensure adequate support within the data for evaluating individual, mutually adjusted, and pairwise joint effects of an extensive set of stressors, we examined variability in exposure assignment within strata of covariates (62). To do this, we used logistic regression to predict for each participant the probability of experiencing each hardship (their propensity score). For the individual effects, propensity models were conditional on maternal age, race or ethnicity, educational attainment, and parity. For the mutually adjusted and pairwise joint effects, propensity models were conditional on the covariates and all remaining hardships. We chose covariates a priori as factors we hypothesized could influence the likelihood of experiencing the hardships considered in this study.
We then identified the area of common support, which is defined as the range between the minimum propensity score among participants who reported experiencing a hardship and the maximum propensity score among participants who did not report experiencing that hardship (Supplemental Figure 2). The area of common support was ascertained separately for each imputed data set and hardship. For each exposure and dataset, only those participants whose propensity scores fell within the area of common support were included in the analyses described below (62). The average sample sizes across imputed datasets for each exposure and effect type (individual, mutually adjusted, or pairwise joint) are provided in the Supplemental Tables 4 and 5.
Analyses were conducted using R version 3.6.0 (63); code is available in the eAppendix.
Results
On average, participants were approximately 32 years old at the time of delivery (Table 1). They were well-educated, the majority were White or Latina, and the index pregnancy was the first child for almost half of the women. Stressful life events (59% of our cohort reporting) and financial hardship (45% of our cohort reporting) were the most commonly experienced hardships. Low community standing was the least common hardship (11% reporting). Descriptive statistics of maternal characteristics by individual hardship exposure status are available in eTable 2. The number of hardships experienced, and the most common combinations of hardships, are described in eTable 3.
Table 1.
Descriptive statistics of study participants.
| Overall (N=510) | |
|---|---|
| Birthweight (grams) | |
| Mean (SD) | 3,331 (587) |
| Birthweight for gestational age z-score | |
| Mean (SD) | 0.07 (1.05) |
| Age (years) | |
| Mean (SD) | 32.4 (5.4) |
| Pre-pregnancy BMI (kg/m2) | |
| Mean (SD) | 25.9 (5.6) |
| Educational attainment | |
| Less than high school | 61 (12%) |
| High school / GED | 78 (15%) |
| Some college | 64 (13%) |
| College degree | 120 (23%) |
| Master's degree | 104 (21%) |
| Doctoral degree | 83 (16%) |
| Household income | |
| Less than $40,000/year | 194 (38%) |
| $40,000-$79,000/year | 66 (13%) |
| $80,000 or more/year | 251 (49%) |
| Marital status | |
| Married | 343 (67%) |
| Widowed, separated, or divorced | 25 (5%) |
| Never married | 141 (28%) |
| Race/ethnicity | |
| Latina | 175 (34%) |
| Asian or Pacific Islander | 86 (17%) |
| Black | 37 (7%) |
| White | 186 (37%) |
| Other or multiple | 25 (5%) |
| Parity | |
| 0 | 251 (49%) |
| 1 | 155 (30%) |
| 2 | 73 (14%) |
| 3+ | 32 (6%) |
| Hardships | |
| Caregiving | 81 (16%) |
| Financial hardship | 231 (45%) |
| Food insecurity | 84 (16%) |
| Job strain | 80 (16%) |
| Low perceived community standing | 56 (11%) |
| Poor perceived neighborhood environment | 124 (24%) |
| Stressful life events | 300 (59%) |
| Unplanned pregnancy | 145 (29%) |
Note: These are averages across the multiply imputed datasets and therefore will not in all cases sum to exactly 510.
Restriction to the area of common support resulted in different sample sizes for each hardship, and for the individual and mutually adjusted effects. For example, we included 507 out of 510 in the individual analysis of stressful life events and 507 out of 510 in the analysis of unplanned pregnancy, whereas the individual analysis of food insecurity included 325 women (eTable 4). Controlling for remaining hardships further restricted the support within the data for all hardships except job strain and caregiving, for which the support increased. The mutually adjusted analysis of stressful life events included 488 women and unplanned pregnancy 500 women, while the mutually adjusted effects of food insecurity included 238 women.
When examined individually, the hardships with the strongest negative relationships with birthweight for gestational age z-scores were food insecurity (−0.16 (95% confidence interval (CI) −0.45, 0.14)) and unplanned pregnancy (−0.10 (95% CI −0.31, 0.11)) (Figure 1 and eTable 4). The associations did not change meaningfully when we controlled for the other exposures—the mutually adjusted associations for −0.21 (95% CI −0.53, 0.11) for food insecurity and −0.12 (95% CI −0.33, −0.10) for unplanned pregnancy. In the individual analysis, financial hardship was associated with slightly reduced birthweight for gestational age z-scores (−0.02 (95% CI −0.35, 0.31)), but was associated with increased birthweight in the mutually adjusted analysis (0.09 (95% CI −0.26, 0.44)), although both estimates were very imprecise. Poor perceived neighborhood was associated with an increase in z-scores in the individual (0.18 (95% CI −0.04, 0.41)) and mutually adjusted analyses (0.24 (95% CI 0.00, 0.48)).
Figure 1.
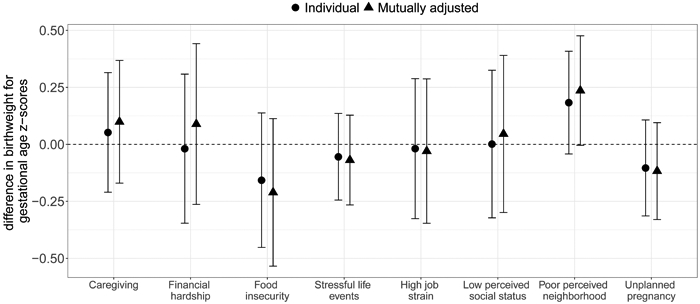
Differences in birthweight for gestational age z-scores and 95% CI associated with reported hardships prior to and during pregnancy, analyzed individually and mutually adjusted for other hardships.
Note: Exposures are binary indicators of experiencing each hardship during pregnancy. Positivity was assessed for each comparison and the sample was restricted to the area of common support (see eTable 3 for the sample sizes for each comparison). Outcome models were adjusted for maternal age, educational attainment, race/ethnicity, parity, marital status, and pre-pregnancy BMI. Mutually adjusted results additionally control for other hardships.
Food insecurity and unplanned pregnancy were both associated with a reduction in birthweight in the individual analyses, with differences of −139.8 g (95% CI −306.7, 27.2) and −78.1 g (95% CI −203.0, 46.9), respectively (eFigure 3 and eTable 4). These patterns were also observed in the mutually adjusted analysis, with differences in birthweight of −154.1 g (95% CI −333.5, 25.3) and −79.5 (95% CI −204.4, 45.5) for food insecurity and unplanned pregnancy, respectively. Job strain was associated with a difference in birthweight of −174.5 g (95% CI −340.6, −8.4) in the individual analyses and −179.0 g (95% CI −350.4, −7.5) in the mutually adjusted. It was not associated with a difference in birthweight for gestational age z-scores in either the individual (−0.02 (95% CI −0.33, 0.29)) or the mutually adjusted analyses (−0.03 (95% CI −0.35, 0.29)).
When we examined pairwise combinations of hardships, we observed the strongest negative associations with birthweight for gestational age z-scores and food insecurity and unplanned pregnancy (−0.45 (95% CI −0.93, 0.02)] and food insecurity and stressful life events (−0.42 g (95% CI −0.90, 0.05)] (Figure 2 and eTable 5). In contrast, poor perceived neighborhood and caregiving and poor perceived neighborhood and financial hardship had the strongest positive relationships (0.47 (95% CI −0.01, 0.95) and 0.38 (95% CI −0.09, 0.85), respectively).
Figure 2.
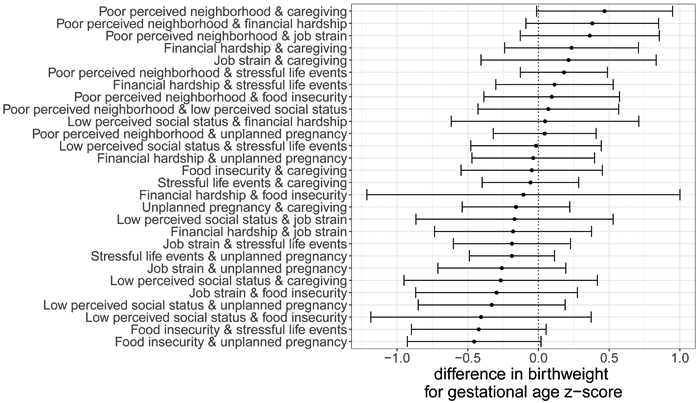
Differences in birthweight for gestational age z-scores and 95% CI associated with pairwise combinations of reported hardships prior to and during pregnancy.
Note: Exposures are binary indicators of experiencing each hardship during pregnancy. Positivity was assessed for each comparison and the sample was restricted to the area of common support (see eTable 4 for the sample sizes for each comparison). Outcome models were adjusted for maternal age, educational attainment, race/ethnicity, parity, marital status, pre-pregnancy BMI, and other hardships.
Food insecurity, unplanned pregnancy, and stressful life events were also among the strongest pairwise associations with birthweight (eFigure 4 and eTable 5). For example, food insecurity and unplanned pregnancy were associated with a difference in birthweight of −314.6 g (95% CI −591.0, −38.3) and food insecurity and stressful life events were associated with a difference of −343.5 g (95% CI −607.0, −79.9). Job strain and unplanned pregnancy (difference of −357.7 g (95% CI −623.2, −92.2) and job strain and stressful life events (−332.7 g; 95% CI −554.3, −111.1) were also among the strongest associations with birthweight.
Discussion
We estimated the individual, mutually adjusted, and pairwise joint effects of hardships experienced by a diverse cohort of pregnant women on fetal growth. We used propensity scores to ensure our analyses did not rely on extrapolation, and applied a methodology associated with chemical mixtures to hardships experienced prior to and during pregnancy. Controlling for hardship co-exposures allowed us to isolate important individual associations with fetal growth. Furthermore, analyzing pairs of hardships permitted the assessment of the potential impact of experiencing multiple hardships simultaneously during pregnancy on fetal growth, which can help identify those most at risk of delivering a growth-restricted infant. Our findings are consistent with the hypothesis that experiencing food insecurity during pregnancy reduces fetal growth. We observed stronger associations with fetal growth for the joint effects of food insecurity and stressful life events, and food insecurity and unplanned pregnancy. We also observed these patterns in associations with birthweight; the magnitudes of association were similar to smoking more than a half a pack of cigarettes a day during the third trimester (64). Thus, this study demonstrates that while investigating the influence of individual hardships can identify key exposures, assessing combinations of hardships holds promise to explain even greater differences in fetal growth.
Our results are consistent with prior studies that have shown food insecurity is associated with reduced birthweight. In particular, exposure to the Dutch famine during pregnancy was famously associated with reduced birthweight (65), and linked to long-term health effects (66, 67). A study of mothers in Illinois found food insecurity associated with elevated odds of delivering a low birthweight baby (8), and evidence from the Supplemental Nutrition Program for Women, Infants, and Children (WIC) estimates that nutritional assistance programs can be effective in improving fetal growth for mothers who receive benefits during pregnancy (68-70). Food insecurity may reduce fetal growth via nutritional deprivation that restricts a fetus’ access to calories or nutrients necessary for sufficient growth (65), stress due to worry about providing for an expanding family (71, 72), or chronic diseases and/or pregnancy complications that may result from poor diet quality (73, 74). Very few participants reported smoking (2%) or drinking (1%) during pregnancy, so these are unlikely to be mechanisms underlying our findings.
Our results did not, however, reflect prior literature’s findings that poor perceived neighborhood quality or financial hardship are associated with reduced birthweight (41, 43, 44). In this study, poor neighborhood quality was associated with higher birthweight for gestational age z-scores, which was unexpected. Our findings also suggest that the association of job strain with birthweight is driven primarily by reduced gestational age, rather than fetal growth. Prior evidence suggests job strain is associated with increased risk of both preterm birth and small for gestational age (42, 75-77), although some studies suggest no effect (78). We only found associations of stressful life events and unplanned pregnancy in combination with food insecurity, which contrasts other studies that have found independent associations of these factors with fetal growth (26, 45, 46).
Differences between our findings and those of prior studies could be due to our unique cohort population. For example, women living in San Francisco may be more likely than women living in other areas of the country to report financial hardship due to the high cost of living. San Francisco women may also be more likely to report their neighborhood is of low quality, despite the fact that they have relatively high income, due to affordable housing shortages in the region. In addition, previous evidence has suggested that neighborhood environment, in particular, is less relevant for immigrant mothers (79). Given that approximately one-third of mothers in our sample were not born in the United States, it is possible that our unexpected findings regarding the neighborhood environment are related to this phenomenon.
Despite addressing some shortcomings of prior research in this area, this analysis had some important limitations. The sample size was modest, especially after restricting to the area of common support for some exposures, and there was a lack of precision for smaller effect sizes. We were also unable to assess exposure to all hardships at once, because only one woman experienced all of them. This lack of exposure variability, in addition to the challenges in capturing social exposures with high accuracy and precision, is a challenge when applying the chemical exposure mixture model to social exposures. Participants in our cohort had predominantly high or low-socioeconomic position; this was a challenge, especially for estimating the effects of poverty-related hardships, as some women were simply not exchangeable with others and had to be excluded to avoid positivity violations.
Furthermore, hardships were assessed based on questions relating to different time periods; for example, caregiving and neighborhood quality were evaluated for the prior 5 years, and food insecurity and stressful life events were evaluated for the prior 12 months, while the other hardships were assessed at the time of the survey. These timeframes may not have been the most appropriate for characterizing hardships relevant to pregnancy for this study population. Furthermore, experience of these hardships before versus during pregnancy may have differential effects, and there may be differential effects based on the timing of hardship experience during pregnancy. Due to our limited sample size, we were not able to evaluate critical windows of exposure for each hardship. In addition, the hardships we examined likely have complex relationships with one another that we were not able to tease apart, since all of the hardships were assessed at the same study visit during the second trimester. We have, however, completed a path analysis in an attempt to better understand how these hardships relate (80). Furthermore, we did not assess potential modifiers of the hardship-fetal growth relationship, such as social support, coping, or resilience.
The cohort study recruited women during prenatal visits, but recruitment was not random and therefore we cannot guarantee the generalizability of our results. We also cannot exclude the possibility that residual confounding or a lack of treatment variability irrelevance (commonly called consistency, see (81-83) for detailed discussion of this issue) affected our findings. The latter is likely to be an issue especially for the composite exposures (84). Furthermore, it is possible women who could have been recruited experienced early pregnancy loss or termination as a result of exposure to one or more of the stressors we examined, which could have induced selection bias. However, we would expect this selection to have biased our results toward the null.
Economic forces are meaningful sources of stress in women’s lives, and this study suggests that food insecurity and job strain among pregnant women are linked to worse fetal growth outcomes at birth. Applying a mixtures analysis approach to this set of social exposures, we found even stronger reductions in birthweight for gestational age z-scores associated with joint exposure to food insecurity and stressful life events, and food insecurity and unplanned pregnancy. The extent of economic hardships experienced by mothers in California, particularly that almost 30% report being food insecure during pregnancy (25), imply potentially large population-level impacts from food insecurity on fetal growth.
Supplementary Material
Acknowledgments
Source of funding: This work was supported by grants RD83543301 from the United States Environmental Protection Agency, and P01ES022841 from the National Institute of Environmental Health Sciences, and UG3OD023272 and UH3OD023272 from the National Institutes of Health Environmental influences on Child Health Outcomes (ECHO) program.
Footnotes
The analysis code is available in the eAppendix and at https://github.com/degoin/Stress-fetal-growth. The sharing of anonymized data from this study is restricted due to ethical and legal restrictions. Data contains sensitive personal health information, which is protected under Health Insurance Portability and Accountability Act (HIPPA), thus making all data requests subject to Institutional Review Board (IRB) approval. Per University of California, San Francisco (UCSF) IRB, the data that support the findings of this study are restricted for transmission to those outside the primary investigative team. Data sharing with investigators outside the team requires IRB approval. Data requests may be submitted to the Program on Reproductive Health and the Environment (PRHE) by contacting Lynn Harvey at Lynn.Harvey@ucsf.edu. All requests for anonymized data will be reviewed by PRHE and then submitted to the UCSF IRB for approval.
References
- 1.Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol. 2004;191(2):481–7. [DOI] [PubMed] [Google Scholar]
- 2.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59(5):624–32. [PubMed] [Google Scholar]
- 3.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108 Suppl 3:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg A, editor The IUGR newborn Seminars in perinatology; 2008: Elsevier. [DOI] [PubMed] [Google Scholar]
- 5.Chaddha V, Viero S, Huppertz B, Kingdom J, editors. Developmental biology of the placenta and the origins of placental insufficiency Seminars in Fetal and Neonatal Medicine; 2004: Elsevier. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon R. Placental insufficiency and its consequences. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2003;110:S99–S107. [DOI] [PubMed] [Google Scholar]
- 7.Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99(3):490–6. [DOI] [PubMed] [Google Scholar]
- 8.Borders AEB, Grobman WA, Amsden LB, Holl JL. Chronic stress and low birth weight neonates in a low-income population of women. Obstetrics & Gynecology. 2007;109(2):331–8. [DOI] [PubMed] [Google Scholar]
- 9.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. American journal of obstetrics and gynecology. 1993;169(4):858–65. [DOI] [PubMed] [Google Scholar]
- 10.Vesterinen HM, Morello-Frosch R, Sen S, Zeise L, Woodruff TJ. Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: systematic-review of the human and animal evidence. PloS one. 2017;12(7):e0176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graignic-Philippe R, Dayan J, Chokron S, Jacquet A, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neuroscience & biobehavioral reviews. 2014;43:137–62. [DOI] [PubMed] [Google Scholar]
- 12.Su Q, Zhang H, Zhang Y, Zhang H, Ding D, Zeng J, et al. Maternal stress in gestation: birth outcomes and stress-related hormone response of the neonates. Pediatrics & Neonatology. 2015;56(6):376–81. [DOI] [PubMed] [Google Scholar]
- 13.Zilversmit Pao L, Harville EW, Wickliffe JK, Shankar A, Buekens P. The Cumulative Risk of Chemical and Nonchemical Exposures on Birth Outcomes in Healthy Women: The Fetal Growth Study. International Journal of Environmental Research and Public Health. 2019;16(19):3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatric and perinatal epidemiology. 2008;22(5):438–66. [DOI] [PubMed] [Google Scholar]
- 15.Paarlberg KM, Vingerhoets J, Passchier J, Dekker GA, Heinen AG, van Geijn HP. Psychosocial predictors of low birthweight: a prospective study. BJOG: An International Journal of Obstetrics & Gynaecology. 1999;106(8):834–41. [DOI] [PubMed] [Google Scholar]
- 16.Heaman M, Kingston D, Chalmers B, Sauve R, Lee L, Young D. Risk Factors for Preterm Birth and Small‐for‐gestational‐age Births among C anadian Women. Paediatric and perinatal epidemiology. 2013;27(1):54–61. [DOI] [PubMed] [Google Scholar]
- 17.Havens JR, Simmons LA, Shannon LM, Hansen WF. Factors associated with substance use during pregnancy: results from a national sample. Drug and alcohol dependence. 2009;99(1–3):89–95. [DOI] [PubMed] [Google Scholar]
- 18.Jesse DE, Graham M, Swanson M. Psychosocial and spiritual factors associated with smoking and substance use during pregnancy in African American and White low-income women. Journal of Obstetric, Gynecologic & Neonatal Nursing. 2006;35(1):68–77. [DOI] [PubMed] [Google Scholar]
- 19.Gouin K, Murphy K, Shah PS. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. American journal of obstetrics and gynecology. 2011;204(4):340. e1–e12. [DOI] [PubMed] [Google Scholar]
- 20.Bailey BA, Byrom AR. Factors predicting birth weight in a low-risk sample: the role of modifiable pregnancy health behaviors. Maternal and Child Health Journal. 2007;11(2):173–9. [DOI] [PubMed] [Google Scholar]
- 21.Shankaran S, Das A, Bauer CR, Bada HS, Lester B, Wright LL, et al. Association between patterns of maternal substance use and infant birth weight, length, and head circumference. Pediatrics. 2004;114(2):e226–e34. [DOI] [PubMed] [Google Scholar]
- 22.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, behavior, and immunity. 2007;21(3):343–50. [DOI] [PubMed] [Google Scholar]
- 23.Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neuroscience & Biobehavioral Reviews. 2012;36(1):350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrix N, Berghella V, editors. Non-placental causes of intrauterine growth restriction Seminars in perinatology; 2008: Elsevier. [DOI] [PubMed] [Google Scholar]
- 25.Braveman P, Marchi K, Egerter S, Kim S, Metzler M, Stancil T, et al. Poverty, near-poverty, and hardship around the time of pregnancy. Maternal and child health journal. 2010;14(1):20–35. [DOI] [PubMed] [Google Scholar]
- 26.Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. American journal of obstetrics and gynecology. 2010;203(1):34. e1–e8. [DOI] [PubMed] [Google Scholar]
- 27.Almeida J, Bécares L, Erbetta K, Bettegowda VR, Ahluwalia IB. Racial/ethnic inequities in low birth weight and preterm birth: the role of multiple forms of stress. Maternal and child health journal. 2018;22(8):1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nkansah-Amankra S, Luchok KJ, Hussey JR, Watkins K, Liu X. Effects of maternal stress on low birth weight and preterm birth outcomes across neighborhoods of South Carolina, 2000–2003. Maternal and child health journal. 2010;14(2):215–26. [DOI] [PubMed] [Google Scholar]
- 29.Holland ML, Kitzman H, Veazie P. The effects of stress on birth weight in low-income, unmarried black women. Women’s Health Issues. 2009;19(6):390–7. [DOI] [PubMed] [Google Scholar]
- 30.Brotnow L, Reiss D, Stover CS, Ganiban J, Leve LD, Neiderhiser JM, et al. Expectant mothers maximizing opportunities: maternal characteristics moderate multifactorial prenatal stress in the prediction of birth weight in a sample of children adopted at birth. PloS one. 2015;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordentoft M, Lou HC, Hansen D, Nim J, Pryds O, Rubin P, et al. Intrauterine growth retardation and premature delivery: the influence of maternal smoking and psychosocial factors. American journal of public health. 1996;86(3):347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henn BC, Coull BA, Wright RO. Chemical mixtures and children’s health. Current opinion in pediatrics. 2014;26(2):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun JM, Gennings C, Hauser R, Webster TF. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environmental health perspectives. 2016;124(1):A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarevic N, Barnett AG, Sly PD, Knibbs LD. Statistical methodology in studies of prenatal exposure to mixtures of endocrine-disrupting chemicals: a review of existing approaches and new alternatives. Environmental health perspectives. 2019;127(2):026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public health reports. 2014;129(1_suppl2):19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of health and social behavior. 1995:80–94. [PubMed] [Google Scholar]
- 37.Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. American journal of obstetrics and gynecology. 2004;191(3):691–9. [DOI] [PubMed] [Google Scholar]
- 38.Kramer MS, Seguin L, Lydon J, Goulet L. Socio‐economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatric and perinatal epidemiology. 2000;14(3):194–210. [DOI] [PubMed] [Google Scholar]
- 39.Kramer M. Socioeconomic determinants of intrauterine growth retardation. European Journal of Clinical Nutrition. 1998;52:S29–32; discussion S-3. [PubMed] [Google Scholar]
- 40.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environmental Health Perspectives. 2020;128(4):047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strully KW, Rehkopf DH, Xuan Z. Effects of prenatal poverty on infant health: state earned income tax credits and birth weight. American Sociological Review. 2010;75(4):534–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BE, Ha M, Park H, Hong YC, Kim Y, Kim YJ, et al. Psychosocial work stress during pregnancy and birthweight. Paediatric and perinatal epidemiology. 2011;25(3):246–54. [DOI] [PubMed] [Google Scholar]
- 43.Schempf A, Strobino D, O’Campo P. Neighborhood effects on birthweight: an exploration of psychosocial and behavioral pathways in Baltimore, 1995–1996. Social science & medicine. 2009;68(1):100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buka SL, Brennan RT, Rich-Edwards JW, Raudenbush SW, Earls F. Neighborhood support and the birth weight of urban infants. American journal of epidemiology. 2003;157(1):1–8. [DOI] [PubMed] [Google Scholar]
- 45.Witt WP, Cheng ER, Wisk LE, Litzelman K, Chatterjee D, Mandell K, et al. Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. American journal of public health. 2014;104(S1):S81–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah PS, Balkhair T, Ohlsson A, Beyene J, Scott F, Frick C. Intention to become pregnant and low birth weight and preterm birth: a systematic review. Maternal and child health journal. 2011;15(2):205–16. [DOI] [PubMed] [Google Scholar]
- 47.Dennis EF, Webb DA, Lorch SA, Mathew L, Bloch JR, Culhane JF. Subjective social status and maternal health in a low income urban population. Maternal and child health journal. 2012;16(4):834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrove JM, Adler NE, Kuppermann M, Washington AE. Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychology. 2000;19(6):613. [DOI] [PubMed] [Google Scholar]
- 49.Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. Jama. 2012;307(4):398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and its association with measures of ill-health in the Whitehall II study. Social science & medicine. 2003;56(6):1321–33. [DOI] [PubMed] [Google Scholar]
- 51.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277(5328):918–24. [DOI] [PubMed] [Google Scholar]
- 52.Schulz AJ, Kannan S, Dvonch JT, Israel BA, Allen A III, James SA, et al. Social and physical environments and disparities in risk for cardiovascular disease: the healthy environments partnership conceptual model. Environmental health perspectives. 2005;113(12):1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133(5):844–53. [DOI] [PubMed] [Google Scholar]
- 54.Land JA. How should we report on perinatal outcome? Human Reproduction. 2006;21(10):2638–9. [DOI] [PubMed] [Google Scholar]
- 55.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. American journal of epidemiology. 2011;174(9):1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: a step-by-step illustration of causal inference methods. American journal of epidemiology. 2009;169(9):1140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snowden JM, Mortimer KM, Dufour M-SK, Tager IB. Population intervention models to estimate ambient NO 2 health effects in children with asthma. Journal of Exposure Science and Environmental Epidemiology. 2015;25(6):567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oulhote Y, Coull B, Bind M-A, Debes F, Nielsen F, Tamayo I, et al. Joint and independent neurotoxic effects of early life exposures to a chemical mixture: A multi-pollutant approach combining ensemble learning and G-computation. Environmental Epidemiology. 2019;3(5):e063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. Journal of agricultural, biological, and environmental statistics. 2015;20(1):100–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brunst KJ, Sanchez Guerra M, Gennings C, Hacker M, Jara C, Bosquet Enlow M, et al. Maternal lifetime stress and prenatal psychological functioning and decreased placental mitochondrial DNA copy number in the PRISM study. American journal of epidemiology. 2017;186(11):1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubin DB. Multiple imputation for nonresponse in surveys: John Wiley & Sons; 2004. [Google Scholar]
- 62.Petersen ML, Porter KE, Gruber S, Wang Y, van der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Statistical methods in medical research. 2012;21(1):31–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Team RC. R: A Language and Environment for Statistical Computing. 3.6.0 ed. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 64.Bernstein IM, Mongeon JA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking and its association with birth weight. Obstetrics & Gynecology. 2005;106(5):986–91. [DOI] [PubMed] [Google Scholar]
- 65.Stein AD, Ravelli ACJ, Lumey LH. Famine, Third-Trimester Pregnancy Weight Gain, and Intrauterine Growth: The Dutch Famine Birth Cohort Study. Human Biology. 1995;67(1):135–50. [PubMed] [Google Scholar]
- 66.Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: the story of the Dutch Famine Study. American journal of epidemiology. 1998;147(3):213–6. [DOI] [PubMed] [Google Scholar]
- 67.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early human development. 2006;82(8):485–91. [DOI] [PubMed] [Google Scholar]
- 68.Currie J, Rajani I. Within‐Mother Estimates of the Effects of WIC on Birth Outcomes in New York City. Economic inquiry. 2015;53(4):1691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bitler MP, Currie J. Does WIC work? The effects of WIC on pregnancy and birth outcomes. Journal of Policy Analysis and Management: The Journal of the Association for Public Policy Analysis and Management. 2005;24(1):73–91. [DOI] [PubMed] [Google Scholar]
- 70.Hamad R, Collin DF, Baer RJ, Jelliffe-Pawlowski LL. Association of Revised WIC Food Package With Perinatal and Birth Outcomes: A Quasi-Experimental Study. JAMA pediatrics. 2019;173(9):845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laraia BA, Siega-Riz AM, Gundersen C, Dole N. Psychosocial factors and socioeconomic indicators are associated with household food insecurity among pregnant women. The Journal of nutrition. 2006;136(1):177–82. [DOI] [PubMed] [Google Scholar]
- 72.Leung CW, Epel ES, Willett WC, Rimm EB, Laraia BA. Household food insecurity is positively associated with depression among low-income supplemental nutrition assistance program participants and income-eligible nonparticipants. The Journal of nutrition. 2015;145(3):622–7. [DOI] [PubMed] [Google Scholar]
- 73.Leung CW, Epel ES, Ritchie LD, Crawford PB, Laraia BA. Food insecurity is inversely associated with diet quality of lower-income adults. Journal of the Academy of Nutrition and Dietetics. 2014;114(12):1943–53. e2. [DOI] [PubMed] [Google Scholar]
- 74.Laraia BA, Siega-Riz AM, Gundersen C. Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain, and pregnancy complications. Journal of the American Dietetic Association. 2010;110(5):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.HENRIKSEN TB, HEDEGAARD M, SECHER NJ. The Relation between Psychosocial Job Strain, and Preterm Delivery and Low Birthweight for Gestational Age. International Journal of Epidemiology. 1994;23(4):764–74. [DOI] [PubMed] [Google Scholar]
- 76.Tuntiseranee P, Geater A, Chongsuvivatwong V, Kor-anantakul O. The Effect of Heavy Maternal Workload on Fetal Growth Retardation and Preterm Delivery: A Study Among Southern Thai Women. Journal of Occupational and Environmental Medicine. 1998;40(11):1013–21. [DOI] [PubMed] [Google Scholar]
- 77.Croteau A, Marcoux S, Brisson C. Work Activity in Pregnancy, Preventive Measures, and the Risk of Delivering a Small-for-Gestational-Age Infant. American Journal of Public Health. 2006;96(5):846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larsen AD, Hannerz H, Juhl M, Obel C, Thulstrup AM, Bonde JP, et al. Psychosocial job strain and risk of adverse birth outcomes: a study within the Danish national birth cohort. Occupational and Environmental Medicine. 2013;70(12):845–51. [DOI] [PubMed] [Google Scholar]
- 79.Urquia ML, Frank JW, Glazier RH, Moineddin R, Matheson FI, Gagnon AJ. Neighborhood context and infant birthweight among recent immigrant mothers: a multilevel analysis. American Journal of Public Health. 2009;99(2):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eick SM, Goin DE, Izano MA, Cushing L, DeMicco E, Padula AM, et al. Relationships between psychosocial stressors among pregnant women in San Francisco: A path analysis. Plos one. 2020;15(6):e0234579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pearl J. On the consistency rule in causal inference: axiom, definition, assumption, or theorem? Epidemiology. 2010;21(6):872–5. [DOI] [PubMed] [Google Scholar]
- 82.Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology. 2009;20(1):3–5. [DOI] [PubMed] [Google Scholar]
- 83.VanderWeele TJ. Concerning the consistency assumption in causal inference. Epidemiology. 2009;20(6):880–3. [DOI] [PubMed] [Google Scholar]
- 84.Rehkopf DH, Glymour MM, Osypuk TL. The consistency assumption for causal inference in social epidemiology: when a rose is not a rose. Current epidemiology reports. 2016;3(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


