Abstract
Hardy kiwifruits (Actinidia arguta) contain various bioactive compounds such as vitamin C and phenolics and can withstand cold temperatures. Changes in soluble solid, vitamin C, total phenolic, and total flavonoid content, and antioxidant capacity of three cultivars of hardy kiwifruits (A. arguta × A. deliciosa cv. Mansu, A. arguta cv. Haeyeon, and A. arguta cv. Chiak) were comparatively evaluated for 8 weeks of storage at 1 ± 0.5 °C. After the 8 weeks of storage, soluble solid content of three cultivars increased, whereas their vitamin C content decreased. Throughout this storage period, total phenolic and flavonoid content of cv. Mansu and cv. Haeyeon remained the same, while antioxidant capacity of these two cultivars also remained similar but with slightly more variations. Cv. Chiak, however, showed a decrease in total phenolic and flavonoid content and antioxidant capacity. These results suggest that cold storage of the hardy kiwifruits maintains levels of bioactive compounds.
Keywords: Actinidia arguta, Antioxidant capacity, Phenolics, Soluble solids, Vitamin C
Introduction
Hardy kiwifruits (Actinidia arguta) are hairless and can be eaten without removing the peel. The size of hardy kiwifruits is smaller than commercial kiwifruits such as the green kiwifruit (A. deliciosa) and golden kiwifruit (A. chinensis) (Lim et al., 2014). Actinidia deliciosa cv. Hayward and A. chinensis cv. Hort16A, the most commercialized kiwifruits, are uncultivable in cold areas due to their low resistance to cold, whereas the hardy kiwifruit is resistant to high frost at − 30 °C, and thus can be cultivated in even cold areas (Fisk et al., 2008).
Hardy kiwifruits contain various chemical compounds, including sugars, organic acids, vitamin C, and phenolics (Wojdyło et al., 2017). Unlike other kiwifruits, hardy kiwifruits contain more sucrose than glucose and fructose (Nishiyama et al., 2008). Hardy kiwifruits have a greater myo-inositol content than green and golden kiwifruits (Nishiyama et al., 2008). In particular, the total phenolic content and antioxidant capacity of hardy kiwifruits are known to be higher than those of green kiwifruits (Lee et al., 2015b). Phenolics including procyanidin B2, (−)-epicatechin, neochlorogenic acid, cryptochlorogenic acid, rutin, hyperoside, isoquercitrin, and astragalin were previously identified in cv. Mansu, cv. Haeyeon, and cv. Chiak hardy kiwifruits (Jeong et al., 2020). Hardy kiwifruits have been reported to have health-promoting effects such as an anti-inflammatory effects on lipopolysaccharide-stimulated RAW 264.7 murine macrophage, a therapeutic effect on atopic dermatitis, and an anti-amnesic effect on trimethyltin-induced learning and memory dysfunction in in vivo mouse models (An et al., 2016; Ha et al., 2015; Park et al., 2007).
Hardy kiwifruits should be harvested when mature and firm in case of damage during transportation and are normally stored at 1–2 °C (Strik and Hummer, 2006). Green kiwifruit is stored at low temperatures for a long-term of 4–6 months (Mitchell, 1990), whereas the hardy kiwifruit should be coldly stored only 1–2 months due to softening (Strik and Hummer, 2006). In recent years, compensating for such storage problems and enhancing the storage period, storage techniques with the control of temperature and humidity and breeding of new cultivars have been developed (Jung et al., 2016; Lim et al., 2016). A fruit’s storage condition and period are important factors that influence its hardness, sweetness, and chemical composition. It was previously reported the changes in total phenolic content, vitamin C content, and antioxidant capacity during storage for three different hardy kiwifruits grown in Poland (Krupa et al., 2011). In recently, the new hardy kiwifruit cultivars including cv. Mansu, one interspecific hybridization cultivar of A. arguta × A. deliciosa, and cv. Haeyeon and cv. Chiak, two cultivars of A. arguta that have been developed in Korea were previously reported to have neuroprotective and anti-inflammatory effects (An et al., 2016; Jeong et al., 2020). However, these are limited information on the changes in total phenolic and flavonoid content, and antioxidant capacity during storage for new hardy kiwifruit cultivars developed and grown in Korea.
The aim of this study was to investigate changes in bioactive phytochemicals and soluble solids of three hardy kiwifruit cultivars: cv. Mansu, cv. Haeyeon, and cv. Chiak. Throughout the storage period, changes in soluble solid, total phenolic, total flavonoid, and vitamin C contents of the three hardy kiwifruit cultivars were comparatively evaluated using colorimetric assays and HPLC. In addition, changes in antioxidant capacities were measured through three different assays: ABTS, DPPH, and oxygen radical absorbance capacity (ORAC) assays.
Materials and methods
Hardy kiwifruits and storage
Three cultivars of hardy kiwifruit, cv. Mansu, cv. Haeyeon, and cv. Chiak, were grown in open field in Gwangyang (35.0114 latitude and 127.5862 longitude), Jeonnam, Republic of Korea. The average temperature in Gwangyang in 2016 was 15.4 °C, and a total precipitation was 134.0 mm (Korea Meteorological Administration, 2016). The hardy kiwifruits of 8–10% of soluble solid content were harvested in October 2016, and then stored for 8 weeks at 1 ± 0.5 °C. After 8 weeks of storage, hardy kiwifruits were frozen at − 80 °C to prevent ripening.
Reagents
Folin-Ciocalteu’s phenol reagent, AAPH, ABTS, DPPH, fluorescein salt, vitamin C, gallic acid, and catechin were purchased from Sigma Chemical Co., LLC (St Louis, MO, USA). All other reagents used were of analytical or HPLC grade.
Measurement of soluble solid content
Soluble solid content of hardy kiwifruits was measured using the digital saccharometer (PR-101α; Atago Co., Ltd., Tokyo, Japan). Soluble solid content was measured ten replicates and expressed in degrees Brix (°Bx).
Extraction of phenolics
Thirty grams of whole hardy kiwifruit were homogenized in 100 mL of 95% ethanol and deionized water (80:20, v/v) using a Polytron homogenizer (PT 10/35; Kinematica, Kriens-Luzern, Switzerland) at 15,000 rpm for 2 min. The homogenate was centrifuged using the centrifugal separator (VS-6000CFi; Vision Science Co., Ltd., Daejeon, Republic of Korea) at 2200 ×g for 10 min. The supernatant was transferred to a chilled Büchner funnel with Whatman no. 2 paper and filtered. The separated residue was re-extracted by repeating the above procedure. Two extracts were combined and evaporated using a rotary evaporator (Eyela, Tokyo Rikakikai Co., Ltd, Tokyo, Japan) at 40 °C. Extraction was done through three independent experiments. The final extracts were stored at − 20 °C until analysis.
Vitamin C extraction and determination of vitamin C content
Vitamin C extraction was carried out according to some modified method reported by Lee et al. (2018). Two grams of each hardy kiwifruit were immersed in 10% (w/v) metaphosphoric acid solution (5 mL) for 10 min. Fifteen milliliters of 5% (w/v) metaphosphoric acid solution were added to the mixture followed by homogenization at 15,000 rpm for 2 min using a homogenizer (PT 10/35; Kinematica). The homogenized mixture was separated using the centrifugal separator (VS-6000Cfi; Vision Science Co., Ltd.) and adjusted to a volume of 50 mL with 5% (w/v) metaphosphoric acid solution.
Vitamin C content was evaluated using the 1200 HPLC system (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a diode array detector, autosampler, and vacuum degasser. The stationary phase was an Agilent Zorbax Eclipse XDB-C18 column (5 μm of particle size, 4.6 mm i.d. × 250 mm; Agilent Technologies, Inc.). The flow rate was set to 0.8 mL/min. The mobile phases were comprised of two solvents: 0.1% (v/v) formic acid in water (solvent A) and 0.1% (v/v) formic acid in acetonitrile (solvent B). The gradient conditions were as follows: 100% A/0% B at 0 min, 97% A/3% B at 8 min, 50% A/50% B at 10 min, 20% A/80% B at 12 min, 100% A/0% B at 15 min, and 100% A/0% B at 20 min. The injection volume was 10 μL, and the detection wavelength was set to 254 nm. Vitamin C content of the three cultivars of hardy kiwifruit was quantified using the vitamin C standard curve.
Determination of total phenolic content
Total phenolic content of the hardy kiwifruit was measured using Folin-Ciocalteu’s phenol reagent (Lee et al., 2019). Hardy kiwifruit extract (200 μL) was mixed with deionized water (2.6 mL) followed by an addition of Folin–Ciocalteu’s phenol reagent (200 μL) and was incubated at 23 °C for 6 min. Two milliliters of 7% (w/v) sodium carbonate solution (2 mL) were added to the mixture and further incubated at 23 °C for 84 min. The absorbance was measured at 750 nm using a spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc., Waltham, MA, USA). Total phenolic content was expressed in mg gallic acid equivalents (GAE)/100 g fresh weight (FW).
Determination of total flavonoid content
Total flavonoid content of hardy kiwifruit extracts was measured using the method described by Lee et al. (2019). Hardy kiwifruit extract (150 μL) was mixed with deionized water (3.2 mL) and 5% (w/v) sodium nitrite solution (150 μL) and incubated at 23 °C for 5 min. One hundred fifty milliliters of 10% (w/v) aluminum chloride solution were added to the mixture and further incubated at 23 °C for 1 min. The mixture was mixed with 1 M sodium hydroxide (1 mL) and measured immediately at 510 nm using the spectrophotometer (SPECTRONIC 200, Thermo Fisher Scientific Inc.). Total flavonoid content of hardy kiwifruits was expressed in mg catechin equivalents (CE)/100 g FW.
Determination of antioxidant capacity using ABTS assay
The antioxidant capacity of hardy kiwifruit extract was assessed using ABTS radicals (Lee et al., 2019). ABTS (2.5 mM) in 100 mL of phosphate-buffered saline (PBS; pH 7.4) was oxidized to ABTS radicals using AAPH (1.0 mM) at 70 °C. ABTS radical solution was adjusted to the absorbance of 0.650 ± 0.020 by diluting with PBS at 734 nm. ABTS radical solution (980 μL) and the hardy kiwifruit extract (20 μL) were mixed together and then reacted at 37 °C for 10 min. The reduced absorbance of the mixture was recorded at 734 nm using the spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc.). Antioxidant capacity of hardy kiwifruits was expressed in mg vitamin C equivalents (VCE)/100 g FW.
Determination of antioxidant capacity using DPPH assay
The DPPH assay was evaluated using the method previously described by Lee et al. (2019). DPPH radical solution was prepared at the absorbance of 0.650 ± 0.020 by dissolving in 80% (v/v) aqueous methanol at 517 nm. The DPPH radical solution (2.95 mL) and hardy kiwifruit extract (50 μL) were mixed and left to react at room temperature for 30 min. The reduced absorbance of the mixture was recorded at 517 nm using the spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc.). The antioxidant capacity of hardy kiwifruits was expressed in mg VCE/100 g FW.
Determination of antioxidant capacity using ORAC assay
The ORAC assay was conducted based on the fluorescent method (Lim et al., 2012). Twenty-five microliters of hardy kiwifruit extract and 150 μL of 81.6 nM fluorescein solution were added to a 96-well plate and incubated at 37 °C for 10 min with 3 min of shaking. After the incubation, 25 μL of 153 mM AAPH solution was added. The fluorescence of the mixture was detected every minute for 90 min using a microplate reader (Infinite M200; Tecan Austria GmbH, Grödig, Austria) with 485 nm excitation and 520 nm emission wavelengths. Antioxidant capacity of hardy kiwifruits was expressed in mg VCE/100 g FW.
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analysis was carried out using IBM SPSS software Version 23 (IBM SPSS Statistics Inc., Chicago, IL, USA). All tests considered the significant difference in mean values using Duncan’s multiple range test (p < 0.05).
Results and discussion
Changes in soluble solid content
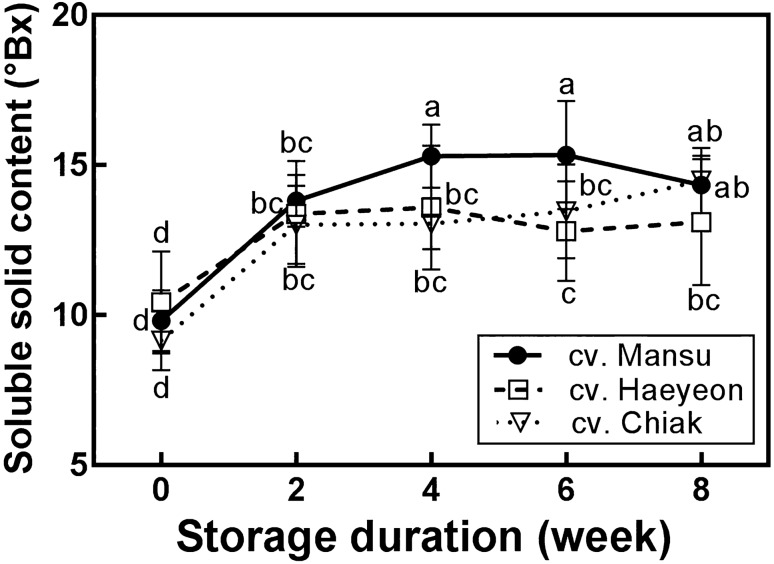
As shown in Fig. 1, soluble solid content of cv. Mansu increased up to the fourth week of cold storage, while soluble solid content of cv. Haeyeon and cv. Chiak increased up to the second week of cold storage. Initially, soluble solid content of cv. Mansu, cv. Haeyeon, and cv. Chiak were 9.8, 10.4, and 9.2°Bx, respectively. After 8 weeks of storage, soluble solid content (°Bx) increased as follows: cv. Chiak (14.5) > cv. Mansu (14.3) > cv. Haeyeon (13.1). Generally, soluble solid content of three hardy kiwifruits after 2 weeks of storage increased significantly (p < 0.05) compared to the initial content. Especially, cv. Mansu showed the highest soluble solid content (15.3°Bx) in the fourth and sixth week among other hardy kiwifruits used in this study.
Fig. 1.
Change in soluble solid content of three cultivars of hardy kiwifruits (cv. Mansu, cv. Haeyeon, and cv. Chiak) during 8 weeks of cold storage at 1 ± 0.5 °C. Data are represented as mean ± standard deviation (bars) of 10 replicate determinations. Different lowercase letters indicated significant differences according to Duncan’s multiple range test (p < 0.05)
Affecting fruit taste and flavor, the soluble solid content is an important factor that unnoticeably sways consumer’s preferences. The increase in soluble solid content is caused by the conversion of starch to sucrose partly due to sucrose phosphate synthase and sucrose synthase activities in kiwifruits during ripening (Langenkämper et al., 1998; Macrae et al., 1992). It was previously reported that sucrose phosphate synthase and sucrose synthase activities varies depending on the cultivar (Choudhury et al., 2009). The difference of soluble solid content in hardy kiwifruits used in this study may be due to different genotypes and glycolytic enzyme activity.
Changes in vitamin C content
The changes of vitamin C content in hardy kiwifruits during storage are shown in Fig. 2. The vitamin C content of cv. Mansu, cv. Haeyeon, and cv. Chiak were 115.9, 21.3, and 26.4 mg/100 g FW at the beginning of storage, respectively. After 8 weeks of storage, vitamin C content (mg/100 g FW) decreased as follows: cv. Mansu (83.5) > cv. Chiak (9.9) > cv. Haeyeon (7.2). Three cultivars of hardy kiwifruit generally showed decreased vitamin C content as storage duration increased. Vitamin C content of cv. Mansu and cv. Chiak showed a significant (p < 0.05) decrease during the storage of 4–8 weeks, whereas cv. Haeyeon had significant (p < 0.05) decrease of vitamin C content by the storage of 8 weeks. It was reported that the vitamin C content in hardy kiwifruit cv. Cheongsan was decreased during storage at 0 °C (Lim et al., 2016). The vitamin C content in hardy kiwifruits during storage was previously reported to be almost unchanged or decrease (Krupa et al., 2011). Similar to the results of vitamin C content during storage in this study, the content of bioactives such as vitamin C and phenolics varies with storage conditions and harvest time of hardy kiwifruits (Lee et al., 2015a; Lim et al., 2016).
Fig. 2.
Change in vitamin C content of three cultivars of hardy kiwifruits (cv. Mansu, cv. Haeyeon, and cv. Chiak) during 8 weeks of cold storage at 1 ± 0.5 °C. Data are represented as mean ± standard deviation (bars) of three replicate determinations. Different lowercase letters indicated significant differences according to Duncan’s multiple range test (p < 0.05)
Vitamin C is a well-known water-soluble antioxidant. Hardy kiwifruits contain a plenty amount of vitamin C (Latocha et al., 2010). It was previously reported that hardy kiwifruits including cv. Otumsense, cv. Chiak, and cv. Skinny Green contained vitamin C of 47.2, 22.6, and 16.1 mg/100 g FW, respectively (Jin et al., 2014). Hardy kiwifruit cv. Cheongsan has been reported to have 147.0 mg/100 g FW of vitamin C at initial storage (Lim et al., 2016). In this study, the range of vitamin C content in hardy kiwifruits was 21.3–115.9 mg/100 g FW at initial storage. The difference of vitamin C content in hardy kiwifruits may be due to the difference in cultivars.
Changes in total phenolic and total flavonoid content
The changes in total phenolic and flavonoid content of three cultivars of hardy kiwifruits during storage are presented in Fig. 3. Three cultivars of hardy kiwifruit, cv. Mansu, cv. Haeyeon, and cv. Chiak, had a total phenolic content of 182.1, 116.4, and 175.4 mg GAE/100 g FW at the beginning of storage, respectively, whereas cv. Mansu, cv. Haeyeon, and cv. Chiak showed a total phenolic content of 184.7, 114.4, and 101.7 mg GAE/100 g FW at 8 weeks of storage (Fig. 3A). The total phenolic content of cv. Mansu and cv. Haeyeon after 8 weeks of storage did not have a significant (p < 0.05) difference compared to that at the beginning of storage, whereas the total phenolic content of cv. Chiak after 8 weeks of storage were significantly (p < 0.05) different compared to that at the beginning of storage. Similar to the results of total phenolic content of cv. Chiak in this study, the total phenolic content of hardy kiwifruit cv. Cheongsan reduced during cold storage (Lim et al., 2016).
Fig. 3.
Change in total phenolic content (A) and total flavonoid content (B) of three cultivars of hardy kiwifruits (cv. Mansu, cv. Haeyeon, and cv. Chiak) during 8 weeks of cold storage at 1 ± 0.5 °C. Data are represented as mean ± standard deviation (bars) of three replicate determinations. Different lowercase letters indicated significant differences according to Duncan’s multiple range test (p < 0.05). GAE, gallic acid equivalents; FW, fresh weight; and CE, catechin equivalents
Total flavonoid content of three cultivars of hardy kiwifruit, cv. Mansu, cv. Haeyeon, and cv. Chiak, were 27.2, 25.9, and 45.6 mg CE/100 g FW at the beginning of storage, and 30.9, 29.2, and 20.7 mg CE/100 g FW at 8 weeks of storage, respectively (Fig. 3B). Cv. Chiak showed a significant (p < 0.05) decrease of total flavonoid content at 8 weeks of storage compared to the beginning of storage. Like the results of total phenolic content in this study, cv. Mansu and cv. Haeyeon had no significant difference of total flavonoid content at 8 weeks of storage compared to the beginning of storage.
Total phenolic and flavonoid content in the kiwifruits are dependent on cultivars, maturity, and storage duration (Latocha et al., 2010; Lee et al., 2015a; Lim et al., 2014). In this study, hardy kiwifruits showed various total phenolic and flavonoid content according to cold storage duration and cultivars (Fig. 3). Cold storage at 1 ± 0.5 °C used in this study effectively maintained total phenolic and flavonoid content of cv. Mansu and cv. Haeyeon except cv. Chiak, suggesting that cold storage of hardy kiwifruits is a good method to preserve bioactive compounds such as phenolics.
Changes in antioxidant capacity
Changes in antioxidant capacities, evaluated using the ABTS, DPPH, and ORAC assays, of the three cultivars of hardy kiwifruit during cold storage are presented in Fig. 4. The antioxidant capacities measured using the ABTS assay of the hardy kiwifruit cv. Mansu, cv. Haeyeon, and cv. Chiak were 195.8, 171.0, and 251.9 mg VCE/100 g FW at the beginning of storage, and were 181.5, 154.5, and 154.5 mg VCE/100 g FW at the end of cold storage, respectively (Fig. 4A). Cv. Mansu and cv. Haeyeon had no significant difference of antioxidant capacities measured using the ABTS assay between the beginning and 8 weeks of cold storage. In contrast, antioxidant capacity of cv. Chiak measured using the ABTS assay after 8 weeks of cold storage was significantly (p < 0.05) lower than that at the beginning of cold storage.
Fig. 4.
Change in antioxidant capacity of three cultivars of hardy kiwifruits (cv. Mansu, cv. Haeyeon, and cv. Chiak) measured using the ABTS (A), DPPH (B), and ORAC (C) assays during 8 weeks of cold storage at 1 ± 0.5 °C. Data are represented as mean ± standard deviation (bars) of three replicate determinations. Different lowercase letters indicated significant differences according to Duncan’s multiple range test (p < 0.05). VCE, vitamin C equivalents; and FW, fresh weight
The antioxidant capacities measured using the DPPH assay of the hardy kiwifruit cv. Mansu, cv. Haeyeon, and cv. Chiak were 181.7, 108.3, and 165.6 mg VCE/100 g FW at the beginning of cold storage, respectively, whereas they were 144.1, 94.5, and 78.9 mg VCE/100 g FW at the 8 weeks of cold storage (Fig. 4B). During cold storage of three hardy kiwifruit cultivars, antioxidant capacities at the 8 weeks of cold storage assayed using the DPPH radicals were significantly (p < 0.05) lower than those at the beginning of cold storage.
The antioxidant capacities measured using the ORAC assay of the hardy kiwifruit cv. Mansu, cv. Haeyeon, and cv. Chiak were 469.1, 412.0, and 448.4 mg VCE/100 g FW at the beginning of cold storage and 485.2, 389.5, and 242.5 mg VCE/100 g FW after 8 weeks of cold storage, respectively (Fig. 4C). Antioxidant capacities of cv. Mansu and cv. Haeyeon measured using the ORAC assay were not significantly different between the beginning and 8 weeks of cold storage. However, antioxidant capacity of cv. Chiak measured using the ORAC assay after 8 weeks of cold storage was significantly (p < 0.05) lower than that at the beginning of cold storage.
After the 8 weeks of cold storage, three cultivars of hardy kiwifruits had antioxidant capacities in the decreasing order as follows: cv. Mansu > cv. Haeyeon > cv. Chiak (Fig. 4) Phenolics and vitamin C are widely known to affect the antioxidative effects of fruits. According to a previous report (Krupa et al., 2011), the reduction in antioxidant capacity during cold storage for hardy kiwifruits grown in Poland differed between cultivars. In this study, the reduction in antioxidant capacity of cv. Mansu, cv. Haeyeon, and cv. Chiak during cold storage was different for each cultivars. The different antioxidant capacities of the hardy kiwifruits in this study are ascribed to a variety of influencing factors, including the reduction and oxidation mechanisms, different assays and solvents, together with their concentrations of antioxidants (i.e., phenolics and viatmin C) (Apak et al., 2013; Yoo et al., 2007).
Relationships among total phenolic, total flavonoid, and vitamin C content and antioxidant capacity
The relationships among total phenolic, total flavonoid, and vitamin C content, and antioxidant capacity were analyzed using Pearson correlation coefficients (r). Antioxidant capacity, measured by the ABTS, DPPH and ORAC assays, had a higher good correlation with total phenolic content than total flavonoid and vitamin C content (Table 1). In previous studies, kiwifruits (Actinidia spp.) showed that total phenolic content was more positively correlated with antioxidant capacity than total flavonoid content (An et al., 2016; Lee et al., 2015a; Lee et al., 2015b).
Table 1.
Pearson correlation coefficients (r) between total phenolic, total flavonoid, and vitamin C content and antioxidant capacity of three cultivars of hardy kiwifruit during 8 weeks of cold storage at 1 ± 0.5 °C
| Total phenolic content | Total flavonoid content | Vitamin C content | ABTSa | DPPHb | ORACc | |
|---|---|---|---|---|---|---|
| Total phenolic content | ||||||
| Total flavonoid content | 0.598* | |||||
| Vitamin C content | 0.774*** | − 0.028 | ||||
| ABTS | 0.767*** | 0.901*** | 0.257 | |||
| DPPH | 0.935*** | 0.558* | 0.771*** | 0.780*** | ||
| ORAC | 0.703*** | 0.506 | 0.526* | 0.479 | 0.678** | |
* indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001
aABTS radical scavenging assay
bDPPH radical scavenging assay
cOxygen radical absorbance capacity
In conclusion, after the 8 weeks of storage, soluble solid content in three cultivars of hardy kiwifruits (cv. Mansu, cv. Haeyeon, and cv. Chiak) gradually increased during cold storage, whereas vitamin C content of these three kiwifruits decreased. Throughout this storage period, cold storage of hardy kiwifruits effectively maintained the total phenolic and flavonoid content as well as antioxidant capacity of cv. Mansu and cv. Haeyeon except cv. Chiak. The results of this study suggest that cold storage of the hardy kiwifruit is an effective approach to maintain bioactive antioxidants such as phenolics before consumers’ consumption. Further study is needed to carry out changes in individual phenolics during cold storage of hardy kiwifruits and to study their beneficial health effects in in vivo models.
Acknowledgements
This research was supported by the Agricultural Biotechnology Development Program (Project No. 114076-3), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ha-Ram Jeong, Email: jiu0hun@naver.com.
Hye-Seung Cho, Email: cometcho@korea.kr.
Youn-Sup Cho, Email: aktis@korea.kr.
Dae-Ok Kim, Email: DOKIM05@khu.ac.kr.
References
- An X, Lee SG, Kang H, Heo HJ, Cho Y-S, Kim D-O. Antioxidant and anti-inflammatory effects of various cultivars of kiwi berry (Actinidia arguta) on lipopolysaccharide-stimulated RAW 264.7 Cells. J. Microbiol. Biotechnol. 2016;26:1367–1374. doi: 10.4014/jmb.1603.03009. [DOI] [PubMed] [Google Scholar]
- Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report) Pure Appl. Chem. 2013;85:957–998. doi: 10.1351/PAC-REP-12-07-15. [DOI] [Google Scholar]
- Choudhury SR, Roy S, Sengupta DN. A comparative study of cultivar differences in sucrose phosphate synthase gene expression and sucrose formation during banana fruit ripening. Postharvest Biol. Technol. 2009;54:15–24. doi: 10.1016/j.postharvbio.2009.05.003. [DOI] [Google Scholar]
- Fisk CL, Silver AM, Strik BC, Zhao Y. Postharvest quality of hardy kiwifruit (Actinidia arguta ‘Ananasnaya’) associated with packaging and storage conditions. Postharvest Biol. Technol. 2008;47:338–345. doi: 10.1016/j.postharvbio.2007.07.015. [DOI] [Google Scholar]
- Ha JS, Jin DE, Park SK, Park CH, Seung TW, Bae D-W, Kim D-O, Heo HJ. Antiamnesic effect of Actinidia arguta extract intake in a mouse model of TMT-induced learning and memory dysfunction. Evid.-Based Complement. Alternat. Med. 2015;2015:876484–345. doi: 10.1155/2015/876484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H-R, Kim KJ, Lee SG, Cho HS, Cho Y-S, Kim D-O. Phenolic profiles of hardy kiwifruits and their neuroprotective effects on PC-12 and SH-SY5Y cells against oxidative stress. J. Microbiol. Biotechnol. 2020;30:912–919. doi: 10.4014/jmb.2001.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DE, Park SK, Park CH, Seung TW, Heo HJ. Nutritional compositions of three traditional Actinidia (Actinidia arguta) cultivars improved in Korea. J. Korean Soc. Food Sci. Nutr. 2014;43:1942–1947. doi: 10.3746/jkfn.2014.43.12.1942. [DOI] [Google Scholar]
- Jung BJ, Cho HS, Park MY, Cho YS. ‘Mansu’, a hardy kiwifruit (Actinidia arguta Planch. et Miq.) cultivar with improved storage life. Korean J. Hortic. Sci. 34: 755-760 (2016)
- Korea Meteorological Administration. 2016. Gwangyang’s temperature and rainfall in 2016. Available from: https://www.weather.go.kr/weather/main.jsp. Accessed July 24, 2020.
- Krupa T, Latocha P, Liwińska A. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci. Hort. 2011;130:410–417. doi: 10.1016/j.scienta.2011.06.044. [DOI] [Google Scholar]
- Langenkämper G, McHale R, Gardner RC, MacRae E. Sucrose-phosphate synthase steady-state mRNA increases in ripening kiwifruit. Plant Mol. Biol. 1998;36:857–869. doi: 10.1023/A:1005964812161. [DOI] [PubMed] [Google Scholar]
- Latocha P, Krupa T, Wołosiak R, Worobiej E, Wilczak J. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int. J. Food Sci. Nutr. 2010;61:381–394. doi: 10.3109/09637480903517788. [DOI] [PubMed] [Google Scholar]
- Lee I, Im S, Jin C-R, Heo HJ, Cho Y-S, Baik M-Y, Kim D-O. Effect of maturity stage at harvest on antioxidant capacity and total phenolics in kiwifruits (Actinidia spp.) grown in Korea. Hortic. Environ. Biote. 56: 841-848 (2015a)
- Lee I, Lee BH, Eom SH, Oh C-S, Kang H, Cho Y-S, Kim D-O. Antioxidant capacity and protective effects on neuronal PC-12 cells of domestic bred kiwifruit. Korean J. Hort. Sci. Technol. 2015;33:259–267. [Google Scholar]
- Lee BH, Nam TG, Cho CH, Cho Y-S, Kim D-O. Functional and sensory characteristics of kiwifruit jangachi cured with traditional Korean sauces, doenjang and kochujang. Korean J. Food Sci. Technol. 2018;50:238–243. [Google Scholar]
- Lee BH, Nam TG, Kim SY, Chun OK, Kim D-O. Estimated daily per capita intakes of phenolics and antioxidants from coffee in the Korean diet. Food Sci. Biotechnol. 2019;28:269–279. doi: 10.1007/s10068-018-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D, Kim W, Lee M-G, Heo HJ, Chun OK, Kim D-O. Evidence for protective effects of coffees on oxidative stressinduced apoptosis through antioxidant capacity of phenolics. Food Sci. Biotechnol. 2012;21:1735–1744. doi: 10.1007/s10068-012-0231-x. [DOI] [Google Scholar]
- Lim YJ, Oh C-S, Park Y-D, Kim D-O, Kim U-J, Cho Y-S, Eom SH. Physiological components of kiwifruits with in vitro antioxidant and acetylcholinesterase inhibitory activities. Food Sci. Biotechnol. 2014;23:943–949. doi: 10.1007/s10068-014-0127-z. [DOI] [Google Scholar]
- Lim S, Han SH, Kim J, Lee HJ, Lee JG, Lee EJ. Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 2016;190:150–157. doi: 10.1016/j.foodchem.2015.05.085. [DOI] [PubMed] [Google Scholar]
- MacRae E, Quick WP, Benker C, Stitt M. Carbohydrate metabolism during postharvest ripening in kiwifruit. Planta. 1992;188:314–323. doi: 10.1007/BF00192797. [DOI] [PubMed] [Google Scholar]
- Mitchell F. Postharvest physiology and technology of kiwifruit. Acta Hortic. 1990;282:291–307. doi: 10.17660/ActaHortic.1990.282.37. [DOI] [Google Scholar]
- Nishiyama I, Fukuda T, Shimohashi A, Oota T. Sugar and organic acid composition in the fruit juice of different Actinidia varieties. Food Sci. Technol. Res. 2008;14:67–73. doi: 10.3136/fstr.14.67. [DOI] [Google Scholar]
- Park E-J, Park KC, Eo H, Seo J, Son M, Kim KH, Chang Y-S, Cho S-H, Min K-U, Jin M, Kim S. Suppression of spontaneous dermatitis in NC/Nga murine model by PG102 isolated from Actinidia arguta. J. Invest. Dermatol. 2007;127:1154–1160. doi: 10.1038/sj.jid.5700658. [DOI] [PubMed] [Google Scholar]
- Strik BC, Hummer KE. ‘Ananasnaya’ hardy kiwifruit. J. Am. Pom. Sci. 2006;60:106–112. [Google Scholar]
- Wojdyło A, Nowicka P, Oszmiański J, Golis T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods. 2017;30:194–202. doi: 10.1016/j.jff.2017.01.018. [DOI] [Google Scholar]
- Yoo K-M, Kim D-O, Lee C-Y. Evaluation of different methods of antioxidant measurement. Food Sci. Biotechnol. 2007;16:177–182. [Google Scholar]