Abstract
The objective of this randomized, double-blind, placebo-controlled, crossover study was to examine the effects of onion extract containing concentrated cysteine sulfoxides on improving sleep quality. In total, 30 healthy men and women who were dissatisfied with their sleep consumed the test food sample (onion extract tablets) for 5 days. The delta power during non-rapid eye movement sleep and the delta power per minute in the first sleep cycle increased significantly with the intake of onion extract containing concentrated cysteine sulfoxides compared with the intake of placebo. A significant decline in sleep latency was also observed. Salivary α-amylase level, a stress indicator, decreased significantly with the intake of onion extract containing concentrated cysteine sulfoxides compared with the intake of placebo. These findings indicate that onion extract containing concentrated cysteine sulfoxides alleviates stress, improves the quality of sleep and promotes smooth transition into sleep.
Keywords: Onion, Cysteine sulfoxide, Non-rapid eye movement sleep, Delta power, Sleep quality
Introduction
Sleep in humans consists of non-rapid eye movement (NREM) sleep and rapid-eye movement (REM) sleep. NREM sleep, which is a stage of deep sleep, is responsible for numerous physiological functions such as consolidation of memory (Born and Wilhelm, 2012), cognitive performance while awake (Ferrara et al., 2000) and glycemic control (Spiegel et al., 2009). Research has shown that when sleep quality declines with light sleep, it increases the risk for developing diseases such as diabetes (Tasali et al., 2008). Therefore, it is essential to maintain and improve the quality of sleep. Improvement in the quality of sleep is assessed by the depth of NREM sleep due to the increased delta power (Monoi et al., 2016).
Poor sleep quality is thought to be caused by various stressors (Fruhstorfer et al., 1984; Pasternak et al., 1992). Increased psychological stress and lack of ability to adequately cope with stress have been reported to increase the risk of insomnia (Kim et al., 2000). This means that alleviating stress is extremely important for improving sleep quality.
Onion contains about 0.2% (dry matter conversion) cysteine sulfoxides (isoalliin, methyiin, and cycloalliin) as characteristic amino acids, which brings about the pungent aroma and taste of onions (Kim et al., 2016). Cysteine sulfoxides are cleaved by cysteine sulfoxidelyase, when onions are cut or crushed, to produce alkyl sulfenic acid which is converted to thiosulfinates and other volatile compound (Kim et al., 2016). It has been reported that intake of onion powder improved depression and anxiety behavior due to stress in animal study (Noreen and Ayesha, 2018). Cysteine sulfoxides are known to have various physiological functions (Kook et al., 2009; Nishimura et al., 2004; Xiao and Parkin, 2002). In addition, cysteine sulfoxides are believed to have effect on improving mental health based on the 36-item Short Form Survey (Nakayama et al., 2017). Healthy participants with subjective dissatisfaction in their sleep quality showed improvements in sleep quality and reductions in psychological stress following ingestion of A. venetum leaf extract (Asami et al., 2018). In other words, cysteine sulfoxides may have stress-reducing effects. Therefore, consuming cysteine sulfoxides may improve sleep quality by reducing psychological stress.
The objective of this study was to examine the effects of consuming onion extract containing concentrated cysteine sulfoxides on sleep quality among healthy participants who were dissatisfied with their sleep quality.
Materials and methods
Test article
As described in previous study (Nakayama et al., 2017), the onion extract used as the test food sample was prepared by cutting, extraction, column purification, and concentration, with heat treatment prior to cutting so that it contained at least 6% cysteine sulfoxides. Nisshin Pharma (Tokyo, Japan) provided the onion extract. The onion extract 500 mg was formulated to contain the following cysteine sulfoxides in a daily dose of three tablets: isoalliin 13 mg, methyiin 3 mg, and cycloalliin 17 mg. After adding the filler agent, tablets with onion extract were formed and used as the test food sample (onion extract tablets). The placebo tablets were prepared by substituting the onion extract with the filler agent and adding the rest of the same ingredients. Blind tests were conducted after both food samples were coated to mask their appearance, flavor, and properties and ensure that they were indistinguishable. Isoalliin, methyiin, and cycloalliin were measured using high-performance liquid chromatography as described in a previous study (Nakayama et al., 2017).
Subjects
The participants were healthy men and women (age 30–50 years) who were dissatisfied with their sleep quality, and those who were deemed to fit for inclusion in the study by the principal investigator physician. Participants with a Pittsburgh Sleep Quality Index (PSQI) score ≥ 6 were classified as being dissatisfied with their sleep (Doi et al. ,1998; Doi et al., 2000; Velez et al., 2013). The main exclusion criteria were: (1) those regularly taking items that may affect the results of the test, e.g., medications, food for specified health uses, foods with function claims, and health foods; (2) those with past or current medical history of serious diseases in organs such as the heart, liver, kidney, or digestive system; (3) those with sensitive skin that develop a rash from adhesive tape; (4) those who were pregnant, suspected of being pregnant, or breastfeeding; (5) those who were heavy alcohol drinkers; (6) those with extremely irregular dietary habits or lifestyle routines such as shift and late-night workers; (7) those who consumed 200 g of onions (at least one onion) per day; (8) those diagnosed with sleep apnea syndrome or who were unaware of their apnea condition;1 (9) those who expected overnight travel for business or leisure (even once) during the test period; (10) those who could not abstain from consuming alcohol during the test period; (11) those with a past medical and treatment history of sleeping disorder; and (12) others deemed unfit to participate in the present study by the principal or sub-investigator physicians.
In compliance with the Declaration of Helsinki, the present study complied with ethical principles of medical research involving human subjects. To protect the human rights of the participants and ensure the safety and reliability of the test data, the ethics committee of Chiyoda Paramedical Clinic reviewed and approved the study protocol (date of approval: December 21, 2017; approval No.: 17122102). All participants were thoroughly briefed on the details of the test, and provided written informed consent. The test protocol was registered beforehand (UMIN000031122) in the University Hospital Medical Information Network (UMIN-CTR: http://www.umin.ac.jp).
The procedures for selecting the participants and conducting the analysis were shown in a flowchart (Fig. 1). Of the 59 clinical test volunteers, 30 (1) met the selection criteria during the screening and pre-intake examination, (2) were not eliminated by the exclusion criteria, and (3) were deemed fit to participate in the study by the principal investigator physician. As for the number of test participants, we calculated that 30 were needed based on clinical test results from our previous studies (data not shown).
Fig. 1.
Flow diagram of the progress through the phases of the present randomized, double-blind, placebo-controlled, crossover study
Study design of clinical test
This was a randomized, double-blind, placebo-controlled, crossover study. The first group was given the onion extract tablets first followed by the placebo tablets, whereas the second group was given the placebo tablets first followed by the onion extract tablets. The participants were asked to consume one sachet containing three tablets per day to be taken with ambient or lukewarm water before bedtime (between 20:00 and 24:00) without chewing. The intake period was 5 days (from Monday to Friday), and a 2-day washout period (as determined previously: data not shown) was allocated. The participants were instructed not to take caffeine (tea, coffee, energy drink, etc.) after 20:00 during the intake period.
Using a stratified block randomization method, the 30 participants were divided into two groups based on age and results from a sleeping questionnaire provided during screening. To maintain blinding from all those involved, the assignment table for the onion extract tablets was sealed and stored until data fixing.
Electroencephalogram (EEG) analysis
The EEG was measured as indicators of efficacy during intake period. EEGs during sleep were measured using Sleep Scope (SleepWell Co., Ltd., Osaka, Japan), a single-channel portable EEG device, in the homes of the participants throughout the 5-day intake period. After the 2-day washout period, the same measurements were repeated. Of all the measured data, EEG analysis and assessments for 3 days were analyzed by EEG analysis laboratory (SleepWell) as described in previous studies (Flexer et al., 2005; Takahashi et al., 2013). To assess the effects on sleep before and after the intake of the onion extract tablets, the changes between the EEG data measured 1 day before the start of the test and the data measured on the last day of intake were compared.
St. Mary’s hospital (SMH) sleep questionnaire
The answers to the SMH sleep questionnaire were tallied for subjective assessment. To assess the subjective quality of sleep, the SMH sleep questionnaire was completed every morning during the 5-day test duration (Ellis et al., 1981). SMH evaluated only Q5, 6, 10, 11, 12, 13 and 14. The others were omitted because they could be replaced by EEG measurements.
Analysis of salivary amylase
Salivary α-amylase levels were measured as a simple method for measuring stress and was adopted as indicators of efficacy during intake period. The pre-intake salivary levels were measured twice (in the morning of the first day and the previous day of intake) and were compared with the post-intake salivary levels (measured twice; in the morning of the last day of intake and the following day.).
Statistical analysis
All measured values showing the participants’ characteristics are expressed as the mean ± standard deviation (SD), and the efficacy of the test results for all measured values are expressed as the mean ± standard error (SE). Comparisons of the participants’ characteristics (age, anthropometric measurements, and physiological and hematology tests) and carry-over effects (order and timing effects) between the two groups were analyzed using the unpaired t test. EEG and salivary α-amylase levels were analyzed using the paired t-test. The blood biochemistry test results and the data from the SMH sleep questionnaire were analyzed using the Wilcoxon signed-rank test. The PSQI score was analyzed using the Wilcoxon rank-sum test. Sex and lifestyle habits were analyzed using the Chi squared test. To analyze efficacy, a per-protocol set analysis was conducted excluding the participants who did not meet the control points. Statistical significance was determined by two-sided tests at the 5% significance level. SPSS 20.0 (IBM Japan Ltd., Tokyo, Japan) and Microsoft Excel (Microsoft Japan Co., Ltd., Tokyo, Japan) were used for all statistical analyses.
Results and discussion
Subjects
There were 30 participants in the present study. The participants’ characteristics were shown in Table 1. Seven participants were excluded because they met the pre-established exclusion criteria (poor health, drug administration, etc.). Therefore, data from 23 participants were included in the efficacy assessment analysis. Using sleep efficiency as the indicator, the carry-over and order effects were analyzed, and no significant differences were observed (p = 0.653, 0.069).
Table 1.
Characteristics of the participants
| Index | Total | Onion extract preceding group | Placebo preceding group | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male/Female | 14 | / | 16 | 6 | / | 9 | 8 | / | 7 | − |
| Age | 43.1 | ± | 5.3 | 42.8 | ± | 6.0 | 43.3 | ± | 4.8 | 0.790 |
| Height (cm) | 165.0 | ± | 8.7 | 163.2 | ± | 7.3 | 166.7 | ± | 9.8 | 0.272 |
| Weight (kg) | 57.7 | ± | 11.7 | 57.6 | ± | 10.6 | 57.9 | ± | 13.0 | 0.948 |
| BMI (kg/m2) | 21.5 | ± | 3.0 | 21.5 | ± | 2.7 | 21.4 | ± | 3.4 | 0.948 |
| PSQI | 8.5 | ± | 1.3 | 8.5 | ± | 1.3 | 8.5 | ± | 1.3 | 0.732 |
BMI: body mass index, PSQI: Pittsburgh Sleep Quality Index
All values are expressed as mean ± SD
Safety evaluation
Adverse events such as symptoms of a common cold were observed with the intake of the onion extract tablets (one participant) and placebo tablets (two participants). However, there seemed to be no safety issues as all the adverse events were considered not to be caused by the onion extract tablets.
EEG measurement
Furthermore, EEG data of the intake tests of the onion extract and placebo tablets were not available for two participants, so their data were also excluded. Consequently, EEG data from 21 participants were analyzed. To examine the effects of the onion extract tablets on sleep objectively, EEGs were measured using a sleep scope. The delta power per minute in the first sleep cycle (p = 0.043) and the total delta power during NREM sleep (p = 0.044) significantly increased after the intake of onion extract tablets compared with the placebo tablets (Table 2). These results suggest that the intake of onion extract tablets containing concentrated cysteine sulfoxides improved sleep quality that is assessed by the depth of NREM sleep due to the increased delta power. However, no significant difference was observed for the other measured EEG parameters.
Table 2.
Objective sleep parameters analyzed by EEG after the intake of onion extract and placebo conditions
| Evaluated factor | Onion extract (n = 21) | Placebo (n = 21) | |||||
|---|---|---|---|---|---|---|---|
| Total sleep time | Min | 335.4 | ± | 9.0 | 323.4 | ± | 8.8 |
| Sleep period time | Min | 375.5 | ± | 11.1 | 358.1 | ± | 10.1 |
| During REM sleep latency | Min | 68.5 | ± | 4.2 | 66.7 | ± | 3.0 |
| Sleep latency | Min | 27.3 | ± | 5.4 | 38.8 | ± | 10.0 |
|
Total time of NREM sleep stage 1 (ratio of NREM sleep stage 1) |
Min (%) |
56.9 (15.0) |
± |
4.3 (0.9) |
53.9 (15.2) |
± |
3.7 (1.1) |
|
Total time of NREM sleep stage 2 (ratio of NREM sleep stage 2) |
Min (%) |
181.0 (48.5) |
± |
5.5 (1.0) |
173.2 (48.3) |
± |
6.9 (1.4) |
|
Total time of NREM sleep stage 3 (ratio of NREM sleep stage 3) |
Min (%) |
14.9 (4.1) |
± |
3.0 (0.8) |
13.5 (3.8) |
± |
3.0 (0.8) |
|
Total time of NREM sleep stage 1–3 (ratio of NREM sleep stage 1–3) |
Min (%) |
252.8 (67.6) |
± |
6.8 (0.8) |
240.6 (67.3) |
± |
6.0 (0.8) |
|
Total time of REM sleep (ratio of REM sleep) |
Min (%) |
82.5 (22.0) |
± |
3.7 (0.8) |
82.9 (23.1) |
± |
3.9 (0.8) |
| Awakening index | Times | 12.5 | ± | 0.9 | 11.5 | ± | 0.9 |
| Wake after sleep onset (WASO) | Min | 40.1 | ± | 3.8 | 34.7 | ± | 3.1 |
| Sleep efficiency | % | 82.4 | ± | 1.7 | 81.3 | ± | 2.0 |
| Delta power during first NREM | µV2 | 100710.6 | ± | 9489.2 | 89714.3 | ± | 9187.7 |
| Delta power during first NREM/min | µV2/min | 1599.0 | ± | 124.5* | 1454.8 | ± | 132.4 |
| Delta power during NREM | µV2 | 286900.5 | ± | 30073.3* | 252816.1 | ± | 21997.3 |
| Delta power during NREM/min | µV2/min | 1134.9 | ± | 109.2 | 1056.0 | ± | 91.0 |
EEG: electroencephalogram, REM: rapid-eye movement, NREM: non-rapid eye movement
All values are expressed as mean ± SE. *Intergroup comparison (p < 0.05, vs. placebo group)
To assess the effects on sleep before and after the intake of the onion extract tablets, changes in EEG data were compared between the measurement taken the day before the start of the intake test and the measurement taken on the last day of intake. When assessing the effects on sleep before and after the intake of the onion extract tablets, EEG data from the day before the start of the intake test were not available from three participants. Subsequently, their data was excluded, and thus, data from 18 participants were included in the EEG analysis of the pre- and post-intake test. The delta power during the first sleep cycle (p = 0.040), the delta power per minute in the first sleep cycle (p = 0.048), the total delta power during NREM sleep (p = 0.015), and the total delta power per minute during NREM sleep (p = 0.028) significantly increased after the intake of onion extract compared with placebo tablets (Table 3). However, there were no significant changes in total sleep time, REM sleep time, and NREM sleep time at each stage. Benzodiazepines, commonly used to treat insomnia, increase stage 2 sleep and decrease slow-wave sleep time and REM sleep time via activation GABA receptor (Borbely et al., 1983; Parrino and Terzano, 1996). Ingestion of onion extract containing concentrated cysteine sulfoxides does not modify the sleep architecture itself, unlike benzodiazepines. Therefore, it is suggested that onion extract containing concentrated cysteine sulfoxides naturally induces deep sleep and is suitable for daily intake. A significant decrease in sleep latency (p = 0.022) was also observed. Improvements in sleep quality as well as smooth transition into sleep were also noted. Therefore, it was indicated that onion extract containing concentrated cysteine sulfoxides was especially effective in the early stages of sleep. However, there was also a significant increase in the awakening index (p = 0.030).
Table 3.
Objective sleep parameters analyzed by EEG under the onion extract and placebo conditions
| Evaluated factor | Before (n = 18) | Onion extract | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amount of change (n = 18) | Amount of change (n = 18) | |||||||||
| Total sleep time | Min | 333.9 | ± | 15.0 | 0.9 | ± | 21.7 | − 19.7 | ± | 17.0 |
| Sleep period time | Min | 369.5 | ± | 17.0 | 12.6 | ± | 27.0 | − 20.8 | ± | 18.9 |
| During REM sleep latency | Min | 70.3 | ± | 5.7 | 6.1 | ± | 9.3 | − 3.8 | ± | 7.5 |
| Sleep latency | Min | 35.6 | ± | 11.4 | − 0.1 | ± | 8.1* | 15.3 | ± | 10.5 |
|
The total time of NREM sleep stage1 (the ratio of NREM sleep stage1) |
Min (%) |
48.7 (13.4) |
± |
4.0 (1.1) |
7.8 (1.0) |
± |
9.2 (1.4) |
1.1 (1.5) |
± |
5.0 (1.7) |
|
The total time of NREM sleep stage2 (the ratio of NREM sleep stage2) |
Min (%) |
189.6 (51.2) |
± |
10.2 (1.8) |
− 8.5 (− 3.2) |
± |
13.3 (1.8) |
− 18.4 (− 2.4) |
± |
13.2 (2.3) |
|
The total time of NREM sleep stage3 (the ratio of NREM sleep stage3) |
Min (%) |
14.0 (4.0) |
± |
3.6 (1.0) |
2.5 (0.6) |
± |
2.7 (1.0) |
− 2.7 (− 0.9) |
± |
2.1 (0.5) |
|
The total time of NREM sleep stage 1–3 (the ratio of NREM sleep stage 1–3) |
Min (%) |
252.4 (68.6) |
± |
11.7 (1.4) |
1.8 (− 1.5) |
± |
18.0 (1.4) |
− 20.0 (− 1.8) |
± |
14.3 (1.3) |
|
The total time of REM sleep (the ratio of REM sleep) |
Min (%) |
81.5 (21.9) |
± |
5.9 (1.2) |
− 0.9 (− 0.8) |
± |
6.7 (1.5) |
0.3 (1.6) |
± |
5.6 (1.4) |
| Awaking index | Times | 11.4 | ± | 1.1 | 2.7 | ± | 1.1* | 0.2 | ± | 1.0 |
| Wake after sleep onset (WASO) | Min | 35.6 | ± | 4.4 | 11.6 | ± | 6.9* | − 1.1 | ± | 4.1 |
| Sleep efficiency | % | 80.9 | ± | 2.9 | − 2.1 | ± | 3.4 | − 1.8 | ± | 2.3 |
| Delta power during first NREM | μV2 | 96270.1 | ± | 10609.4 | 14323.4 | ± | 8307.1* | − 19564.8 | ± | 11231.7 |
| Delta power during first NREM/min | μV2/min | 1495.6 | ± | 144.9 | 126.2 | ± | 107.1* | − 186.9 | ± | 168.4 |
| Delta power during NREM | μV2 | 269253.8 | ± | 29710.7 | 23817.1 | ± | 27443.6* | − 47071.9 | ± | 24713.5 |
| Delta power during NREM/min | μV2/min | 1068.6 | ± | 105.7 | 106.8 | ± | 103.8* | − 127.4 | ± | 81.7 |
Each value compared before and after treatment
EEG: electroencephalogram, REM: rapid-eye movement, NREM: non-rapid eye movement
All values are expressed as mean ± SE. *Intergroup comparison (p < 0.05, vs. placebo group)
The above findings suggest that onion extract containing concentrated cysteine sulfoxides promotes smooth transition into sleep and deepens sleep in the first sleep cycle. In Japan, 20% of adults have sleeping problems (Kim et al., 2000). A decline in sleep quality has been shown to increase the risk of diseases such as diabetes (Tasali et al., 2008). Studies have also shown that sleep quality during the first sleep cycle is related to the levels of growth hormone secretion (Gronfier et al., 1996; Monoi et al., 2016). There may be similar health benefits with the improvement of sleep quality due to the intake of onion extract containing concentrated cysteine sulfoxides. However, further studies are needed on the direct effects of the index compounds.
SMH sleep questionnaire
Sleep quality can also be subjectively assessed using questionnaires (Ellis et al., 1981). Therefore, the SMH sleep questionnaire was administered to determine the subjective effects of the onion extract tablets on NREM sleep. The results of the sleep questionnaire were shown in Table 4. According to the results, no significant differences were found between the onion extract and placebo tablets.
Table 4.
Subjective sleep parameters as rated by the SMH sleep questionnaire under the onion extract and placebo conditions
| Subjective symptom after awake | Onion extract (n = 23) |
Placebo(n = 23) | |||||
|---|---|---|---|---|---|---|---|
| Q.5 How was your sleep? | Light < deep | 4.0 | ± | 0.1 | 4.2 | ± | 0.2 |
| Q.6 How many times did you wake up? | Times | 2.6 | ± | 0.2 | 2.5 | ± | 0.2 |
| Q.9 How well did you sleep last night? | Badly < well | 3.6 | ± | 0.1 | 3.7 | ± | 0.1 |
| Q.10 How clear-headed did you feel after getting up this morning? |
Still very drowsy < alert |
3.4 | ± | 0.1 | 3.2 | ± | 0.1 |
| Q.11 How satisfied were you with last night’s sleep? |
Unsatisfied < satisfied |
3.3 | ± | 0.1 | 3.3 | ± | 0.1 |
| Q.12 Were you troubled by waking early and being unable to go to sleep again? | No < yes | 1.1 | ± | 0.0 | 1.1 | ± | 0.0 |
| Q.13 How much difficulty did you have in going to sleep last night? | None or very little < difficult | 1.5 | ± | 0.1 | 1.5 | ± | 0.1 |
| Q.14 How long did it take you to fall asleep last night? | Mins | 23.4 | ± | 2.5 | 26.4 | ± | 3.0 |
SMH: St. Mary’s Hospital
All values are expressed as mean ± SE
The present study consisted of participants consuming onion extract tablets for 5 days, and some parameters, such as sleep latency, became evident through the analysis of data obtained before and after the intake period. With extended test period, it may be able to identify significant improvement in subjective sleep parameters as well as objective ones.
Analysis of salivary amylase
Stress is increased by different causes and is known to affect sleep quality (Fruhstorfer et al., 1984; Pasternak et al., 1992). The mechanism of gamma-aminobutyric acid (GABA) in improving sleep works on the stress-reducing effects (Nakamura et al., 2009). Therefore, stress was measured to assess the mechanism of sleep improving effects of cysteine sulfoxides (contained in onion extract tablets). Research has shown that salivary α-amylase levels increase with stress (Chatterton et al., 1996). Therefore, salivary α-amylase levels were used as the indicator to determine the stresses related to sleep. Salivary α-amylase levels significantly decreased (p = 0.035) after the intake of onion extract compared with placebo (Fig. 2).
Fig. 2.
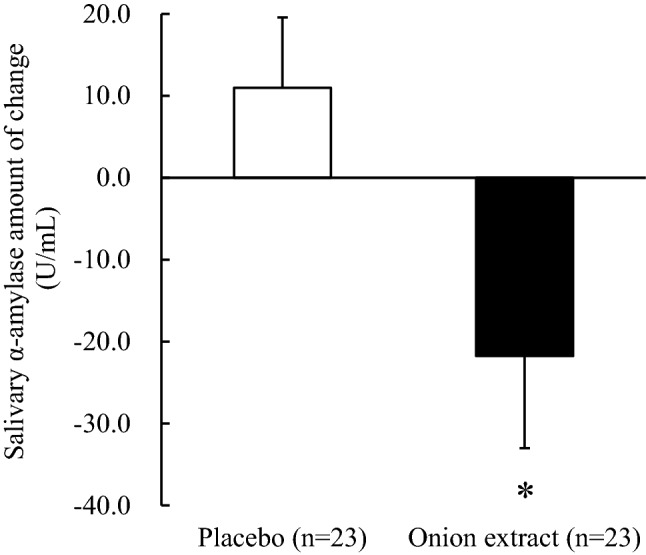
Salivary α-amylase amount of change under the onion extract and placebo conditions. All values are expressed as mean ± SE. *Intergroup comparison (p < 0.05, vs. placebo group)
This finding suggests that the improvement of sleep quality from the intake of onion extract tablets containing concentrated cysteine sulfoxides may be related to the stress-reducing effect. Former studies have shown that some amino acids have sleep-improving effects (Ito et al., 2014; Yamadera et al., 2007; Yamatsu et al., 2008). The mechanism of action of GABA in improving sleep works on the GABAB receptors in the peripheral ganglia (Hayakawa et al., 2002), and in addition to the stress-reducing effects (Nakamura et al., 2009), it may also work on the autonomic nervous system to suppress the sympathetic nervous system (Okita et al., 2009). Adenosine receptors (Oishi et al., 2008), prostaglandin D2 (Ueno et al., 1983), and orexin-mediated pathways (Chemelli et al., 1999) have been reported as regulatory mechanisms of sleep. The effect of improving sleep quality of the onion extract tablets containing concentrated cysteine sulfoxides, which are amino acids, may occur through these receptors. Further investigation is needed to elucidate the action mechanism and functional constituents.
In conclusion, the present study showed that the intake of onion extract tablets containing concentrated cysteine sulfoxides improved sleep quality and promoted smooth transition into sleep. The intake of cysteine sulfoxides deepened daily sleep and improved sleep quality, thereby showing potential to improve the quality of life.
Acknowledgements
We thank Yoshida Masaki from SleepWell Co., Ltd., Osaka, Japan, and Ando Toshiki from CPCC Co., Ltd., Tokyo, Japan for their helpful discussions and valuable comments throughout this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Asami N, Shin-ichiro Y, Naoko S, Tingfu L, Tomoko K, Jinwei Y, Tsuyoshi T. Effect of an Apocynum venetum Leaf Extract (VENETRON®) sleep quality and psychological stress improvement -A randomized, double-blind, placebo-controlled crossover study- Jpn. Pharmacol. Ther. 2018;46:117–125. [Google Scholar]
- Born J, Wilhelm I. System consolidation of memory during sleep. Psychol. Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Mattmann P, Loepfe M, Fellmann I, Gerne M, Strauch I, Lehmann D. A single dose of benzodiazepine hypnotics alters the sleep EEG in the subsequent drug-free night. Eur. J. Pharmacol. 1983;89:157–161. doi: 10.1016/0014-2999(83)90622-2. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary α-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097X.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/S0092-8674(00)81973-X. [DOI] [PubMed] [Google Scholar]
- Doi Y, Minowa M, Okawa M, Uchiyama M. Development of the Japanese version of the Pittsburgh sleep quality index. (in Japanese).) Jpn. J. Psychiatry Treat. 13: 755–763 (1998).
- Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui, Kamei Y. Psychometric assessment of subjective sleep quality using Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 97: 165–172 (2000) [DOI] [PubMed]
- Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary’s hospital sleep questionnaire: A study of reliability. Sleep 4: 93–97 (1981) [DOI] [PubMed]
- Ferrara M, De Gennaro L, Casagrande M, Bertini M. Selective slow-wave sleep deprivation and time-of-night effects on cognitive performance upon awakening. Psychophysiology. 2000;37:440–446. doi: 10.1111/1469-8986.3740440. [DOI] [PubMed] [Google Scholar]
- Flexer A, Gruber G, Dorffner G. A reliable probabilistic sleep stager based on a single EEG signal. Artif. Intell. Med. 2005;33:199–207. doi: 10.1016/j.artmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer B, Fruhstorfer H, Grass P. Daytime noise and subsequent night sleep in man. Eur. J. Appl. Physiol. Occup. Physiol. 1984;53:159–163. doi: 10.1007/BF00422580. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. A quantitative evaluation of the relationships between growth hormone secretion and delta wave electroencephalographic activity during normal sleep and after enrichment in delta waves. Sleep. 1996;19:817–824. doi: 10.1093/sleep/19.10.817. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Kimura M, Kamata K. Mechanism underlying gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur. J. Pharmacol. 2002;438:107–113. doi: 10.1016/S0014-2999(02)01294-3. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takahashi S, Shen M, Yamaguchi K, Satoh M. Effects of l-serine ingestion on human sleep. Springerplus. 2014;3:456. doi: 10.1186/2193-1801-3-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41–47. doi: 10.1093/sleep/23.1.1a. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee S, Shin D, Yoo M. Change in organosulfur compounds in onion (Allium cepa L.) during heat treatment. Food Sci. Biotechnol. 2016;25:115–119. doi: 10.1007/s10068-016-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook S, Kim GH, Choi K. The antidiabetic effect of onion and garlic in experimental diabetic rats: meta-analysis. J. Med. Food. 2009;12:552–560. doi: 10.1089/jmf.2008.1071. [DOI] [PubMed] [Google Scholar]
- Monoi N, Matsuno A, Nagamori Y, Kimura E, Nakamura Y, Oka K, Sano T, Midorikawa T, Sugafuji T, Murakoshi M, Uchiyama A, Sugiyama K, Nishino H, Urade Y. Japanese sake yeast supplementation improves the quality of sleep: a double-blind randomised controlled clinical trial. J. Sleep Res. 2016;25:116–123. doi: 10.1111/jsr.12336. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Takishima T, Kometani T, Yokogoshi H. Psychological stress-reducing effect of chocolate enriched with gamma-aminobutyric acid (GABA) in humans: assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009;60(Suppl 5):106–113. doi: 10.1080/09637480802558508. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Inagawa Y, Nukui K, Tanaka K, Hiramoto S, Yahata N, Horie S. Alleviation of the aging males’ symptoms by the intake of onion-extracts containing concentrated cysteine sulfoxides for 4 Weeks -randomized, double-blind, placebo-controlled, parallel-group comparative study- Jpn. Pharmacol. Ther. 2017;45:595–608. [Google Scholar]
- Nishimura H, Higuchi O, Tateshita K. Antioxidative activity of sulfur-containing compounds in Allium species for human low-density lipoprotein (LDL) oxidation in vitro. Biofactors. 2004;21:277–280. doi: 10.1002/biof.552210154. [DOI] [PubMed] [Google Scholar]
- Noreen S, Ayesha S. Administration of Allium cepa L. bulb attenuates stress-produced anxiety and depression and improves memory in male mice. Metab. Brain Dis. 33: 271–281. (2018) [DOI] [PubMed]
- Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. USA. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita Y, Nakamura H, Kouda K, Takahashi I, Takaoka T, Kimura M, Sugiura T. Effects of vegetable containing gamma-aminobutyric acid on the cardiac autonomic nervous system in healthy young people. J. Physiol. Anthropol. 2009;28:101–107. doi: 10.2114/jpa2.28.101. [DOI] [PubMed] [Google Scholar]
- Parrino L, Terzano MG. Polysomnographic effects of hypnotic drugs. Psychopharmacology. 1996;126:1–16. doi: 10.1007/BF02246405. [DOI] [PubMed] [Google Scholar]
- Pasternak RE, Reynolds CF, 3rd, Hoch CC, Buysse DS, Schlemitzauer M, Machen M, Kupfer DJ. sleep in spousally bereaved elders with subsyndromal depressive symptoms. Psychiatry. Res. 1992;43:43–53. doi: 10.1016/0165-1781(92)90140-X. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat. Rev. Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Masaki C, Makino M, Yoshida M, Mukaibo T, Kondo Y, Nakamoto T, Hosokawa R. Management of sleep-time masticatory muscle activity using stabilisation splints affects psychological stress. J. Oral. Rehabil. 2013;40:892–899. doi: 10.1111/joor.12110. [DOI] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl. Acad. Sci. USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno R, Honda K, Inoué S, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc. Natl. Acad. Sci. USA. 1983;80:1735–1737. doi: 10.1073/pnas.80.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez JC, Souza A, Traslavina S, Barbosa C, Wosu A, Andrade A, Frye M, fitzpatrick AL, Gelaye B, Williams MA. The epidemiology of sleep quality and consumption of stimulant beverages among Patagonian Chilean college student. Sleep Disorders 910104 (2013) [DOI] [PMC free article] [PubMed]
- Xiao H, Parkin KL. Antioxidant functions of selected Allium thiosulfinates and s-alk(en)yl-l-cysteine sulfoxides. J. Agric. Food Chem. 2002;50:2488–2493. doi: 10.1021/jf011137r. [DOI] [PubMed] [Google Scholar]
- Yamadera W, Inagawa K, Chiba S, Bannai M, Takahashi M, Nakayama K. Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes. Sleep Biol. Rhythm. 2007;5:126–131. doi: 10.1111/j.1479-8425.2007.00262.x. [DOI] [Google Scholar]
- Yamatsu A, Yamashita Y, Maru I, Yang J, Tatsuzaki J, Kim M. The improvement of sleep by oral intake of GABA and Apocynum venetum leaf extract. J. Nutr. Sci. Vitaminol. 2008;61:182–187. doi: 10.3177/jnsv.61.182. [DOI] [PubMed] [Google Scholar]



