Abstract
Crutzen (1974) and Crutzen and Ehhalt (1977) presented two key papers in Ambio that in Ambioexemplify how science first revealed to humankind the potential for damage to our ozone shield in the Anthropocene. Crutzen’s (1974) review is a sweeping summary of the risks to the ozone layer from supersonic aircraft, chlorofluorocarbons, as well as nuclear weapons testing and nuclear war. Crutzen and Ehhalt (1977) described how the nitrous oxide produced from fertilizers could pose another threat to the stability of the stratospheric ozone layer. The two papers are part of a body of influential scientific work that led to the pioneering Montreal Protocol to Protect the Earth’s Ozone Layer to phase out production of chlorofluorocarbons (in 1987), as well as national decisions that slowed or stopped production of supersonic planes (in the 1970s). They remain guideposts today for ongoing international negotiations regarding reducing emissions from fertilizer and limiting nuclear testing.
Keywords: Chlorofluorocarbons, Fertilizers, Montreal Protocol, Nuclear, Ozone, Policy
Introduction
The 1970s witnessed an explosion of scientific understanding of stratospheric ozone that rocked both the scientific and political spheres, and continues even to the present day. A few key scientific leaders and their papers initiated this remarkable period of scholarly advancement and societal response to scientific information. Among the foremost are Paul Crutzen and Dieter Ehhalt, in papers published in Ambio. In a tour-de-force review, Crutzen (1974) summarized his own and other modeling work demonstrating that the stratospheric ozone layer (the Earth’s protective shield against damaging ultraviolet sunlight) was at risk due to human activities. Ehhalt is best known for critical measurements that provided an early bedrock database for stratospheric trace species that affect the ozone layer, including nitrous oxide and chlorofluorocarbons. Together they analyzed the sensitivities of the ozone layer to human activities involving nitrous oxide increases from human use of fertilizer in a landmark paper (Crutzen and Ehhalt 1977).
Humans have taken action to protect the ozone layer, guided in part by this scientific information. Protection of the ozone layer against chlorofluorochemicals (chlorofluorocarbons, or CFCs) is widely considered the signature environmental success story of the twentieth century. But the story has not yet ended. Policy discussions continue today regarding whether and by how much fertilizer-related emissions of nitrous oxide (N2O) could be and should be constrained because of impacts on both the ozone layer and the climate system. Below I briefly discuss the remarkable history of these two papers, the ozone protection policies that flowed from them, and the unfinished business of N2O emission.
Evolution of the science and policy of the supersonic transport (SST), chlorofluorochemicals, and ozone
By the late 1960s, scientists were deeply puzzled by a large mismatch between observations of the vertical profile of stratospheric ozone and the then-understood chemistry. Crutzen (1970) published a landmark paper (while still a postdoctoral associate at the time) in which he postulated that a new catalytic cycle involving nitrogen oxides could explain the conundrum. He drew in part upon the work of Bates and Hays (1967), who had postulated that nitrous oxide produced by microbial processes in soil could be transported to the stratosphere, where its breakdown could yield NO and NO2; Bates and Hays focused on potential natural sources of stratospheric nitrogen oxides but did not make the connections to ozone chemistry identified by Crutzen, nor did they discuss fertilizers.
Johnston (1971) in turn drew on Crutzen’s work to argue that then-proposed fleets of supersonic transport (SST) aircraft that would cruise through the lower stratosphere and emit nitrogen oxides could deplete the ozone layer far more than had yet been imagined. He calculated the steady-state concentrations of ozone for scenarios with and without the proposed 500 fleet using a hand calculator, and suggested that by 1985, the ozone layer would be depleted by 20 to 50%, a catastrophic amount that would wreak havoc on surface biology (including direct effects on humans in the form of skin cancer and eye damage). Johnston’s result was heavily criticized by meteorologists regarding whether the influence of stratospheric winds and transport would invalidate the steady-state assumptions that he had made, and reduce the estimated losses.
Molina and Rowland (1974) were the first to draw attention to the fact that chlorofluorocarbon chemicals that were used mainly as the propellants in spray cans at that time might pose risks for the ozone layer. Other uses of these chemicals included as cooling agents in refrigeration and air conditioning, as solvents, and to blow foams such as Styrofoam. Molina and Rowland suggested that ozone losses from the chlorine released by continued human use of chlorofluorochemicals would cause future stratospheric ozone losses comparable to those estimated for the nitrogen oxides from SSTs.
Crutzen’s (1974) paper in Ambio introduced his one-dimensional global average stratospheric model, one of the first of its day to attempt to simulate transport along with chemistry using a computer (albeit with the limitations of a single dimension in the vertical), and applied it to improve the estimates of these risks to the ozone layer. He was one of a handful of international scientists who worked with astonishing speed in the early 1970s to provide decision makers with information needed for policy change. His 1974 paper built on earlier work by Crutzen (1972) and provided estimates of mid-latitude total ozone losses from the proposed fleet of 500 SSTs of about 14%, smaller than Johnston’s figure without including transport at all, but still quite substantial. Every one percent decrease in mid-latitude total ozone is projected to cause a 2 to 3% increase in, for example, skin cancer in light skinned people (along with other damaging biological impacts, such as to certain food crops and marine organisms; see Slaper et al. 1996; Bais et al. 2018). While other considerations such as the noise pollution from a supersonic passenger fleet and its economic cost contributed to national decision making about SSTs, the potential for ozone layer damage was likely among the factors that led the US not to manufacture such planes. In particular, efforts to revive the SST failed despite strong support for the project from then-President Nixon (Dotto and Schiff 1978) after the publication of these papers. France and the UK did construct the Concorde SST as a joint project, and the USSR (now Russia) also built some of their own, but the SSTs never flew in large numbers and were later entirely discontinued (Drake and Purvis 2001).
Regarding the CFCs, in 1978, Sweden became the first nation to ban their use entirely in non-essential spray cans, followed rapidly by the US, Canada, Norway, and Denmark (New York Times 1978; Morrisette 1989). However, many other countries continued to use CFCs in sprays for many years, and global use of CFCs in refrigeration and air conditioning not only continued but also increased. The risk of ozone depletion led to the signing of the landmark UN Montreal Protocol by dozens of nations in 1987 (Birmpili 2018) taking the policy action to the global level and beginning the global process that ultimately led to the agreement to entirely phase out manufacture of the ozone-depleting substances (ODS), including CFCs, halons (compounds containing bromine) and later, the HCFC and HFC substitutes for the CFCs (hydrochlorofluorocarbons and hydrofluorocarbons (Morrisette 1989; Birmpili 2018). Although there is evidence that some illegal production of CFC has been occurring over 2014–2018 (Montzka et al. 2018; Rigby et al. 2019), it is too small so far to substantially hinder ozone recovery (Dhomse et al. 2019; Fleming et al. 2020), and discernible healing of ozone hole that formed in the Antarctic in the 1980s has already begun (Solomon et al. 2016; WMO/UNEP 2018). Figure 1 depicts the expected recovery of the global ozone layer.
Fig. 1.
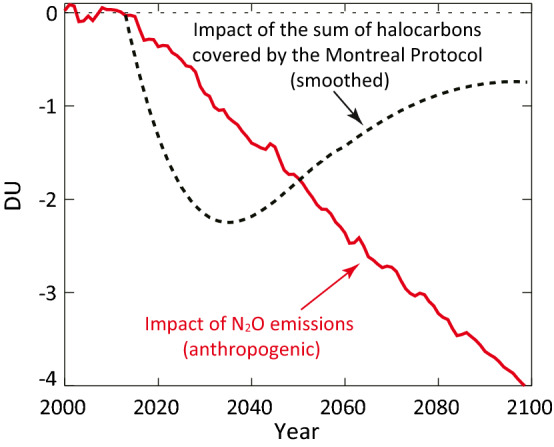
Impact on global ozone from future emissions of N2O and the sum of halocarbons covered by the Montreal Protocol (after Daniel et al. 2010; from UNEP 2013, Fig. 2.3 and used with permission). The figure shows that eliminating N2O emissions after 2010 would lead to a 4 Dobson Unit (DU) increase in global average total ozone by 2100, or about 1.25% of the average value of 300 DU. Eliminating halocarbon emissions after 2010 has a small impact by 2100 because these emissions were nearly all phased out before 2010
Crutzen (1974) also presented an analysis of the potential for nitrogen oxides from nuclear weapons to deplete stratospheric ozone. He carefully considered the nuclear tests of the 1960s, and wisely avoided trying to interpret the observed ozone record as reflecting their impact, given that meteorological variability leads to significant year to year fluctuations in total ozone. But he also noted that the far larger production of nitrogen oxides that could be produced in the event of nuclear war between major world powers could have much larger and catastrophic impacts; for example, a 500 megatonne TNT detonation would be expected to yield a 50% loss of total ozone. Crutzen did not consider the influence of soot. Later studies (e.g., Mills et al. 2008) suggested that even a much smaller and limited nuclear exchange (e.g., between India and Pakistan) could inject enough soot into the stratosphere to cause ozone losses rivaling those of the ozone hole, due to the heating of stratospheric air and influence of warmer temperatures on ozone chemistry (especially the nitrogen oxide reactions identified in Crutzen 1970). Thus, while there are many reasons to avoid nuclear wars, testing, or exchanges, the risks they pose to the stability of the global ozone layer are among them and Crutzen (1974) was one of the first to quantify this issue.
The fertilizer challenge
The sustainability challenges related to human use of fertilizers extend from its important use to increase food supplies to its influence on nitrous oxide emissions which affect climate change and ozone. It was Crutzen who first pointed out that application of nitrogen fertilizers could increase the abundance of stratospheric N2O and thereby represent a new mechanism for ozone depletion. According to Johnston (1977), Crutzen first proposed this in a memorandum in 1975, and Crutzen and Ehhalt’s (1977) paper in Ambio presented the issue in detail. They emphasized that certain types of bacteria in soil can reduce nitrate (NO3−) to gaseous nitrogen and nitrous oxide. While the natural rate at which denitrification processes occur must equal the rate of nitrogen fixation, addition of nitrogen fertilizers to the soil increases denitrification and releases increased N2O, depending upon factors such as acidity, temperature, and especially the oxygen availability. In anaerobic conditions where little oxygen is available, the fraction of fertilizer converted to N2O is largest. Crutzen and Ehhalt projected an increase in the application of nitrogen fertilizers to better nourish the expected populations of a growing and modernizing developing world, from about 40 Tg/year in 1977 to almost 200 Tg/year by 2010. A recent survey puts the 2010 number closer to 100 Tg/year (Heffer and Prud’homme 2016). Interestingly, Crutzen and Ehhalt (1977) suggested that global human population might stop increasing around 2010 and that global development would also be well advanced, so that further N2O increases from agriculture might hence be limited. These two assumptions were overly optimistic, and N2O emissions from increasing fertilizer use remains a twenty-first century challenge.
Crutzen and Ehhalt (1977) were conscious of large scientific uncertainty in the atmospheric turnover time (or lifetime) of N2O at the time of their study. Using laboratory data on its photolysis and chemical loss with O1D together with transport times in Crutzen’s or other models of the day yielded atmospheric lifetimes of the order of 150 years. On the other hand, in-situ measurements of the tropospheric variability of N2O suggested a much shorter lifetime of the order of only 5 to 10 years (Junge 1974). Analytical issues plagued those early in-situ data, and their paper certainly highlighted the urgency of resolving this key discrepancy through improved measurements. Measurement techniques have greatly improved, and current studies indicate that the lifetime of N2O is indeed around 120 years (UNEP 2013). Using their 160-year lifetime estimate, Crutzen and Ehhalt suggested that by 2100, the increased use of nitrogen fertilizer could deplete the global ozone column by around 10%, and their pioneering paper was among the first to establish this link.
Perhaps because of the difficult politics of linkages of N2O emissions from use of fertilizers to food supply, perhaps because of scientific and projection uncertainties, or perhaps because the production and use of fertilizers involves a broader range of practical and procedural issues than for the chlorofluorochemicals (e.g., see Kanter et al. 2013), N2O has not thus far been included among the ozone-depleting substances regulated by the Montreal Protocol. Discussions have been ongoing to this date as to whether or not it ought to be included in adjustments and amendments to the Protocol. A key point is that now that emissions of chlorofluorochemicals have markedly decreased, it is expected that N2O will be the primary driver of future ozone depletion in the twenty-first century as shown in Fig. 1 (Daniel et al. 2010; UNEP 2013). Anthropogenic emissions of N2O, largely from fertilizers, are therefore undermining some of the gains that would otherwise be achieved by the historic Montreal Protocol. Further, because of its relatively long lifetime and efficient absorption of infrared radiation, N2O is currently the third most important long-lived anthropogenic greenhouse gas (after CO2 and CH4), so its growing concentrations are one of the drivers of change in the Earth’s climate system (UNEP 2013). As the world pays increasing attention to avoiding warming that exceeds the 2 °C and 1.5 °C targets of the Paris Agreement, the potential to reduce warming by N2O along with the range of other molecules and aerosols is a subject of scrutiny. Among the practical measures that can reduce N2O emissions from fertilizers, a recent comprehensive UN report highlighted enhanced efficiency fertilizers that increase uptake of nitrogen by plants while minimizing soil emissions, as well as crop and nutrient management practices that ensure efficient use of animal manure and fertilizers (UNEP 2013).
Closing thoughts
For both the policy decisions on SSTs and on CFCs, Crutzen’s (1974) paper is part of the backbone of the collective science by an active group of researchers that fostered public understanding and policy actions at both the national and international levels. Decisions were made by various nations to build or not build SST aircraft, considering a variety of factors among which the potential for ozone loss highlighted by that paper along with others was one. While current models suggest that SST aircraft pose less of a risk to the ozone layer than originally projected (due mainly to changes in laboratory kinetic understanding), the risks posed by the chlorolofluorochemicals proved to be far greater than the early projections (not only at mid-latitudes and the Arctic, but also in the spectacular Antarctic ozone hole). The risks from nitrous oxide emissions from fertilizers remain broadly similar to the early estimates. In the Anthropocene some risks may be overestimated, while others are underestimated by the best available science at any given time, and human society is constantly forced to make decisions while recognizing that science is incomplete. For CFCs, non-essential national spray can bans followed scientific work of the early 1970s in several countries (and usages deemed essential such as the small amounts in asthma inhalers continued, in flexible policies designed around practicality). The 1987 landmark Montreal Protocol began an historic global agreement that has averted massive ozone losses (Newman et al. 2009), and the healing of the Antarctic ozone hole has already begun despite evidence for a small amount of likely illicit new production. Finally, more than four decades after Crutzen and Ehhalt’s (1977) paper, policymakers and scientists continue to work on understanding N2O from fertilizers and to mitigate its impacts on ozone depletion (and climate). Thus, the intellectual shadows cast by these two great papers continue to influence twenty-first century science and policy.
Susan Solomon
is the Lee and Geraldine Martin Professor of Environmental Studies at the Massachusetts Institute of Technology in Cambridge, MA. Her major interests include stratospheric ozone chemistry, chemistry–climate coupling, and the timescales of climate change processes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bais AF, Lucas RM, Bornman JF, Williamson CE, Sulzberger B, Austin AT, Wilson SR, Andrady AL, Bernhard G, McKenzie RL, Aucamp PJ, Madronich S, Neale RE, Yazar S, Young AR, de Gruij FR, Norval M, Takizawa Y, Barnes PW, Robson TM, Robinson SA, Ballaré CL, Flint SD, Neale PJ, Hylander S, Rose KC, Wängberg S-Å, Häder D-P, Worrest RC, Zepp RG, Paul ND, Cory RM, Solomon KR, Longstreth J, Pandey KK, Redhwi HH, Torikai A, Heikkilä AM. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochemical & Photobiological Sciences. 2018;17:127–179. doi: 10.1039/C7PP90043K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DR, Hays PB. Atmospheric nitrous oxide. Planetary and Space Science. 1967;15:189–197. doi: 10.1016/0032-0633(67)90074-8. [DOI] [Google Scholar]
- Birmpili T. Montreal Protocol at 30: The governance structure, the evolution, and the Kigali Amendment. Comptes Rendus Geoscience. 2018;350:425–431. doi: 10.1016/j.crte.2018.09.002. [DOI] [Google Scholar]
- Crutzen PJ. The influence of nitrogen oxides on the atmospheric ozone content. Quarterly Journal of the Royal Meteorological Society. 1970;96:320–325. doi: 10.1002/qj.49709640815. [DOI] [Google Scholar]
- Crutzen PJ. SST's: A threat to the earth's ozone shield. Ambio. 1972;1:41–51. [Google Scholar]
- Crutzen PJ. Estimates of possible variations in total ozone due to natural causes and human activities. Ambio. 1974;3:201–210. [Google Scholar]
- Crutzen PJ, Ehhalt DH. Effects of nitrogen fertilizers and combustion on the stratospheric ozone layer. Ambio. 1977;6:112–117. [Google Scholar]
- Daniel JS, Fleming EL, Portmann RW, Velders GJM, Jackman CH, Ravishankara AR. Options to accelerate ozone recovery: Ozone and climate benefits. Atmospheric Chemistry & Physics. 2010;10:7697–7707. doi: 10.5194/acp-10-7697-2010. [DOI] [Google Scholar]
- Dhomse SS, Feng W, Montzka SA, Hossaini R, Keeble J, Pyle JA, Daniel JS, Chipperfield MP. Delay in recovery of the Antarctic ozone hole from unexpected CFC-11 emissions. Nature Communications. 2019;10:1–12. doi: 10.1038/s41467-019-13717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto L, Schiff H. The ozone war. New York: Doubleday Books; 1978. [Google Scholar]
- Drake F, Purvis M. The effect of supersonic transports on the global environment: A debate revisited. Science, Technology, & Human Values. 2001;26:501–528. doi: 10.1177/016224390102600406. [DOI] [Google Scholar]
- Fleming EL, Newman PA, Liang Q, Daniel JS. The impact of continuing CFC-11 emissions on stratospheric ozone. Journal of Geophysical Research: Atmospheres. 2020;125:e2019JD031849. [Google Scholar]
- Heffer, P., and M. Prud’homme. 2016. Global nitrogen fertilizer demand and supply: trend, current level, and outlook. Proceedings of the 2016 International Nitrogen Initiative Conference, “Solutions to improve nitrogen use efficiency for the world”, 4–8, 1 December 2016, Melbourne, Australia. www.ini2016.com.
- Johnston H. Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust. Science. 1971;173:517–522. doi: 10.1126/science.173.3996.517. [DOI] [PubMed] [Google Scholar]
- Johnston HS. Analysis of the independent variables in the perturbation of stratospheric ozone by nitrogen fertilizers. Journal of Geophysical Research. 1977;82:1767–1772. doi: 10.1029/JC082i012p01767. [DOI] [Google Scholar]
- Junge CE. Residence time and variability of tropospheric trace gases. Tellus. 1974;26:477–488. [Google Scholar]
- Kanter D, Mauzerall DL, Ravishankara AR, Daniel JS, Portmann RW, Grabiel PM, Moomaw WR, Galloway JN. A post-Kyoto partner: Considering the stratospheric ozone regime as a tool to manage nitrous oxide. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4451–4457. doi: 10.1073/pnas.1222231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills MJ, Toon OB, Turco RP, Kinnison DE, Garcia RR. Massive global ozone loss predicted following regional nuclear conflict. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5307–5312. doi: 10.1073/pnas.0710058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina MJ, Rowland FS. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature. 1974;249:810–812. doi: 10.1038/249810a0. [DOI] [Google Scholar]
- Montzka SA, Dutton GS, Yu P, Ray E, Portmann RW, Daniel JS, Kuijpers L, Hall BD, Mondeel D, Siso C, Nance JD, Rigby M, Manning AJ, Hu L, Moore F, Miller BR, Elkins JW. An unexpected and persistent increase in global emissions of ozone-depleting CFC-11. Nature. 2018;557:413–417. doi: 10.1038/s41586-018-0106-2. [DOI] [PubMed] [Google Scholar]
- Morrisette PM. The evolution of policy responses to stratospheric ozone depletion. Natural Resources Journal. 1989;29:793–820. [Google Scholar]
- Newman PA, Oman LD, Douglass AR, Fleming EL, Frith SM, Hurwitz MM, Kawa SR, Jackman CH, Krotkov NA, Nash ER, Nielsen JE, Pawson S, Stolarski RS, Velders GJM. What would have happened to the ozone layer if chlorofluorocarbons (CFCs) had not been regulated? Atmospheric Chemistry & Physics. 2009;9:2113–2128. doi: 10.5194/acp-9-2113-2009. [DOI] [Google Scholar]
- New York Times. 1978. Most aerosols face a Swedish ban. https://timesmachine.nytimes.com/timesmachine/1978/01/30/110785008.html?pageNumber=17. Accepted 9 Aug 2020.
- Rigby M, Park S, Saito T, Western LM, Redington AL, Fang X, Henne S, Manning AJ, Prinn RG, Dutton GS, Fraser PJ, Ganesan AL, Hall BD, Harth CM, Kim J, Kim K-R, Krummel PB, Lee T, Li S, Liang Q, Lunt MF, Montzka SA, Mühle J, O'Doherty S, Park M-K, Reimann S, Salameh PK, Simmonds P, Tunnicliffe RL, Weiss RF, Yokouchi Y, Young D. Increase in CFC-11 emissions from eastern China based on atmospheric observations. Nature. 2019;569:546–550. doi: 10.1038/s41586-019-1193-4. [DOI] [PubMed] [Google Scholar]
- Slaper H, Velders GJ, Daniel JS, de Gruijl FR, van der Leun JC. Estimates of ozone depletion and skin cancer incidence to examine the Vienna Convention achievements. Nature. 1996;384:256–258. doi: 10.1038/384256a0. [DOI] [PubMed] [Google Scholar]
- Solomon S, Ivy DJ, Kinnison D, Mills MJ, Neely RR, III, Schmidt A. Emergence of healing in the Antarctic ozone layer. Science. 2016;353(6296):269–274. doi: 10.1126/science.aae0061. [DOI] [PubMed] [Google Scholar]
- UNEP. 2013. Drawing down N2O to protect climate and the ozone layer. A UNEP Synthesis Report. United Nations Environment Programme (UNEP), Nairobi, Kenya.
- WMO (World Meteorological Organization). 2018. Scientific Assessment of Ozone Depletion: 2018. Global Ozone Research and Monitoring Project—Report No. 58, 588 pp., Geneva, Switzerland.


