Abstract
The search for enzymes with histamine-degrading activity is of great interest, since it has great potential in the way of solving the problem of high histamine levels in food. In this study, the gene of a novel histamine-degrading enzyme, i.e., glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Lactobacillus plantarum was cloned and successfully expressed in Escherichia coli DH5α, with the recombinant host determined as histamine-degrading active. The recombinant GAPDH was then purified to homogeneity by ammonium sulfate fraction and gel filtration. The optimum pH and temperature were 5.5 and 40 °C and it was strongly resistant to SO2 and ethanol. Afterwards, the histamine degradative activity of partially purified GAPDH in actual wine environments (grape and cherry wines) was examined by incubating the enzymes in the middle, near the end and completion of malolactic fermentation, and histamine in the corresponding contaminated wines was decreased by 36.8–52.4%, 59.6–66.9% and 83.1–85.5%, respectively.
Electronic supplementary material
The online version of this article (10.1007/s10068-020-00838-z) contains supplementary material, which is available to authorized users.
Keywords: Histamine, Degradation, Glyceraldehyde-3-phosphate dehydrogenase, Characterization, Application
Introduction
Biogenic amines (BA) are non-volatile low molecular weight nitrogenous organic bases, widely present in fermented food and beverages including wine (Doeun et al., 2017; Guo et al., 2015). Histamine is the most toxic amine found in wine, and the consumption of wine containing a high concentration of histamine may cause serious human health problems, such as hot flush, difficulty in breathing, urticaria and rashes (Kim and Kim, 2016; Kovacova-Hanuskova et al., 2015). Concerning the widespread BA contamination problem in wine (Choi et al., 2019; Guo et al., 2015; Henríquez-Aedo et al., 2016), an effective method is proposed and seems to gain more interest nowadays, which is the use of food microorganisms or enzymes that are able to degrade BA already present in wine (Alvarez and Moreno-Arribas, 2014; Callejón et al., 2014). And some Lactobacillus plantarum species have been proved quite effective in the reduction of BAs in synthetic or actual wine environment (Callejón et al., 2014; García-Ruiz et al., 2011; Sun et al., 2020). However, regarding the nature of BA-degrading enzyme in L. plantarum, different results were reported. Callejón et al. (2014) found that the enzyme in their isolated L. plantarum J16 could be a laccase. Later, the gene of the laccase was cloned and expressed in E. coli, and recombinant laccase was found being active in the oxidization of BAs (Callejón et al., 2016). In our previous study, a novel histamine-degrading enzyme, i.e., glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from our selected L. plantarum strain, whose identity was verified by N-terminal sequence analysis of the pure enzyme (Sun et al., 2020).
GAPDH is a glycolytic enzyme. Its main function is to catalyze the oxidative phosphorylation of glyceraldehyde-3-phosphate to 1,3-diphosphoglycerate coupled with reduction of NAD+ to NADH (Sirover, 2014). Later, such enzyme is identified as a multifunctional protein, because it has been verified involving in numerous other cell processes, such as intracellular membrane trafficking, regulation of gene expression and nuclear tRNA export, iron uptake and transport, etc. (Garcin, 2019; Hui et al., 2014; Ong et al., 2019; Zhang et al., 2017). Our previous result demonstrated that GAPDH from selected L. plantarum strain can catalyze the degradation of histamine, therefore adding a new enzymatic activity to the GAPDH (Sun et al., 2020).
It should be pointed out that understanding how L. plantarum GAPDH function towards histamine is of great interest, as it has the potential to be used to solve the problem of high histamine amounts in food and wine. In order to assure the function of our purified GAPDH from L. plantarum and further characterize its properties, in this study the GAPDH gene of L. plantarum will be cloned and overexpressed in E. coli, followed by purification and characterization. Subsequently, partially purified GAPDH will be tested for its ability for histamine degradation in actual wine environment. As far as we know, this is the first report concerning the application of the novel GAPDH in wine-making. We hope our study can gain a deeper insight into this enzyme and lay the basis for its use in wine industry.
Materials and methods
Materials
Histamine was purchased from Sigma-Aldrich. Restriction endonuclease Sal I and Sph I were from TAKARA Biomedical Technology (Beijing, China). T4 DNA ligase was provided by Biolabs (Beijing, China). The antibiotic ampicillin and isopropyl-d-thiogalactoside (IPTG) were all obtained from Solarbio (Beijing, China).
Escherichia coli DH5α and pUC19 were used for DNA cloning and sequencing. E. coli DH5α (Novagen) was used for protein expression.
Cloning of GAPDH and expression
Lactobacillus plantarum PP02 was cultured in MRS medium at 30 °C for 24 h, the genomic DNA of which was used as template to PCR amplify GAPDH coding sequence with N1 (CTAGCATGCAGACGTG TTTGATCAC) and N2 (GCGTCGACCAAATATTAGAGAGTGG) as primers. The plasmid DNA in E. coli was isolated using TIANprep Mini Plasmid Kit (TIANGEN, Beijing, China). After amplification, the GAPDH gene was digested with Sal I and Sph I at 37 °C for 6 h; plasmid pUC19 was digested with the same enzymes. Digested GAPDH and pUC19 were purified by TIANgel Midi Purification Kit (TIANGEN, Beijing, China) and ligated with T4 DNA ligase for 6 h at 16 °C. Subsequently, this recombinant pUC19-GAPDH was transferred into E. coli DH5α which was then cultured on LB agar plate containing 100 μg/mL ampicillin at 37 °C for 12 h. Positive bacterial strains were picked up by Blue-White Screening test (Hanahan, 1983). Positive clones were identified by colony PCR as well as restriction digestion of purified plasmid DNA with Sal I and Sph I. Verified positive clone was sent to Shanghai Sangon Co., Ltd. and confirmed by sequencing.
Expression of GAPDH was conducted on Luria–Bertani (LB) medium (Hope Bio-Technology, Qingdao, China) containing 100 μg/mL ampicillin at 37 °C till the absorbances at 600 nm reached 0.6-0.8, followed by addition of 0.5 mM IPTG and moved to 27 °C for another 8 h. After cultivation, cells were harvested by centrifugation at 8952×g for 5 min at 4 °C, washed with 50 mM PBS (phosphate buffer saline, pH 7.4), recentrifuged and subjected to subsequent purifications.
Purification of recombinant GAPDH
Cells (5 g) was resuspended in 25 mL PBS, and disrupted by ice-bath sonication at 630 W for 1 h (1 time for 10 s with 5 s interval) using a SCIENTZ Ultrasound Cell Disintegrator (Ningbo Scientz Biotechnology, Ningbo, China). The supernatant obtained by centrifugation at 12,880×g, 5 min, 4 °C was treated with ammonium sulfate at a saturation fraction of 70–80% (Barinova et al., 2017). And the precipitate was collected by centrifugation (12,880×g, 5 min, 4 °C) and resuspended in 10 mL 20 mM, pH 7.4 PBS buffer.
Further purification was performed on a Hiload Superdex 200 column (2.6 × 60 cm) equilibrated with 3 column volume of 20 mM, pH 7.4, PBS buffer, on AKTA pure (GE Healthcare, Piscataway, USA). Fractions resulting from above ammonium sulfate fractionation was filtered with 0.22 μm filter membrane, injected into this column and eluted with the same buffer. Peak fractions were pooled and subjected to desirable activity assay.
Assay for GAPDH activity during purification
Reaction mixture consisting of 0.25 mL of GAPDH resulting from purification and 0.75 mL histamine solution (10 mg/L), was placed in a shaking bath (60 rpm, 37 °C, 40 min), followed by HIS Elisa Kit (Mlbio, Shanghai, China) determination. The details of the Kit was: 50 μL GAPDH diluted with pH 7.4, 0.01 mol/L PBS plus 5% BSA was added to 12 × 8 wells of microtiter plates coated with purified HIS antibody (16 ng/mL); after gently mixing, the plate was incubated at 37 °C for 30 min and washed 5× with pH 7.4, 0.01 mol/L PBS plus 0.05% Tween 20, and 50 μL HRP-conjugated rabbit polyclonal antibodies (8 ng/mL) was added, followed by another incubation at 37 °C for 30 min and washing 5×; thereafter 100 μL of HRP substrate 3, 3′, 5, 5′-tetramethylbenzidine (TMB) solution was added, avoiding light, and incubated for 10 min at 37 °C. The reaction was stopped using 50 μL/well 1 mol/L H2SO4 and absorbance was measured at 450 nm with a microplate reader. And histamine concentration was calculated from standard curve previously built using pure histamine at different concentrations (10–50 ng/L).
One unit of enzymatic activity was defined as the amount of enzyme that decreased 1 mg/L histamine per minute under standard assay conditions.
Electrophoresis
Fractions resulting from purification process were analyzed on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE, 12.5% separating and 4.5% stacking) according to Laemmli (1970) to check the homogeneity and the subunit molecular weight. Protein bands were colorized by Coomassie brilliant blue R250 and destained with 10% acetic acid.
Characterization of recombinant GAPDH
Optimal temperature and thermostability
The optimal temperature of GAPDH was determined ranging from 15 to 70 °C in pH 7.4, 50 mM PBS buffer. Thermostability was measured by incubation of pure enzyme at different temperatures (15–70 °C) for 1 h, followed by enzymatic activity assay under the standard condition.
Optimal pH and pH stability
The pH dependence of GAPDH was conducted at 37 °C among the pH ranges of 3.0–9.5, and buffers used included acetic acid-sodium acetate (pH 3.0–5.5, 50 mM), sodium phosphate (pH 6.5–7.5, 50 mM) and Tris–HCl (pH 8.5–9.5, 50 mM). After incubation, the concentration of residual histamine in solution was determined according to GB/T 5009.208-2008, as previously described by Sun et al. (2018). pH stability was assessed by keeping pure enzyme at different pH (3.0–9.5) for 1 h, followed by enzymatic activity assay by HIS Elisa Kit.
Effect of ethanol and sulfur dioxide
The effect of ethanol on purified GAPDHs was measured by incubating pure enzyme with ethanol–water solution at different concentrations (5, 10 and 15%, v/v) for 1 h. The effect of SO2 was determined with different concentrations of SO2 (20, 30, 40 and 50 mg/L) for 1 h followed by enzymatic activity assay under standard condition.
Application of partially purified GAPDH for fruit wine production
Grape wine production
Carmenere grapes (initial must composition: 20.6 oBrix, total acidity 6.79 g/L, pH 3.60) were crushed, poured into 5 L bottles, supplemented with SO2 (to 50 mg/L) and pectinase (20 mg/L), and inoculated with commercial S. cerevisiae strain of GRE (300 mg/L) to start alcoholic fermentation. This fermentation was kept at 25 °C for 6–7 days and yeast cells were removed by centrifugation at 12,880×g for 15 min at the end. Then, triplicate malolactic fermentations (MLF) were begun in 20 mL tubes by the introduction of 30 mg/L O. oeni strain of Viniflora Oenos (Chr.-Han, Horsholm, Denmark), and external histamine (10 mg/L) was simultaneously added. MLF was carried out at 20 °C for about 28–30 d, and the progress was followed by measuring L-malic acid content (L-malic acid Enzymatic BioAnalysis, Boehringer-Mannheim/R-Biopharm, Darmstadt, Germany) until its concentration decreased below 0.5 g/L. Such a contaminated wine was used for the determination of histamine removal ability of GAPDH in actual wine environment. Partially purified GAPDH resulting from (NH4)2SO4 precipitation at a saturation fraction of 70–80% was obtained following the procedure as described above, and it was added to the contaminated wine at a concentration of 2 mg/L at three different times, i.e., in the middle of MLF (15 d), near the end of MLF (25 d) and after MLF (bacterial biomass removed by centrifugation at 20,000×g followed by crude GAPDH addition and incubation for 5 d). And for simplicity, we defined GM15, GM25 and GMC5 as the wines that received the above and corresponding treatments. A control wine was taken following the same fermentation process as the treated wines but free of GAPDH incubation. All wines were analyzed for conventional enological parameters and histamine concentration at the time point of 5 days after MLF, and they were all subjected to centrifugation at 20,000×g for 20 min prior to analyses.
Cherry wine production
Cherries of Early Richmond (initial must composition: 15.7 oBrix, total acidity 6.65 g/L, pH 3.72) were used as the materials for cherry wine-making. The procedure of cherry wine-making was mostly the same as Carmenere grape wine production, including supplementation of SO2 and pectinase, inoculation of S. cerevisiae GRE and yeast cells removement, addition of histamine, culture of MLF starter, determination of L-malic acid and incubation of crude GAPDH. The difference of cherry wine-making from grape wine making was the period of MLF, 23 days in total. Therefore, partially purified GAPDH was incubated in the middle of MLF (12 d), near the end of MLF (20 d) and after MLF. And for simplicity, we used codes of CM12, CM20 and CMC5 as the cherry wine resulting from above treatments.
Analytical method of fruit wines
Basic analysis
The enological wine parameters including total reducing sugars, total acidity, ethanol and volatile acid, were measured by the official methods (OIV, 2018).
Histamine determination in wine
The histamine concentration in grape and cherry wines were determined according to the Chinese national standards GB/T 5009.208-2008, as previously stated by Sun et al. (2018). The pre-treatment involves fat removal by hexane, followed by the addition of solid sodium chloride (to saturation) and pH adjustment (to 12.0). Equal amount of extraction solvent (trichlormethane: butanol = 1:1, v/v) was added to fat-free sample. After stirring and centrifugation (3100×g, 10 min), the precipitate was discarded and 1 M HCl was added. Excessive extraction solvent was removed by nitrogen stream, and the residue was dissolved in 1 mL of 0.1 M HCl, ready for the derivatization process.
A 10 mL vial containing 0.5 mL pretreated wine sample, 1.5 mL saturated sodium bicarbonate and 1 mL Dns-Cl solution (10 g/L in acetone) was incubated for 30 min at 60 °C, with mixing at 10 and 20 min. 0.1 mL sodium glutamate (50 g/L in saturated sodium bicarbonate) was added, with incubation at 60 °C for 15 min. After derivatization, acetone was removed by the stream of nitrogen, and 3 mL ether was added which was also then removed by nitrogen flowing. The residue was reconstituted to 1 mL of methanol, filtered through 0.2 μm filters and injected into the HPLC.
The histamine quantification was carried out on an Inertsil ODS-SP (4.6 × 250 mm, particle diameter 5 μm) column (GL Sciences, Tokyo, Japan) equipped in a Shimadzu Prominence LC-20A HPLC (Shimadzu Corporation, Kyoto, Japan) coupled with a variable wavelength UV–Vis spectrophotometer. Methanol and ultra-pure water were solvent A and B, respectively. The gradient profile was: 0–7 min, 55–65% A; 7–14 min, 65–70% A; 14–27 min, 70–90% A; 27–30 min, 90–100%A; 30–36 min, 100–55% A; 36–45 min, 55%A). And the dansylamides were detected by monitoring their UV absorbance at 254 nm.
Statistical analyses
Data are presented as the mean ± SE. Percentage data were normalized by arcsine transformation prior to the analysis. All analyses were carried out using SPSS 25.0 (SPSS Inc., Chicago, Illinois).
Result and discussion
Expression and purification of GAPDH
After cloning, sequencing and target gene verification of pUC19-GAPDH plasmids (Fig.S1), GAPDH expression was performed in E. coli DH5α cells in LB medium, induced by the supplement of 0.5 mM IPTG, and a large quantity of recombinant proteins was obtained after incubation at 30 °C for 12 h. Histamine-degrading activity test using recombinant E. coli DH5α and original E. coli host free of pUC19-GAPDH plasmids showed that the former bacteria had desirable activity [4 U/g (wet weight)] while the latter did not. From these results, it was concluded that GAPDH indeed had the ability to degrade histamine and its cloning and expression in E. coli DH5α was successful.
The purification of GAPDH to homogeneity was accomplished by cell disruption, ammonium sulfate fractionation and gel filtration. And the purification progress was visualized by SDS-PAGE gel (Fig. 1). Among those steps, ammonium sulfate fractionation is the most effective procedure for the removal of redundant proteins and concentration of target protein. Such step has been reported in the purification of recombinant human untagged GAPDH by Barinova et al., using 70–80% saturation ammonium sulfate, which facilitated the concentration of the target enzyme and exhibited a relatively larger specific activity (Barinova et al., 2017). With the final purification step on AKTA Superdex 200 (elution peak at 125 mL), a single band of around 36 kDa of GAPDH was generated (Fig. 1). The purification flow sheet was listed in Table 1, with an overall 2500-fold of purification, 0.1% of activity yield and 12.5 U/mg of specific activity. The value of this specific activity was comparable with that of pure GAPDH from L. plantarum (17.0 U/mg) (Sun et al., 2020).
Fig. 1.
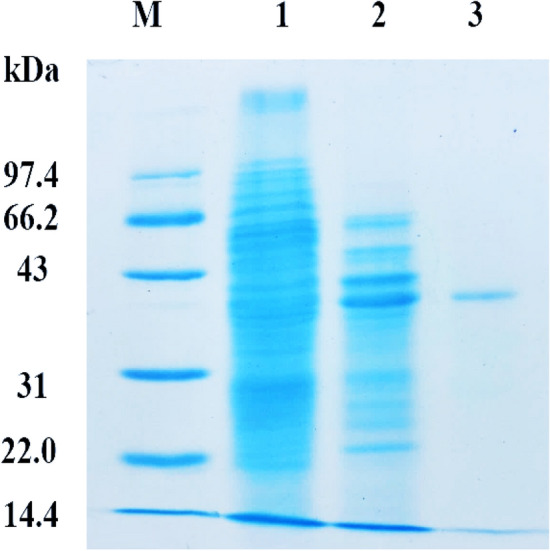
Purification of GAPDH from E.coli DH5α. M: protein molecular weight marker; lane 1: crude extract; lane 2: fractions from (NH4)2SO4 fractionation; lane 3: fractions from Superdex 200
Table 1.
Purification sheet of recombinant GAPDH from E. coli DH5α
| Purification step | Protein concentration (mg/mL) | Activity (U/mL) | Specific activity (U/mg) | Yield (%) | Purification fold |
|---|---|---|---|---|---|
| Crude extract | 10.48 | 0.043 | 0.005 | 100 | 1 |
| Ammonium sulfate fractionation | 0.79 | 0.044 | 0.056 | 20.7 | 11.2 |
| Superdex 200 gel filtration | 0.004 | 0.050 | 12.50 | 0.1 | 2500 |
Characterization of recombinant GAPDH
Optimum temperature and thermostability
Data in Fig. 2 demonstrated that the recombinant GAPDH showed maximum activity at 40 °C, and was sensitive to high temperature, as evidenced by the significant activity loss of 92% at 70 °C in comparison with its activity at 40 °C. Here, it should be pointed out the performances of GAPDH at low temperatures (15–25 °C) should be paid more attention, since a relative cold and moderately warm environment (< 30 °C) will be encountered in a realistic wine-making process. However, only 10.3–30.7% activity was found at the temperature range of 15–25 °C for GAPDH, suggesting a higher amount of GAPDH will be required if used in actual production.
Fig. 2.
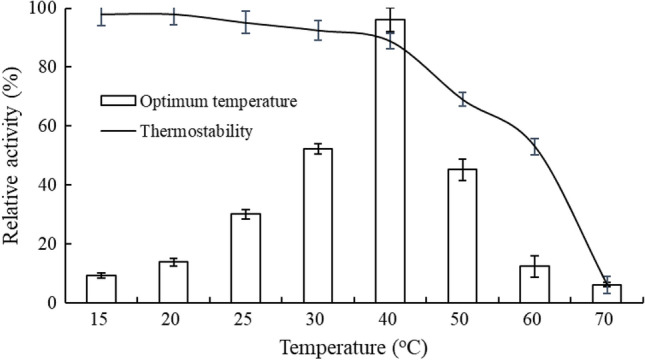
Optimum temperature and thermostability of GAPDHs. Enzyme activity was plotted as a percentage relative to the maximum value (% relative activity)
For the determination of thermostability, purified enzyme was incubated at the temperature range of 15–70 °C for 1 h, followed by enzymatic assay. Results in Fig. 2 showed that GAPDH exhibited higher tolerance to lower temperatures (≤ 40 °C) and highly sensitive to higher temperature (≥ 60 °C), as seen over 90% of activity was maintained after incubated at 40 °C for 1 h, but a complete loss of activity was observed when incubated at 70 °C. Our result was in agreement with Gani et al. (2016) who found GAPDH from pea seeds was unstable at higher temperatures, although the enzymatic activity determined and corresponding methods were completely different.
Optimum pH and pH stability
In order to determine optimum pH, small aliquots of purified GAPDH was mixed with different buffers to adjust to different pH values (3.0–9.5), followed by activity assay under standard condition. As shown in Fig. 3, the optimum pH was 5.5, and the activities were decreased along with the increase of pH values. This indicates that for optimal conditions, the pH in enzyme activity measurements could have been higher (pH 7.4). An almost complete loss of activity was found in the pH range of 8.5–9.5, especially at pH 8.5, indicative of the weak alkali resistance of GAPDH. Another possibility that can not be excluded is that low activity at pH 8.5 was related to inactivation and decreased solubility of GAPDHs near their pI (8.8) as reported by author from Tetrahymena pyriformis (Hafid et al., 1998).
Fig. 3.
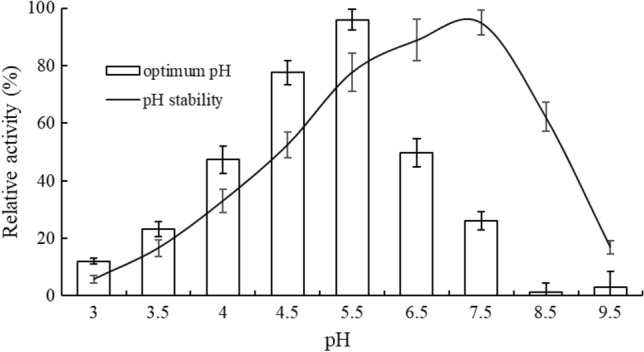
Optimum pH and pH stability of GAPDH. Enzyme activity was plotted as a percentage relative to the maximum value (% relative activity)
Here, it should be highlighted that the optimum pH of recombinant GAPDH was lower than its purified counterpart (pH 7.5) from the initial L. plantarum strain (Sun et al., 2020), which implied that the expression in E. coli may influence the space conformation of the recombinant GAPDH that led to changes in the optimal pH value. Such change is unanticipated but satisfactory, since a higher activity in an acidic environment is demanded in the realistic food processing. For wine-making, the common pH of wine is around 3.0–4.0, and in this study 12.1–47.3% activity was found during this pH range for recombinant GAPDH, suggesting again a higher amount of GAPDH is still required if used in actual wine production. In our future study, more efforts will be devoted to the genetic modification of the recombinant GAPDH for its activity enhancement in those environments.
As for pH stability which was determined at 37 °C at the pH range of 3.0–9.5 for 1 h, data in Fig. 3 showed that over 50% of the degradative activity was maintained after incubated at a pH range of 4.5–8.5 for 1 h for this enzyme, and it was most stable at the pH ranges of 6.5–7.5.
Effect of ethanol and sulfur dioxide
Ethanol and sulfur dioxide are two wine stressing factors present in wines (Cinquanta et al., 2018; Sun et al., 2016). To address the effects of these two factors, pure enzymes were mixed with each compound at different concentrations for 1 h, followed by residual activity determination under standard condition. Data in Fig. S2 showed that GAPDH was stable at an ethanol concentration of 5–15%, and the maximum activity was found even at 15%. As for the effect of sulfur dioxide (Fig. S3), similar phenomenon was observed. This GAPDH was active at a SO2 concentration ranging from 20 mg/L to 50 mg/L and over 90% activity was retained after 1 h of incubation. These results showed that the recombinant GAPDH was negligibly affected by the presence of ethanol and SO2, indicative of the potential of such enzyme in its application in wine-making industry.
With respect to the ability of GAPDH to the resist these two stressing factors, it was assumed that such enzyme was originally expressed in a L. plantarum strain which was isolated from wines with spontaneous malolactic fermentation; this LAB could survive in harsh wine environments and showed strong resistance to various wine restriction factors, which may have a significant effect on the induction of the resisting ability of GAPDH.
Preliminary application of partially purified GAPDH for wine-making
In order to determine the histamine degradative activity of GAPDH in actual wine environment and its effect on wine enological properties, GAPDH was introduced into the fermenting musts at different time points followed by incubation and subsequent wine analyses. Partially purified GAPDH was used instead of the pure due to a very low yield of purification via gel filtration as well as the unstable form of GAPDH as a pure enzyme. Besides, the tested fermentation stage was mainly about MLF, which was ascribed to the following reason. Although many conventional vinification stages, such as skin maceration, alcoholic fermentation, malolactic fermentation and aging, and sometimes contaminated by spoilage bacteria, all probably result in the formation or accumulation of BAs, MLF has been proposed as the most important stage, the amount of BAs synthesized during MLF normally larger than other steps (Martínez-Pinilla et al., 2013; Restuccia et al., 2018; Smit and du Toit, 2013).
Table 2 summarized the results for the basic compositional parameters of grape wines assayed all at the time point of 5 d after MLF. It was seen that negligible differences were found for the most of the enological wine parameters, with an average value for the content of ethanol, total acidity, reducing sugar and pH as 11.1% (v/v), 5.76 g/L, 1.21 g/L and 3.70, respectively. Only volatile acid concentration in the control wine was slightly higher compared to the others. However, histamine concentration showed significant variations among these samples, with the highest detected in the control wine and the lowest in the GMC5 sample. Compared to the control wine, the concentrations of histamine in the GM15, GM25 and GMC5 wines were evidently decreased by 36.8%, 59.6% and 83.1%, respectively.
Table 2.
Basic composition and histamine concentration in grape wines
| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | GM15 | GM25 | GMC5 | |
| Ethanol* (%, v/v) | 11.1 ± 0.1a | 11.1 ± 0.1a | 11.0 ± 0.1a | 11.1 ± 0.1a |
| Total acidity** (g/L) | 5.73 ± 0.01a | 5.80 ± 0.02ab | 5.72 ± 0.02a | 5.77 ± 0.02ab |
| Total reducing sugars (g/L) | 1.33 ± 0.02c | 1.06 ± 0.02a | 1.29 ± 0.02c | 1.15 ± 0.02b |
| pH | 3.69 ± 0.01a | 3.71 ± 0.01a | 3.70 ± 0.01a | 3.71 ± 0.01a |
| Volatile acidity (g/L) | 0.27 ± 0.01b | 0.23 ± 0.01a | 0.24 ± 0.01a | 0.24 ± 0.01a |
| Histamine (mg/L) | 13.6 ± 0.9d | 8.6 ± 0.5c | 5.5 ± 0.3b | 2.3 ± 0.2a |
*Results represent the mean ± SD. Values with different roman letters (a–d) in the same row are significantly different according to the Duncan test (p < 0.05)
**Expressed as g/L of tartaric acid
Table 3 shows the means and standard deviations of the enological parameters of cherry wines measured at the time point of 5 d after MLF. As seen, similar tendency was observed in cherry wine as in grape wine, since basic compositional parameters varied insignificantly amongst the tested samples while histamine content showed great differences. Compared to the control cherry wine, the concentrations of histamine in CM12, CM20 and CMC5 was reduced by 52.4, 66.9 and 85.5%, respectively.
Table 3.
Basic composition and histamine concentration in cherry wines
| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | CM12 | CM20 | CMC5 | |
| Ethanol* (%, v/v) | 9.0 ± 0.1a | 8.9 ± 0.1a | 8.9 ± 0.1a | 9.0 ± 0.1a |
| Total acidity** (g/L) | 5.69 ± 0.02a | 5.70 ± 0.02a | 5.71 ± 0.01a | 5.72 ± 0.02a |
| Total reducing sugars (g/L) | 0.88 ± 0.02a | 1.01 ± 0.02b | 0.85 ± 0.02a | 0.97 ± 0.02b |
| pH | 3.82 ± 0.01a | 3.80 ± 0.01a | 3.80 ± 0.01a | 3.81 ± 0.01a |
| Volatile acidity (g/L) | 0.21 ± 0.01a | 0.18 ± 0.01a | 0.19 ± 0.01a | 0.19 ± 0.01a |
| Histamine (mg/L) | 12.4 ± 0.7d | 5.9 ± 0.6c | 4.1 ± 0.3b | 1.8 ± 0.1a |
*Results represent the mean ± SD. Values with different roman letters (a–d) in the same row are significantly different according to the Duncan test (p < 0.05)
**Expressed as g/L of malic acid
From these results, it is interesting to note that the incubation of GAPDH after the completion of MLF generated the best result, as the histamine concentration in both GMC5 and CMC5 was significantly degraded by over 80% compared to their respective controls. As for the reason, it was speculated that the development and accumulation of malolactic bacteria during MLF inhibited the activity of GAPDH or prevented the contact of histamine with GAPDH, thus leading to a relatively lower histamine degradative percentage; when the biomass was removed, the wine environment was then more suitable for the performance of GAPDH. Therefore, in the actual wine-making process, GAPDH could be recommended to be used during the elaboration period.
In conclusion, GAPDH was heterologously expressed in E. coli host in this study to prove its ability to degrade histamine. The recombinant GAPDH was most active at 40 °C and pH 5.5, and showed higher resistance to ethanol and SO2. Preliminary application of partially purified GAPDH incubated in the middle, near the end and completion of MLF of wine-making was successful, and the most significant effect was observed in the last case, indicative of its most possible use in the elaboration period. However, a higher amount of GAPDH in its present form will be required if used in actual production. In our future study, more efforts will be devoted to the genetic modification of the recombinant GAPDH for its activity enhancement in acidic environments at lower temperatures. Moreover, studies on the selection or synthesis of appropriate immobilized carrier will be carried out, in order to lay the theorical basis for its large-scale application in wine or food industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the Science and Technology Project of Yantai (No. 2019XDHZ091) and the National Natural Science Foundation of China (No. 31501577).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dongqi Jiang, Email: 1203317539@qq.com.
Huamin Li, Email: lihm01@163.com.
Shuyang Sun, Email: sysun81@ldu.edu.cn.
References
- Alvarez MA, Moreno-Arribas MV. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Tech. 2014;39:146–155. doi: 10.1016/j.tifs.2014.07.007. [DOI] [Google Scholar]
- Barinova KV, Eldarov MA, Khomyakova EV, Muronetz VI, Schmalhausen EV. Isolation of recombinant human untagged glyceraldehyde-3-phosphate dehydrogenase from E. coli producer strain. Protein Expres. Pur. 2017;137:1–6. doi: 10.1016/j.pep.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Callejón S, Sendra R, Ferrer S, Pardo I. Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl. Microbiol. Biotechnol. 2014;98:185–198. doi: 10.1007/s00253-013-4829-6. [DOI] [PubMed] [Google Scholar]
- Callejón S, Sendra R, Ferrer S, Pardo I. Cloning and characterization of a new laccase from Lactobacillus plantarum J16 CECT 8944 catalyzing biogenic amines degradation. Appl. Microbiol. Biotechnol. 2016;100:3113–3124. doi: 10.1007/s00253-015-7158-0. [DOI] [PubMed] [Google Scholar]
- Choi JS, Jeong ST, Lee HS, Lee B-H, Kim SM. Biogenic amine production of makgeollis with controlled alcohol concentrations. Food Sci. Biotechnol. 2019;28:923–930. doi: 10.1007/s10068-018-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinquanta L, De Stefano G, Formato D, Niro S, Panfili G. Effect of pH on malolactic fermentation in southern Italian wines. Eur. Food Res. Technol. 2018;244:1261–1268. doi: 10.1007/s00217-018-3041-4. [DOI] [Google Scholar]
- Doeun D, Davaatseren M, Chung M-S. Biogenic amines in foods. Food Sci. Biotechnol. 2017;26:1463–1474. doi: 10.1007/s10068-017-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gani Z, Boradia VM, Raghu Ram J, Suryavanshi PM, Patil P, Kumar S, Singh R, Raje M, Raje CI. Purification and characterization of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) from pea seeds. Protein Express. Purif. 2016;127:22–27. doi: 10.1016/j.pep.2016.06.014. [DOI] [PubMed] [Google Scholar]
- García-Ruiz A, González-Rompinelli EM, Bartolomé B, Moreno-Arribas MV. Potential of wine-associated lactic acid bacteria to degrade biogenic amines. Int. J. Food Microbiol. 2011;148:115–120. doi: 10.1016/j.ijfoodmicro.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Garcin ED. GAPDH as a model non-canonical AU-rich RNA binding protein. Semin. Cell Dev. Biol. 2019;86:162–173. doi: 10.1016/j.semcdb.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Guo Y-Y, Yang Y-P, Peng Q, Han Y. Biogenic amines in wine: a review. Int. J. Food Sci. Technol. 2015;50:1523–1532. doi: 10.1111/ijfs.12833. [DOI] [Google Scholar]
- Hafid N, Valverde F, Villalobo E, Elkebbaj MS, Torres A, Soukri A, Serrano A. Glyceraldehyde-3-phosphate dehydrogenase from Tetrahymena pyriformis: enzyme purification and characterization of a gapC gene with primitive eukaryotic features. Comp. Biochem. Physiol. B. 1998;119:493–503. doi: 10.1016/S0305-0491(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henríquez-Aedo K, Durán D, Garcia A, Hengst MB, Aranda M. Identification of biogenic amines-producing lactic acid bacteria isolated from spontaneous malolactic fermentation of chilean red wines. LWT-Food Sci. Technol. 2016;68:183–189. doi: 10.1016/j.lwt.2015.12.003. [DOI] [Google Scholar]
- Hui S, Hong W, Qiao L, Huihui L, Maikun T, Xu L. Structural insights into RNA recognition properties of glyceraldehyde-3-phosphate dehydrogenase 3 from Saccharomyces cerevisiae. IUBMB Life. 2014;66:631–638. doi: 10.1002/iub.1313. [DOI] [PubMed] [Google Scholar]
- Kim Y-J, Kim Y-W. Optimizing the preparation conditions and characterization of cross-linked enzyme aggregates of a monoamine oxidase. Food Sci. Biotechnol. 2016;25:1421–1425. doi: 10.1007/s10068-016-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkova J. Histamine, histamine intoxication and intolerance. Allergol. Immunopath. 2015;43:498–506. doi: 10.1016/j.aller.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martínez-Pinilla O, Guadalupe Z, Hernández Z, Ayestarán B. Amino acids and biogenic amines in red varietal wines: the role of grape variety, malolactic fermentation and vintage. Eur. Food Res. Technol. 2013;237:887–895. doi: 10.1007/s00217-013-2059-x. [DOI] [Google Scholar]
- OIV. Compendium of international methods of wine and must analysis. Organisation Internationale de la Vigne et du Vin, Paris. (2018)
- Ong JS, Taylor TD, Wong CB, Khoo BY, Sasidharan S, Choi SB, Ohno H, Liong MT. Extracellular transglycosylase and glyceraldehyde-3-phosphate dehydrogenase attributed to the anti-staphylococcal activity of Lactobacillus plantarum USM8613. J. Biotechnol. 2019;300:20–31. doi: 10.1016/j.jbiotec.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Loizzo M, Spizzirri U. Accumulation of biogenic amines in wine: role of alcoholic and malolactic fermentation. Fermentation. 2018;4:6. doi: 10.3390/fermentation4010006. [DOI] [Google Scholar]
- Sirover MA. Structural analysis of glyceraldehyde-3-phosphate dehydrogenase functional diversity. Int. J. Biochem. Cell B. 2014;57:20–26. doi: 10.1016/j.biocel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A, du Toit M. Evaluating the influence of malolactic fermentation inoculation practices and ageing on lees on biogenic amine production in wine. Food Bioproc. Technol. 2013;6:198–206. doi: 10.1007/s11947-011-0702-8. [DOI] [Google Scholar]
- Sun S, Jiang D, Fan M, Li H, Jin C, Liu W. Selection of a versatile Lactobacillus plantarum for wine production and identification and preliminary characterization of a novel histamine-degrading enzyme. Int. J. Food Sci. Technol. 2020;55:2608–2618. doi: 10.1111/ijfs.14514. [DOI] [Google Scholar]
- Sun SY, Chen ZX, Jin CW. Combined influence of lactic acid bacteria starter and final pH on the induction of malolactic fermentation and quality of cherry wines. LWT-Food Sci. Technol. 2018;89:449–456. doi: 10.1016/j.lwt.2017.11.023. [DOI] [Google Scholar]
- Sun SY, Gong HS, Liu WL, Jin CW. Application and validation of autochthonous Lactobacillus plantarum starter cultures for controlled malolactic fermentation and its influence on the aromatic profile of cherry wines. Food Microbiol. 2016;55:16–24. doi: 10.1016/j.fm.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sun W, Huang L, Zhu K, Pei F, Zhu L, Wang Q, Lu Y, Zhang H, Jin H, Zhang L-H, Zhang L, Yue J. Identifying glyceraldehyde 3-phosphate dehydrogenase as a cyclic adenosine diphosphoribose binding protein by photoaffinity protein–ligand labeling approach. J. Am. Chem. Soc. 2017;139:156–170. doi: 10.1021/jacs.6b08088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


