Abstract
Background
There is scarce evidence on the feasibility, safety and resource utilisation of active mobilisation in critically ill patients on extracorporeal life support (ECLS).
Methods
This prospective observational single-centre study included all consecutive critically ill patients on ECLS admitted to an academic centre in Germany over a time period of one year. The level of mobilisation was categorised according to the ICU Mobility Scale (IMS). Primary outcome was complications during mobilisation.
Results
During the study period, active mobilisation with an activity level on the IMS of ≥ 3 was performed at least on one occasion in 43 out of 115 patients (37.4%). A total of 332 mobilisations with IMS ≥ 3 were performed during 1242 ECLS days (26.7%). ECLS configurations applied were va-ECMO (n = 63), vv-ECMO (n = 26), vv-ECCO2R (n = 12), av-ECCO2R (n = 10), and RVAD (n = 4). Femoral cannulation had been in place in 108 patients (93.9%). The median duration of all mobilisation activities with IMS ≥ 3 was 130 min (IQR 44–215). All mobilisations were undertaken by a multi-professional ECLS team with a median number of 3 team members involved (IQR 3–4). Bleeding from cannulation site requiring transfusion and/or surgery occurred in 6.9% of actively mobilised patients and in 15.3% of non-mobilised patients. During one mobilisation episode, accidental femoral cannula displacement occurred with immediate and effective recannulation. Sedation was the major reason for non-mobilisation.
Conclusions
Active mobilisation (IMS ≥ 3) of ECLS patients undertaken by an experienced multi-professional team was feasible, and complications were infrequent and managed successfully. Larger prospective multicentre studies are needed to further evaluate early goal directed sedation and mobilisation bundles in patients on ECLS.
Keywords: Mobilisation, Extracorporeal life support, ECLS, ECMO, ECCO2R
Background
Prolonged immobilisation during critical illness often promotes neuromuscular and neuropsychiatric syndromes such as intensive care unit-acquired weakness (ICU-AW) and ICU delirium, which in turn is associated with relevant long-term post-ICU morbidity [1]. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium have been published in order to enhance liberation from mechanical ventilation, target adequate sedation, increase patients’ functional status, and reduce neuromuscular and neuropsychiatric side-effects [2, 3]. Early and active mobilisation is associated with higher chances of returning to independent functioning, lower incidence of delirium, as well as decreased duration of mechanical ventilation, ICU and hospital length of stay [4]. Despite growing evidence that active mobilisation of critically ill patients is not only beneficial, but also safe and feasible in the general ICU population, many barriers to its implementation in routine clinical practice have been identified [5–8].
Over the last decade, extracorporeal life support (ECLS) is increasingly being applied to critically ill patients with severe circulatory and/or respiratory failure [9, 10]. Technological advances improving the quality and safety of membranes, circuits, cannulas, and pumps as well as promising clinical research results on the potential benefit of ECLS on survival have all motivated this trend [9]. However, patients receiving ECLS are often considered too unstable for active mobilisation [11, 12]. Moreover, concerns about specific complications associated with active mobilisation of patients on ECLS, such as circuit malfunction and/or cannula complications, are potential barriers to active mobilisation of these patients [11, 12]. So far, only a few retrospective cohort studies and one recent small pilot randomised controlled study have been published on the feasibility and safety of actively mobilising critically ill patients on ECLS [13, 14].
This prospective observational single-centre study was initiated within a quality assurance programme to evaluate the feasibility and safety of active mobilisation in ECLS patients by a multi-professional team. Further goals were to assess resource requirements for active mobilisations, as well as to investigate reasons for withholding active mobilisation to patients on ECLS.
Methods
Study design and population
This prospective observational study was conducted in a university hospital and ECLS referral centre in Germany. All consecutive adult patients admitted to the department of intensive care medicine from January to December 2014 treated with percutaneous ECLS for severe circulatory and/or respiratory failure were included. The study protocol was approved by the institutional ethics committee (protocol number PV4685) and was conducted according to the amended declaration of Helsinki.
ECLS configurations under investigation were veno-arterial extracorporeal membrane oxygenation (va-ECMO), veno-venous extracorporeal membrane oxygenation (vv-ECMO), veno-venous extracorporeal carbon dioxide removal (vv-ECCO2R), arterio-venous extracorporeal carbon dioxide removal (av-ECCO2R), and right ventricular assist device (RVAD).
Clinical setting and protocol
The multi-professional ECLS team comprised critical care nurses, physiotherapists, perfusionists, intensive care physicians, and cardiac surgeons. Local protocols for ECLS management, anticoagulation, mechanical ventilation, analgosedation, weaning and mobilisation were followed. All ECLS cannulas were secured by means of cutaneous sutures in combination with adhesive tapes and bandages. In va-ECMO a distal perfusion catheter was placed in addition to the arterial cannula to enhance distal limb perfusion.
All ECLS patients were screened daily for readiness for mobilisation. The standardised screening procedure included assessment of neurological ability, cardiopulmonary stability, stability of the ECLS circuit flow, and securement of the ECLS cannulas. In patients with femoral cannulation a 90° hip flexion was performed to ensure stable ECLS blood flow under flexed conditions. Thereafter, a daily individualised mobilisation plan was implemented by the interprofessional team, led by the physiotherapists, mobilising ECLS patients as far as clinically feasible and appropriate. The mobilisation sessions included functional strengthening, breathing exercises, active upper and lower limb exercises, endurance exercises, and progressing functional mobility. The level of mobilisation on each day on ECLS was categorised according to the ICU Mobility Scale (IMS; Table 1) [15].
Table 1.
ICU mobility scale [15]
| ICU mobility scale (IMS) |
|---|
| IMS 0: no mobilisation or passively exercised by staff |
| IMS 1: sitting in bed and actively exercising |
| IMS 2: passively moved to chair without standing |
| IMS 3: sitting over edge of bed |
| IMS 4: standing in front of bed |
| IMS 5: transferring bed to chair |
| IMS 6: marching on spot |
| IMS 7: walking with assistance of more than one person |
| IMS 8: walking with assistance of one person |
| IMS 9: walking independently with a gait aid |
| IMS 10: walking independently without a gait aid |
ICU intensive care unit, IMS ICU mobility scale
Reasons for deferring active mobilisation per protocol included severe hypoxemia, hemodynamic instability, unstable dysrhythmia, bleeding or high risk of bleeding, sedation or coma precluding active mobilisation and the use of neuromuscular blockade.
To manage equipment and continuously monitor haemodynamic and respiratory state, the multi-professional team mobilising the patient typically consisted of a physiotherapist, a critical care nurse, and an intensivist. Prior to each intervention in patients with femoral cannulas the team performed a hip flexion manoeuvre to ascertain stable extracorporeal blood flow and secure cannulas. Non-essential treatments were temporarily discontinued during active mobilisation. Based on clinical judgement extracorporeal respiratory and/or circulatory support was temporarily increased during active mobilisation. Active mobilisation was interrupted or terminated for unexpected changes including sustained haemodynamic instability, hypoxaemia, weakness, chest pain or other relevant clinical symptoms or signs.
Data collection
Baseline demographic and clinical data including ECLS characteristics and functions, as well as ICU-mortality was collected in the departments’ electronic patient data management system (Integrated Care Manager®, Dräger, Lübeck, Germany). As part of a one-year quality assurance project, the following data were prospectively collected before, during and after all active mobilisations at an activity level of equal to or more than side-of-bed activities (IMS ≥ 3): ECLS and cannulation settings, mechanical ventilation, concurrent renal replacement therapy, haemodynamic and respiratory status, level of sedation according to the Richmond Agitation Sedation Scale (RASS), and complications potentially related to mobilisation on ECLS.
Furthermore, the time from start to end of mobilisation and the number of team members involved in each mobilisation activity at an activity level of an IMS ≥ 3 were observed. Additionally, daily reasons for non-mobilisation as indicated by the attending ECLS team were prospectively recorded through a questionnaire.
Outcomes recorded were all ECLS-associated complications that occurred during active mobilisation as well as length of ECLS treatment, length of ICU and hospital stay, and ICU-mortality. Complications included patient-related complications (fall, haemodynamic instability, desaturation to < 85%, cardiac arrest, arrhythmia, acute limb ischemia, bleeding at cannulation site, cannula dislodgement) and circuit-related complications (oxygenator and pump failure, tubing rupture, critical drop or interruption of extracorporeal blood flow, clotting in the extracorporeal circuit). Major complications were defined as adverse events requiring abrupt discontinuation of mobilisation for medical and/or surgical treatment. Minor complications were defined as self-limiting or instantly reversible adverse events not requiring discontinuation of mobilisation.
Statistical analyses
Continuous variables are expressed as medians (with range). Categorical variables are expressed as counts and percentages. In order to compare outcome variables between subgroups, non-parametric analyses were performed. Depending on the number of groups compared and the type of data, Chi-squared test, Fisher’s exact test, Mann–Whitney U test, or Kruskal–Wallis one-way analysis of variance were applied. A two-sided p < 0.05 was considered significant. The software used for analyses was SPSS (version 23, IBM SPSS Statistics, USA) and STATA v.14.2 (StataCorp LLC, College Station, TX, USA).
Results
Baseline clinical characteristics
Details of demographic baseline data, diagnostic categories and clinical severity scores differentiating for mobilised (Mob, IMS ≥ 3) and non-mobilised (Non-Mob, IMS < 3) patients are displayed in Table 2. During the study period, a total of 115 patients (32.2% female) with a median age of 57.0 years (IQR 46.0–67.5) were treated with ECLS. Mobilisation at an activity level of IMS ≥ 3 was performed at least on one occasion in 43 patients (37.4%). These patients had significant lesser severity of illness scores than those patients not mobilised to that activity level.
Table 2.
Baseline demographics, diagnostic categories, severity of illness scores, type of primary ECLS, non-intubated patients, cannulation configuration according to level of mobilisation
| Variable | Mob | Non-Mob | All | p value |
|---|---|---|---|---|
| n = 43 (37.4%) | n = 72 (62.6%) | n = 115 | ||
| Age, BMI, and sex—median (IQR), n (%) | ||||
| Age (years) | 53.0 (45.0–68.0) | 58.5 (47.8–67.0) | 57.0 (46.0–67.5) | 0.51 |
| Body mass index (BMI) | 24.6 (21.2–27.3) | 26.2 (23.2–29.3) | 25.6 (22.5–27.8) | 0.37 |
| Female sex (% within subgroup) | 15 (34.9) | 22 (30.6) | 37 (32.2) | 0.68 |
| Primary diagnosis—n (% Mob/Non-Mob per subgroup; % between diagnostic groups for ALL) | ||||
| ARDS | 13 (34.2) | 25 (65.8) | 38 (33,0) | 0.08 |
| AHF without CS | 10 (37.0) | 17 (63.0) | 27 (23,5) | 0.96 |
| AHF peri/post-CS | 6 (35.3) | 11 (64.7) | 17 (14,8) | 0.84 |
| eCPR | 3 (17.6) | 14 (82.4) | 17 (14,8) | 0.10 |
| COPD | 7 (63.6) | 4 (36.4) | 11 (9,6) | 0.09 |
| ILD | 3 (75.0) | 1 (25.0) | 4 (3,5) | 0.14 |
| PAH | 1 (100.0) | 0 (0.0) | 1 (0,9) | 0.37 |
| ECLS as bridge-to-transplant | 2 (100.0) | 0 (0.0) | 2 (1.7) | 0.14 |
| Severity of illness scores—median (IQR) | ||||
| SAPS-II score on ICU admission | 38 (31–47) | 44 (39–54) | 42 (36–50) | 0.01 |
| APACHE II score on ICU admission | 19 (16–22) | 26 (20–30) | 23 (18–28) | < 0.01 |
| SOFA score on ICU admission | 8 (6–11) | 11 (8–13) | 10 (7–13) | < 0.01 |
| Type of primary ECLS—n (% Mob/Non-Mob per subgroup; % within ECLS types for ALL) | ||||
| va-ECMO (non-eCPR) | 14 (29.8) | 33 (70.2) | 47 (40.9) | < 0.01 |
| eCPR (va-ECMO) | 3 (17.6) | 14 (82.4) | 17 (14.8) | 0.01 |
| vv-ECMO | 12 (46.2) | 14 (53.8) | 26 (22.6) | 0.84 |
| vv-ECCO2R | 7 (58.3) | 5 (41.7) | 12 (10.4) | 0.77 |
| av-ECCO2R | 3 (33.3) | 6 (66.7) | 9 (7.8) | 0.75 |
| RVAD | 4 (100) | 0 (0) | 4 (2.6) | 0.02 |
| Non-intubated ECLS—n (% Mob/Non-Mob per subgroup; % within ECLS types for ALL) | ||||
| All non-intubated patients on ECLS | 9 (75.0) | 3 (25.0) | 12 (10.4) | 0.14 |
| Cannulation—n (% Mob/Non-Mob per subgroup; % within cannulation categories for ALL) | ||||
| Femoro-femoral | 22 (31.0) | 49 (69.0) | 71 (61.7) | < 0.01 |
| DL femoral | 2 (100) | 0 (0) | 2 (1.7) | 0.14 |
| Femoro-jugular | 12 (46.2) | 14 (53.8) | 26 (22.6) | 0.85 |
| DL jugular | 3 (75.0) | 1 (25.0) | 4 (3.5) | 0.63 |
| Femoro-central | 4 (44.4) | 5 (55.6) | 9 (7.8) | 0.98 |
| Central | 0 (0) | 3 (100) | 3 (2.6) | 0.29 |
Data are presented as n (%) for categorical variables and median (25th and 75th percentile) for continuous variables
Mob mobilised with IMS ≥ 3, Non-Mob never mobilised with IMS ≥ 3, ARDS acute respiratory distress syndrome, COPD chronic obstructive pulmonary disease, ILD interstitial lung disease, PAH pulmonary arterial hypertension, AHF acute heart failure, CS cardiac surgery, eCPR extracorporeal cardiopulmonary resuscitation, ECLS extracorporeal life support, ICU intensive care unit, SAPS Simplified Acute Physiology Score, APACHE Acute Physiology and Chronic Health Evaluation, SOFA Sepsis-related Organ Failure Assessment, eCPR extracorporeal cardiopulmonary resuscitation, ECLS extracorporeal life support, va-ECMO veno-arterial extracorporeal membrane oxygenation, vv-ECMO veno-venous extracorporeal membrane oxygenation, vv-ECCO2R veno-venous extracorporeal carbon dioxide removal, av-ECCO2R arterio-venous extracorporeal carbon dioxide removal, RVAD right ventricular assist device, DL double lumen
Study patients were treated with the following types of ECLS: va-ECMO (n = 63), vv-ECMO (n = 26), vv-ECCO2R (n = 12), av-ECCO2R (n = 10), and RVAD (n = 4). In 12 patients (10.4%) ECLS was performed in spontaneously breathing patients without invasive mechanical ventilation and in 17 patients (14.8%) more than one type of ECLS was used during their clinical course. Femoral cannulation was in place in 108 patients (93.9%). Details of respiratory, haemodynamic, and renal variables before initiation of ECLS are presented in Additional file 1
Mobilisations
Details on how many mobilisation units were applied for each type of ECLS and cannulation configuration are given in Table 3. On 310 out of 1242 ECLS days (24.9%) a total of 332 mobilisations with an IMS ≥ 3 were undertaken. On all other ECLS days the level of mobilisation was below IMS 3. Of all 332 mobilisation units, 313 (94.3%) mobilisations were undertaken with femoral ECLS cannulation and 91 (27.4%) mobilisations with concurrent renal replacement therapy through an additional Shaldon catheter. A total of 239 (72.0%) mobilisations were performed on spontaneously breathing patients without an artificial airway, 71 (21.4%) mobilisations on patients ventilated through a tracheal tube, and 22 (6.6%) mobilisations on patients with orotracheal intubation.
Table 3.
Active mobilisation units (IMS ≥ 3) according to ECLS type and cannulation
| Variable | Mobilisation units |
|---|---|
| n = 332 | |
| ECLS type—n (%) | |
| va-ECMO | 72 (21.7) |
| vv-ECMO | 100 (30.1) |
| vv-ECCO2R | 48 (14.5) |
| av-ECCO2R | 63 (19.0) |
| RVAD | 49 (14.7) |
| Cannulation configuration—n (%) | |
| DL jugular (vv-ECCO2R) | 19 (5.7) |
| Femoral any cannulation | 313 (94.3) |
| Femoro-femoral | 154 (46.4) |
| Femoro-femoral (va-ECMO) | 72 (21.7) |
| Femoro-femoral (av- ECCO2R) | 63 (19.0) |
| Femoro-femoral (vv-ECMO) | 19 (5.7) |
| DL femoral (vv-ECCO2R) | 26 (7.8) |
| Femoro-jugular | 84 (25.3) |
| Femoro-jugular (vv-ECMO) | 81 (24.4) |
| Femoro-jugular (vv-ECCO2R) | 3 (0.9) |
| Femoro-central | 49 (14.8) |
| Femoro-central (va-ECMO) | 8 (2.4) |
| Femoro-central (RVAD) | 41 (12.4) |
Data are presented as n (%) for categorical variables. ECLS extracorporeal life support, va-ECMO veno-arterial extracorporeal membrane oxygenation, vv-ECMO veno-venous extracorporeal membrane oxygenation, vv-ECCO2R veno-venous extracorporeal carbon dioxide removal, av-ECCO2R arterio-venous extracorporeal carbon dioxide removal, RVAD right ventricular assist device, DL double lumen
Duration of mobilisation and team resources
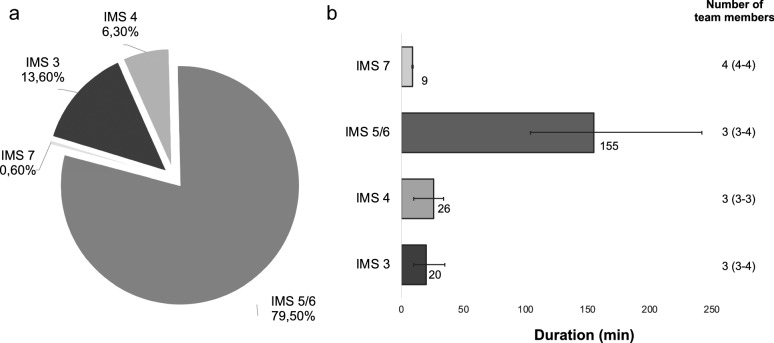
Duration and team resources of active mobilisations IMS 3–7 are presented in Fig. 1. The median duration of all mobilisation activities IMS ≥ 3 was 130 min (IQR 44–215) including the duration of sitting in a chair (IMS 5). Of these mobilisation episodes, 45 were side-of-bed activities (13.6%), 21 standing activities (6.3%), 264 mobilisations into the chair via standing and brief walking (79.5%), and 2 walking exercises (0.6%). All mobilisations were undertaken by a multi-professional ECLS team with a median number of 3 team members involved (IQR 3–4).
Fig. 1.
Percentage, duration and team resources according to different levels of active mobilisation (IMS 3–7). Detailed analysis of a the percentage of each level of mobilisation of all mobilisation units (n = 332), and b duration of different levels of mobilisation and the respective number of team members used for mobilisation. IMS ICU mobility scale, data presented as percent (%) and median (IQR)
Reasons for non-mobilisation
The reasons for non-mobilisation on 932 ECLS days were as follows: sedation with RASS ≤ − 2 (65.0%), “poor general condition” (21.1%), coma without sedation (4.6%), lack of manpower (4.0%), haemodynamic instability (3.4%), respiratory instability (3.4%), agitation with RASS ≥ 2 (2.7%), and fear of cannula complications (2.6%). In 5.9% of non-mobilisation days no specific reason was documented.
Complications
Circuit malfunction and cardiopulmonary instability
Critical blood flows of < 2 L/min in high-flow ECLS and of < 0.5 L/min in low-flow ECLS, all lasting below 60 s, occurred in 3 (0.9%) and 1 (0.3%) mobilisation episodes, respectively. Acute low-flow alarms of the ECLS console were recorded during 12 mobilisation episodes (3.4%). Median variation of ECLS blood flow from before to during active mobilisation for high-flow ECLS (va-ECMO, vv-ECMO, RVAD; n = 224) was − 0.1 L/min (range − 3.8–1.9 L/min). For low-flow ECLS (vv-/av-ECCO2R; n = 111) the median variation of blood flow was − 0.08 L/min (range − 1.7–0.5 L/min).
Desaturation of peripheral oxygen saturation (SpO2) below 85% occurred during 63 mobilisations (19.0%), a drop in mean arterial blood pressure below 50 mmHg during 25 mobilisations (7.5%), and tachycardia (heart rate > 140/min) during 19 mobilisations (5.7%). Bradycardia (heart rate < 40/min) and ventricular arrhythmia were not noted.
All of the described clinical changes during mobilisation were brief and either self-limiting or successfully managed by the ECLS team. They did not require discontinuation of mobilisation and were therefore considered minor complications. Median alterations in heart rate, mean arterial pressure, arterial O2 saturation, and ECLS blood flow over the course of each mobilisation (IMS ≥ 3) are listed in Additional file 2.
Bleeding from cannulation site
Major bleeding from cannulation site requiring transfusion, surgery and/or discontinuation of mobilisation occurred in 3 out of 43 actively mobilised patients (6.9%) as opposed to 11 out of 72 non-mobilised patients requiring transfusion and/or surgery (15.3%). The three major bleeding complications in actively mobilised (IMS ≥ 3) patients all concerned bleeding from a femoral cannulation site and occurred in two patients without invasive mechanical ventilation and one patient on invasive mechanical ventilation. The types of ECLS used were va-ECMO, vv-ECMO and av-ECCO2R. Minor bleeding from cannulation site managed non-surgically and not requiring transfusion or discontinuation of mobilisation occurred in 9 out of 43 actively mobilised patients (20.9%) and in 1 out of 72 non-mobilised patients (1.4%).
Cannula displacement
During one mobilisation episode out of 332 active mobilisations (0.3%) an accidental displacement of the venous femoral cannula in a patient on va-ECMO occurred (2.3% of all actively mobilised ECLS patients). Subsequent rapid and effective recannulation with reestablishment of the va-ECMO circuit was achieved without sequelae. The patient was discharged later to a rehabilitation centre.
Treatment duration and survival
The median length of all ECLS treatments was 7 days (IQR 3–12), treatment durations for respiratory ECLS (vv-ECMO and vv-/av-ECCO2R), non-eCPR circulatory ECLS (va-ECMO and RVAD) and eCPR (va-ECMO) were 9.0 (IQR 5–15), 6.5 (IQR 2–12), and 3.5 (IQR 1–7) days, respectively. More details on ECLS treatment durations are presented in Additional file 3.
The median length of stay in ICU and hospital for all ECLS treatments were 16 days (IQR 7–37) and 33 days (IQR 10–72), respectively.
The ICU-mortality rate for all ECLS patients was 59.1 and 41.6% for non-intubated patients on ECLS (“awake ECLS”). For respiratory ECLS, non-eCPR circulatory ECLS and eCPR the ICU-mortality rates were 57.4, 54.9, and 76.5%, respectively.
Discussion
In our prospective observational study, active mobilisation (IMS ≥ 3) of critically ill patients on ECLS was undertaken in 37% of patients and found to be feasible and safe if performed by an experienced ECLS team that routinely provides mobilisation treatment and is well equipped to manage major complications. Of note, mobilised ECLS patients predominantly had femoral ECLS cannulation, concurrent renal replacement therapy and did not have additional invasive mechanical ventilation. During active mobilisation, changes in extracorporeal circuit function and cardiopulmonary variations were limited and did not lead to discontinuation of active mobilisation. Four major complications—three major bleedings and one cannula displacement—occurred in a total of 332 active mobilisations in 43 patients actively mobilised. All four adverse events were successfully managed without further sequelae.
In daily practice, clinicians are often concerned about ECLS malfunction, cannula displacement and bleeding from cannula insertion sites during active mobilisation of patients on ECLS [11, 12]. To our knowledge, this is the largest prospective observational study investigating the feasibility, safety, and resource utilisation of active mobilisation of ECLS patients. Several retrospective observational studies and one small pilot randomised controlled study on active mobilisation on ECLS have been published elsewhere [13, 14]. Abrams et al. retrospectively studied the feasibility and safety of active mobilisation of 35 patients on veno-venous and veno-arterial ECMO and found no relevant complications associated with mobilisation [16]. Wells et al. studied 167 patients with active mobilisation on veno-arterial ECMO and also found no major complications with a minor event rate of < 0.5% per mobilisation [17]. Bonizzoli et al. retrospectively studied 101 patients on veno-venous ECMO, of whom 40 patients (39%) were mobilised with an ICU Mobility Scale (IMS) ≥ 3 (sitting over edge of bed) [18]. More than 90% of these patients had a double lumen cannula and no complications were recorded. Munshi et al. evaluated 61 patients with severe ARDS requiring veno-venous ECMO [19]. The ICU physiotherapy team mobilised 82% (50) of these patients. A maximum activity level of ≥ 2 (active exercises in bed) was achieved in 39% [18] of these patients and 17% [8] achieved a maximum activity level ≥ 4 (actively sitting over the side of the bed) without relevant complications. Univariate analysis revealed severity of illness factors differentiating higher intensity and lower intensity physiotherapy. Gottschalk et al. retrospectively studied 37 actively mobilised patients on veno-arterial ECMO and observed no severe adverse events, such as cannula dislocation, bleeding, or cardiorespiratory deterioration [20]. In a recently published small pilot randomised controlled study 4 out of 10 ECMO patients in the early mobilisation group were actively mobilised with an activity level of IMS > 3 on a total of 7 occasions [14]. No serious adverse events and a signal for improved functional independence in the activities of daily living at hospital discharge were reported.
It is encouraging that no major complications were associated with active mobilisation of ECLS patients in the aforementioned studies, which supports further implementation of active mobilisation in this specific group of critically ill adult patients. However, the design of the retrospective observational studies and the small sample size with limited number of active mobilisations in the randomised study may have underestimated adverse clinical events, such as bleeding complications. As opposed to the above described findings, our prospective study is the first study to report major bleeding complications associated with active mobilisation in a larger cohort. Interestingly, in non-mobilised patients the rate of major bleeding from the cannulation site was higher than in mobilised patients. However, groups were not balanced according to coagulation state and severity of illness. Our case of cannula displacement during active mobilisation demonstrates that this potentially life-threatening complication can occur, and that great caution is necessary during active mobilisation with respect to securing ECLS cannulas. It shows that the ECLS teams’ competency for immediate and efficient complication management is crucial for patient safety and survival. As a consequence, drawn from this incident, the training of the ECLS team regarding strict adherence to the local standardised screening protocol before mobilising ECLS patients including accurate cannula securement and safety management has been further intensified.
Active mobilisation was resource intense in terms of personnel and time, demonstrating the need for sufficient staffing of an ECLS team. Our observational data are in line with the results of an international survey by Marhong et al. reporting that most ECMO centres require 3–5 team members for active mobilisation [11]. In addition, and in line with the results of our study population comprising 37% actively mobilised ECLS patients (IMS ≥ 3), the rate of mobilisation reported in our study is in line with the aforementioned studies. Specifically, Abrams et al. reported a 26% mobilisation rate while Wells et al. 28%, Bonizzoli et al. 37%, and Munshi et al. 39% [16–19]. In the international survey of Marhong et al. physical therapy in patients on ECLS was reported by 84% respondents, with 41% initiating it within 72 h after cannulation and mobilisation goals varied from range of motion exercises (81%) to ambulation (22%) [11].
In our study, patients actively mobilised had significantly lower severity of illness scores on ICU admission, lower pre-ECLS lactate levels and catecholamine support, and lower ICU-mortality. This may be explained by the likely perception within the ECLS team that sicker patients are deemed less appropriate for active mobilisation. Considering our study design and research question, and the expected baseline treatment indication bias (e.g. confounding), a possible association between mobilisation and mortality was thus not studied and cannot be inferred. To validly assess the efficacy of mobilisation on survival and also on the important clinical outcome of long-term functional status, randomised controlled studies are warranted.
Further, potential changes in sedation and mobilisation practices in ECLS patients over recent years cannot be excluded. Even though in our institution protocols for cannulation, anticoagulation, sedation and mobilisation were not substantially altered within the last years, it is difficult to assess potential subtle changes of practice in our routine clinical care over time with respect to sedation and mobilisation, as subjective clinical judgement plays an important role for such interventions. To assess global contemporary practice, further surveys and observational studies evaluating these important aspects of current routine clinical care of ECLS patients in other centres are warranted.
Of note, active mobilisation (IMS ≥ 3) was not performed in 63% of the ICU patients. The main reason provided by our ICU team was sedation. Therefore, as expected, most mobilisations were performed in awake patients without concurrent invasive mechanical ventilation. Assumptions on whether the individual daily level of sedation was related to the severity of illness or possible over sedation cannot be made due to the design of the study. However, this result highlights the importance of targeting light or no sedation where clinically feasible and safe to facilitate active mobilisation. As opposed to the international survey of Marhong et al. on barriers to mobilising ECMO patients, haemodynamic and respiratory instability, the level of dependence on ECLS, femoral cannulation, shortage of staff or fear of unintentional decannulation were not frequent reasons for non-mobilisation [11].
Finally, the initial selection of patients into our observational study, together with the fact that our report is based on a single centre with specific expertise in the care of ECLS patients may limit the external validity and generalisability of our findings to a broader context.
Conclusions
In conclusion, our study shows that active mobilisation of critically ill patients on ECLS with predominantly femoral cannulation is feasible and safe if performed by an experienced ECLS team that routinely provides mobilisation treatment and is well equipped to manage major complications. However, randomised controlled multicentre studies are needed to evaluate the effect of early goal directed sedation and mobilisation bundles in this specific patient population and to validly assess its efficacy on survival and long-term functional outcomes.
Supplementary information
Additional file 1: Table 1. Respiratory, haemodynamic, and renal variables within 6 hours before initiation of ECLS according to level of mobilisation.
Additional file 2: Table 2. Variation of haemodynamic state, oxygenation, and ECLS blood flow over the course of each active mobilisation unit IMS ≥ 3.
Additional file 3: Table 3. Duration of ECLS treatments according to type of ECLS and ICU-mortality.
Acknowledgements
Not applicable.
Abbreviations
- AHF
Acute heart failure
- APACHE
Acute Physiology and Chronic Health Evaluation
- ARDS
Acute respiratory distress syndrome
- av-ECCO2R
Arterio-venous extracorporeal carbon dioxide removal
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CS
Cardiac surgery
- DL
Double lumen
- ECLS
Extracorporeal life support
- ECMO
Extracorporeal membrane oxygenation
- eCPR
Extracorporeal cardiopulmonary resuscitation
- ICU
Intensive care unit
- ILD
Interstitial lung disease
- IMS
ICU mobilisation scale
- IQR
Interquartile range
- PAH
Pulmonary arterial hypertension
- RASS
Richmond Agitation Sedation Scale
- RVAD
Right ventricular assist device
- SAPS
Simplified Acute Physiology Score
- SOFA
Sepsis-related Organ Failure Assessment
- va-ECMO
Veno-arterial extracorporeal membrane oxygenation
- vv-ECCO2R
Veno-venous extracorporeal carbon dioxide removal
- vv-ECMO
Veno-venous extracorporeal membrane oxygenation
Authors’ contributions
SB, PB, GS and SK contributed substantially to the conception and design of the study, the acquisition of data, the analysis and interpretation of the data, and the drafting of the manuscript. AM, FA, KW, DW, MK, SB and HR contributed substantially to the analysis and interpretation of the data. All authors provided critical revision of the article and final approval of the version submitted for publication.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The institutional ethics committee approved of pseudonymised data collection and anonymised analysis and the requirement for individual patient consent was waived as this study was based on observational data from quality assurance and routine clinical care (Protocol Number PV4685).
Consent for publication
Not applicable.
Competing interests
SB has received lecture honoraria from Gettinge and Xenios. SK is a member of the advisory board of Xenios and has received consulting fees or lecture honoraria and travel costs from Xenios, ArjoHuntleigh, Baxter, Fresenius, Novalung, and Sorin. DW has received lecture honoraria from AstraZeneca, Bayer, and Novartis. All other authors have declared no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13613-020-00776-3.
References
- 1.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43:171–183. doi: 10.1007/s00134-016-4612-0. [DOI] [PubMed] [Google Scholar]
- 2.Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 3.Baron R, Binder A, Biniek R, Braune S, Buerkle H, Dall P, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015)—short version. German Med Sci. 2015;13:Doc19. doi: 10.3205/000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber EA, Everard T, Holland AE, Tipping C, Bradley SJ, Hodgson CL. Barriers and facilitators to early mobilisation in intensive care: a qualitative study. Aust Crit Care. 2015;28:177–182. doi: 10.1016/j.aucc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Berney SC, Rose JW, Denehy L, Granger CL, Ntoumenopoulos G, Crothers E, et al. Commencing out-of-bed rehabilitation in critical care-what influences clinical decision-making? Arch Phys Med Rehabil. 2019;100(261–269):e262. doi: 10.1016/j.apmr.2018.07.438. [DOI] [PubMed] [Google Scholar]
- 6.Dubb R, Nydahl P, Hermes C, Schwabbauer N, Toonstra A, Parker AM, et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc. 2016;13:724–730. doi: 10.1513/AnnalsATS.201509-586CME. [DOI] [PubMed] [Google Scholar]
- 7.Nydahl P, Sricharoenchai T, Chandra S, Kundt FS, Huang M, Fischill M, et al. Safety of patient mobilization and rehabilitation in the intensive care unit systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14:766–777. doi: 10.1513/AnnalsATS.201611-843SR. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson CL, Stiller K, Needham DM, Tipping CJ, Harrold M, Baldwin CE, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 2014;18:658. doi: 10.1186/s13054-014-0658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63:2769–2778. doi: 10.1016/j.jacc.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Karagiannidis C, Bein T, Weber-Carstens S. Indications and limitations of ECMO therapy: considerations on evidence, treatment decisions and ethical challenges. Med Klin Intensivmed Notfmed. 2019;114:207–213. doi: 10.1007/s00063-019-0533-3. [DOI] [PubMed] [Google Scholar]
- 11.Marhong JD, DeBacker J, Viau-Lapointe J, Munshi L, Del Sorbo L, Burry L, et al. Sedation and mobilization during venovenous extracorporeal membrane oxygenation for acute respiratory failure: an international survey. Crit Care Med. 2017;45:1893–1899. doi: 10.1097/CCM.0000000000002702. [DOI] [PubMed] [Google Scholar]
- 12.Abrams D, Garan AR, Brodie D. Awake and fully mobile patients on cardiac extracorporeal life support. Ann Cardiothorac Surg. 2019;8:44–53. doi: 10.21037/acs.2018.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira DDC, Marcolino MAZ, Macagnan FE, Plentz RDM, Kessler A. Safety and potential benefits of physical therapy in adult patients on extracorporeal membrane oxygenation support: a systematic review. Rev Bras Ter Intensiva. 2019;31:227–239. doi: 10.5935/0103-507X.20190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ECMO-PT Investigators Early mobilisation during extracorporeal membrane oxygenation was safe and feasible: a pilot randomised controlled trial. Intensive Care Med. 2020;46:1057. doi: 10.1007/s00134-020-05994-8. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, et al. Feasibility and inter-rater reliability of the ICU mobility scale. Heart Lung. 2014;43:19–24. doi: 10.1016/j.hrtlng.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014;18:R38. doi: 10.1186/cc13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells CL, Forrester J, Vogel J, Rector R, Tabatabai A, Herr D. Safety and feasibility of early physical therapy for patients on extracorporeal membrane oxygenator: University of Maryland Medical Center Experience. Crit Care Med. 2018;46:53–59. doi: 10.1097/CCM.0000000000002770. [DOI] [PubMed] [Google Scholar]
- 18.Bonizzoli M, Lazzeri C, Drago A, Tadini Boninsegni L, Donati M, Di Valvasone S, et al. Effects of a physiotherapic program in patients on veno-venous extracorporeal membrane oxygenation: an 8-year single-center experience. Minerva Anestesiol. 2019;85:989–994. doi: 10.23736/S0375-9393.19.13287-7. [DOI] [PubMed] [Google Scholar]
- 19.Munshi L, Kobayashi T, DeBacker J, Doobay R, Telesnicki T, Lo V, et al. Intensive care physiotherapy during extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Am Thorac Soc. 2017;14:246–253. doi: 10.1513/AnnalsATS.201606-438OC. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk A, Bräunig J, Bause D, Volkert T, Lanckohr C, Ellger B. Early goal directed physiotherapy in patients undergoing extracorporeal cardiac life support. Int J Sci Eng Res. 2016;7:1589–1600. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. Respiratory, haemodynamic, and renal variables within 6 hours before initiation of ECLS according to level of mobilisation.
Additional file 2: Table 2. Variation of haemodynamic state, oxygenation, and ECLS blood flow over the course of each active mobilisation unit IMS ≥ 3.
Additional file 3: Table 3. Duration of ECLS treatments according to type of ECLS and ICU-mortality.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.