Abstract
Objectives:
The aim of this study was to modify the surface of fillers used in dental composites by the synthesis of two novel thiourethane oligomeric silanes, used to functionalize the silica-containing inorganic particles. Several thiourethane silane concentrations were tested during the silanization process to systematically assess the effect of silane coverage on experimental composite conversion, polymerization stress and fracture toughness.
Materials and Methods:
Two different thiourethane silanes were synthesized based either on 1,6-hexanediol-diissocynate (HDDI), or 1,3-bis(1-isocyanato-1-methylethyl) benzene (BDI). Conventional 3-(Trimethoxysilyl)propyl methacrylate was used as the control. Glass fillers were silanized with 1, 2 or 4 wt% of each thiourethane silane, then evaluated by thermogravimetrical analysis. Photopolymerizable resin composites were prepared with Bis-GMA/UDMA/TEGDMA and 50 wt% silanized glass filler. Polymerization kinetics and degree of conversion were tested using Near-IR. Bioman was used to test polymerization stress. Data were analyzed with two-way ANOVA/Tukey’s test (α=5%)
Results:
The mass of silane coupled to the filler increased with the concentrations of thiourethane in the silanizing solution, as expected. Thiourethane-containing groups exhibited significantly higher degree of conversion compared to control groups, except for BDI 4%. HDDI 4%, BDI 2% and BDI 4% showed significantly lower polymerization stress than control groups. HDDI 4% exhibited significantly higher fracture toughness.
Conclusions and Clinical significance:
Novel filler functionalization with thiourethane silanes may be a promising alternative for improving dental composites properties by significantly increasing the degree of conversion, fracture toughness and reducing the polymerization stress.
Keywords: Composite resins, Mechanical strength, Polymerization stress, Glass filler, Filler functionalization
Introduction
Resin composites have significantly evolved since their introduction and are widely used in Dentistry. Direct restorations using resin composites have low annual failure rates in long term follow-up studies (Da Rosa Rodolpho et al. 2011), but some studies still show that their average life-span is about 10 years (Demarco et al. 2012). The most common reasons for failure reported are restoration fracture and secondary decay and fracture (Da Rosa Rodolpho et al. 2011; Demarco et al. 2012). Even though factors such as operator technique and patient’s dietary and hygiene habits greatly contribute to outcomes, material properties still play an important role on the restoration survival. Most of the advances in composite materials have been accomplished on the inorganic filler component, with the organic matrix receiving significantly more attention in the past 15-20 years (Pfeifer 2017). Novel monomers and polymeric additives have been proposed, some with encouraging results in vitro (Bacchi et al. 2014; Moraes et al. 2011; Najeeb et al. 2016), and some with commercial examples (Park et al. 2012).
One example of polymeric additives proposed for composite materials are thiourethane oligomers (Bacchi et al. 2014). Thiourethanes oligomers are macromolecules comprising thiol pendant groups, which are capable of chain-transfer reactions with the vinyl in methacrylates, creating covalent bonds with the resin matrix (Bacchi et al. 2014). Chain-transfer is a chain-breaking mechanism, which in this case leads to delayed gelation and vitrification, extended limiting conversion and stress reduction (Berchtold et al. 2001). Because the chain-transfer is accomplished via a multi-functional thiol, the final crosslinking density is not significantly affected (Bacchi and Pfeifer 2016). The addition of thiourethanes in the matrix of dental resin composites has shown promising results with higher conversion, lower polymerization stress and improved mechanical properties (Bacchi et al. 2015; Bacchi et al. 2014). In particular, the addition of thiourethanes directly into the matrix has been shown to improve fracture toughness and toughness in highly filled composites (Bacchi and Pfeifer 2016). This toughening effect has also been demonstrated in other thiol-isocyanate applications where impact resistance is needed (Li et al. 2007). However the addition of thiourethanes to the organic matrix is limited by the increase in viscosity, and potential deleterious effects beyond 30 wt% of the organic matrix (Bacchi et al. 2015). Therefore, there is a clear concentration-dependent effect, so a maximum of overall 6 wt% thiourethane has been added (Bacchi et al. 2014).
More recently, with the aim of circumventing the increase in viscosity, these oligomers have been used to functionalize the surface of inorganic filler particles (Faria-e-Silva et al. 2018; Fugolin et al. 2019). The inorganic filler is in general a mixture of different heavy metal glasses, added at 60-80 % in weight, depending on the required consistency (Habib et al. 2016). The filler content and size distribution directly affects mechanical properties such as flexural strength, modulus, hardness and fracture toughness (Habib et al. 2016). In addition, light transmission, depth of cure, surface roughness and maximum loading are also affected by filler size (Habib et al. 2016; Marghalani 2010). Fillers may also serve as stress concentrators in the matrix (Fu et al. 2008; Sowan et al. 2018; Üstündag et al. 2000), which is why functionalization of the surface with moieties other than methacrylates has been proposed to potentially provide a toughening mechanism on this critical region for crack growth in the composite. In fact, recent studies have demonstrated the ability of thiourethane-functionalized fillers to increase fracture toughness and reduce polymerization stress in highly filled composites (Faria-e-Silva et al. 2018; Faria-e-Silva and Pfeifer 2017; Fugolin et al. 2019), which is achieved with overall lower thiourethane concentration and no prejudice to the conversion or handling characteristics (Fugolin et al. 2019). In addition, the thickness of the silane layer is well known to play a role in stress relaxation (Johnson and Stafford 2010; Kubát et al. 1988). However, a systematic evaluation of the thiourethane concentration on the filler surface as it affects composite properties is still lacking.
Another potential advantage of the use of thiourethanes is increased depth of cure (Fugolin et al. 2019). Increased conversion in depth has been demonstrated in highly filled composites containing this additive, both as part of the matrix and as a silane-coupling agent (Bacchi and Pfeifer 2016; Faria-e-Silva et al. 2018; Faria-e-Silva and Pfeifer 2017; Fugolin et al. 2019). This was attributed to the increase in refractive index provided by the presence of thiol, thiocarbamate and aromatic groups (the latter in selected compositions), which minimizes the mismatch with the refractive index of the filler during polymerization, and improves light penetration in depth (Faria-e-Silva and Pfeifer 2017). However, to date, this has only been tested for one concentration of the oligomers used as silanes, and the effect of different thicknesses of the oligomer on the filler surface remains unexplored.
Therefore, the objective of this study was to systematically vary the concentration of oligomeric thiourethane silanes used to functionalize inorganic filler particles, and evaluate its effects on polymerization kinetics, final composite conversion, polymerization stress and fracture toughness. Two thiourethanes with different backbone structures (one flexible and other more rigid) were synthesized and used to functionalize the surface of silica-containing fillers. The null hypothesis was that different thiourethane silane types and concentrations do not affect the different material properties tested.
Materials and methods
Two oligomeric thiourethane silanes were synthesized for this study. The reaction was done at room temperature in 60 ml of methylene chloride by combining pentaerythritol tetra-3-mercaptopropionate with either 1,6-hexanediol-diissocynate (HDDI) or 1,3-bis(1-isocyanato-1-methylethyl) benzene (BDI), and 3-(Triethoxysilyl)propyl isocyanate (Sigma–Aldrich, St. Louis, MO, USA) at 1/2.5/1 molar ratio, resulting in pendant thiols and alkoxy silane groups from the oligomer structure. Triethylamine was used to catalyze the reaction and oligomers were purified by precipitation in hexane. Solvent evaporation was done in a rotary evaporator. Formation of thiourethane bonds and the absence of starting materials were verified by H-NMR and mid-IR spectroscopy (Bacchi et al. 2014). After this, aromatic (BDI) and aliphatic (HDDI) thiourethane silanes were obtained to use for filler silanization.
The functionalization process was performed in 65 ml solutions of 80 vol% ethanol and 20 vol% distilled water. Each solution was adjusted to pH 4.5 with the addition of glacial acetic acid. After this, 1, 2 or 4 wt% of HDDI or BDI oligomers were added to each silanizing solution together with 5 grams of unsilanized Ba-Al-SiO2 glass filler with 1.5 μm average particle size (Kavo Kerr Corporation, Orange, CA). The solution was kept under mechanical agitation for 24 hours, filtered, and dried for 4 days in an oven at 37°C. Two controls were included: negative control (control −), consisting of un-treated particles, and a positive control (control +), consisting of analogue methacrylate commercially supplied particles. In total, 8 experimental resin composites were mixed.
Composites were formulated with a resin matrix consisting of a mixture of 50 wt% bis-phenol A diglycidyl dimethacrylate, 30 wt% urethane dimethacrylate and 20 wt% triethylene glycol dimethacrylate (Esstech, Essington, PA, USA). The photo-initiator system was added to this resin matrix by total matrix weight. It consisted of 0.2 wt% dl-camphoroquinone, 0.8 wt% ethyl 4-dimethylaminobenzoate, and 0.2 wt% 2,6-di-tert-butyl-4-methylphenol (Sigma-Aldrich, St. Louis, MO, USA). Mixing was performed in a room with yellow light with a mechanical mixer (DAC 150 Speed mixer, Flacktek, Landrum, SC, USA). Experimental composites were obtained by adding 50 wt% one of the fillers described above to the resin matrix.
Thermogravimetric analysis (TGA, Pyris 7, Perkin Elmer, Waltham, MA, USA) was used to verify the mass loss from silane-coated fillers. The mass variation was measured in a 10 mg sample while the temperature was increased from 50 to 850 °C at 10 °C/min, under nitrogen flow (20 mL/min). Samples from fillers from two different synthesis batches were analyzed in TGA (n=2).
For polymerization kinetics and degree of conversion, composite discs (0.8 mm thick and 6 mm diameter) were tested between two glass slides (n=3) using near-infrared spectroscopy (NIR). Each sample was photopolymerized with a mercury arc lamp (EXFO Acticure 4000 UV Cure; Mississauga, Ontario, Canada) filtered at 320-500 nm for 300 seconds. Incident irradiance was 100 mW/cm2 reaching the surface of the specimen. A relatively low light irradiance was used for polymerization kinetics in this study as an attempt to minimize the effect of heat generation on the polymerization reaction. Polymerization kinetics was assessed by recording the decrease in area of the methacrylate absorption peak at 6165 cm−1 during light curing (Stansbury and Dickens 2001). Degree of conversion (DC, in %) was calculated with the following equation:
Rate of polymerization was calculated as the first derivative of the conversion × time curve.
Fracture toughness was determined according to the American Society for Testing Materials standard (E399-90) (ASTM 1997). Single-edge notch beam specimens (n=6) were fabricated in a split steel mold (5 x 2 x 25 mm) with a razor blade insert of 2.5 mm and light cured for 120 seconds each face at 800 mW/cm2 (Acticure 4000, 320-500 nm). Specimens were subjected to the 3-point bending test at a crosshead speed 0.5 mm/min on a universal testing machine (MTS Criterion, MTS Inc., Eden Prairie, MN, USA). Fracture toughness was calculated from the critical stress intensity factor (KIC) as described previously (Ferracane and Berge 1995).
To assess the polymerization stress, a cantilever beam method (Bioman instrument) was used as previously described (Watts and Satterthwaite 2008). A standard amount of composite (n=5) with 0.5 mm thickness was polymerized between a silica slab treated with a commercial silane (Ceramic Primer, 3M ESPE, St. Paul, MN, USA) and a steel piston that was sanded with 600 grit sandpaper and treated with a metal primer to improve adhesion to the metal (Z-prime, Bisco, Schaumburg, IL, USA). The test settings corresponded to a C-factor of 4. Composites were photopolymerized through the silica slab for 300 s with the mercury arc lamp at 800 mW/cm2.
The degree of conversion in depth was mapped using an IR-coupled microscope (Continuum, ThermoScientific, Madison, WI, USA). Rectangular specimens (5 mm x 2 mm) of 5 mm in thickness were produced by inserting the different composites in custom-made polyvinyl siloxane molds, sandwiched between glass slides. The light source was positioned 5 cm away from the surface of the specimen to ensure uniform light distribution at the surface. The light intensity was adjusted to produce an irradiance of 800 mW/cm2 reaching the surface of the specimen. After curing, the specimens were embedded in epoxy resin (Buehler, Lake Bluff, IL, USA) and sectioned along the long axis using a high-speed diamond saw to produce slices with 300 μm thickness. This procedure was carried out under copious water cooling to avoid potentially increased conversion due to heating. The slices were then stabilized on glass slides with silly putty, and placed on the automated stage of the microscope. Near-IR was used to obtain spectra at 500 μm intervals from the surface, with 3 measurements per depth, totaling 30 points in each map. Conversion at each point was determined as described above. The three points at each depth were averaged, and the means were used to build a 2D conversion map as a function of depth.
Light transmission through a 2 mm thick sample was followed in real-time (n=3) using a portable spectrometer instrument (Check MARC, BlueLight Analystics, Halifax, NS, Canada). The sample was irradiated with the same mercury arc lamp used for all photocuring procedures at 800 mW/cm2, and the irradiance (between 360 and 540 nm) reaching the sensor through the 2 mm thickness was recorded for 60 s. The 2 mm thickness was selected for two reasons: first, it is a clinically relevant thickness for most conventional composites. The second reason was technical: the light transmission through 5 mm specimens (such as the ones used in the conversion mapping experiment described above) was very low and the results were not reproducible due to the sensitivity limit of the Check MARC device)
After cleaning in ultrasonic bath, toughness bars were mounted on aluminum stubs with carbon tape, and fractured surfaces were coated with a 6 nm layer thickness of gold/palladium (Leica EM ACE600 High Vacuum Sputter Coating). Scanning electron micrographs were obtained under high vacuum, at 20 kV and working distance of 10 mm (JEOL, JSM-5600 LV, SEM, Japan).
Data were analyzed using the statistical software SPSS (IBM Corp, Armonk, NY, USA). Data were tested for normality (Anderson-Darling) and homoscedasticity (Bartlett/Levene). One-way ANOVA and Tukey post-hoc test for multiple comparisons were used at a significance level of 95%.
Results
Thermogravimetric analysis results are shown in Figure 1. The mass loss ranged between 2.3% and 12.7%, for the positive control and HDDI 4% (Figure 1A). Mass loss increased monotonically with the increase in the initial concentration of thiourethane silane in the functionalization process for both aliphatic and aromatic thiourethanes (Figure 1B).
Figure 1.
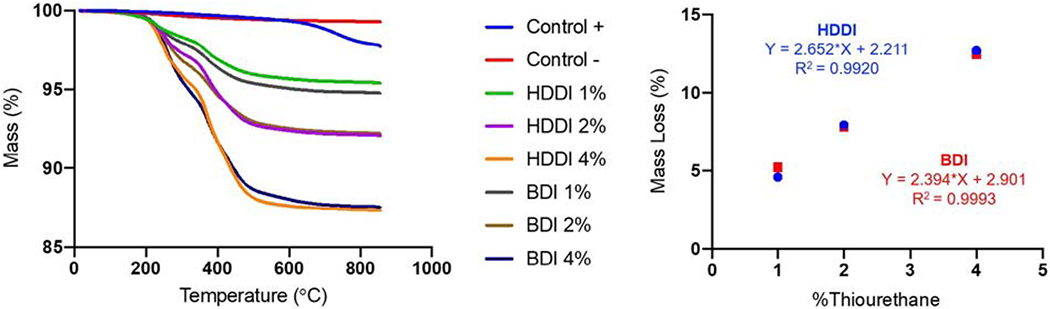
Left: Mass loss (%) as a function of the temperature (°C) for all inorganic filler particles tested in this study obtained by thermogravimetrical analysis. Right: Linear regression curves for inorganic filler mass loss (%) as a function of the initial concentration of thiourethane oligomers used for the functionalization procedures. A strong, positive linear correlation was observed between those parameters.
Results of polymerization kinetics and degree of conversion are presented in Table 1 and Figure 2. Thiourethane containing groups exhibited statistically higher degree of conversion compared to control groups, except for BDI 4% (p=0.002). The negative control (unsilanized particles) and BDI 4% presented similar conversion, higher than the positive control. RPmax in general decreased for thiourethane-containing materials, but was only significantly lower than the controls for BDI 4% (p=0.023). The degree of conversion at RPmax serves as a mark for the onset of vitrification (Odian 2004). In general, the thiourethane-containing materials presented higher DC at RPmax, but only the BDI 4% was statistically different from the positive control (p=0.001).
Table 1.
Degree of conversion (DC, %), maximum rate of polymerization (RPmax, %.s−1) and conversion at RPmax (%) for the groups tested, containing the aromatic (BDI) or aliphatic (HDDI) thiourethane oligomers. The methacrylate silane (control +) and the unsilanized particles (control −) are also shown. Values followed by the same superscript on the same column are statistically similar (α=5%).
| DC (%) | RPmax (%.s−1) | DC at RPmax (%) | |
|---|---|---|---|
| Control + | 46.6 (0.18)d | 1.06 (0.06)ab | 9.89 (0.92)b |
| Control − | 48.2 (0.29)c | 1.10 (0.05)ab | 12.49 (1.44)a |
| HDDI 1% | 50.9 (0.44)ab | 1.06 (0.06)ab | 10.55 (0.84)ab |
| HDDI 2% | 51.2 (0.42)a | 1.16 (0.02)a | 10.25 (1.00)ab |
| HDDI 4% | 50.3 (0.24)b | 1.03 (0.07)ab | 9.09 (0.54)b |
| BDI 1% | 50.7 (0.23)ab | 1.09 (0.03)ab | 10.61 (2.46)ab |
| BDI 2% | 50.4 (0.38)ab | 0.97 (0.06)b | 10.59 (2.83)ab |
| BDI 4% | 48.0 (0.16)c | 0.71 (0.03)c | 13.83 (4.89)a |
Figure 2.
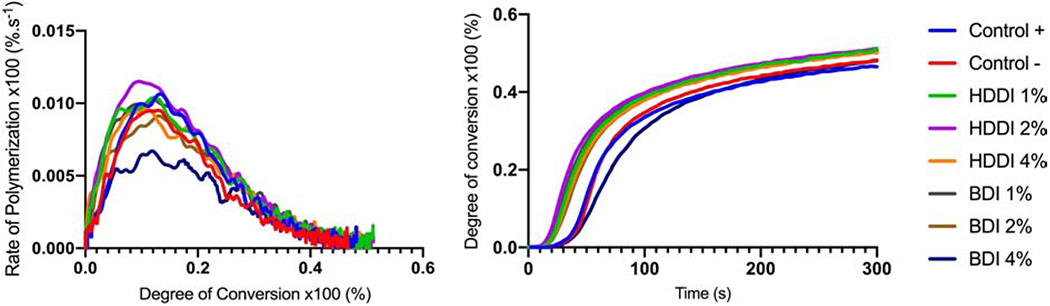
Left: Rate of polymerization (x100, %.s−1) as a function of the degree of conversion (x100, %), and Right: degree of conversion (x100, %) as a function of the time (s) for all tested resin composite formulations. The kinetics profiles were assessed by near-IR spectroscopy in real time during photoactivation for 300s at 100 mW/cm2.
In general, fracture toughness increased with the presence of thiourethane, and that increase was greater for higher concentrations of the oligomer on the filler (p=0.001). The material with unsilanized fillers (control −) showed the lowest fracture toughness value and the material containing the filler functionalized with HDDI 4% showed the highest fracture toughness value, statistically greater than the positive control (Figure 3).
Figure 3.

Left: Fracture toughness (MPa.m1/2) obtained from single notch specimens and Right: Polymerization stress (MPa) obtained in a cantilever apparatus (Bioman) made from experimental composites containing different silanized fillers with the aromatic (BDI) or aliphatic (HDDI) thiourethane oligomers. The initial concentrations of silane used in the synthesis procedure varied from 1-4 wt% of the mass of solvents used. Different letters indicate statistically significant difference among the groups (p ≤ 0.05).
Polymerization stress (Figure 3) was lower for thiourethane-containing materials, in general, with a few examples presenting statistically lower values than the controls (HDDI 4%, BDI 2% and BDI 4%, p=0.005). BDI 4% led to 50% lower stress compared to the control groups, and it was statistically the lowest value among the thiourethanes. All the other thiourethane materials had statistically similar stress results.
Degree of conversion (%) averages as a function of depth (mm) are depicted in the 2-D heat maps and presented in the table insert (Figure 4). Formulations containing thiourethane-functionalized particles presented similar or higher conversion in comparison with the control groups at all tested depths. In general, HDDI 4% presented the highest results of conversion (ranging from 72.2±1.4 to 78.8±0.9%), and the positive control (ranging from 64.6±2.3 to 70.7±0.1%) had the lowest values.
Figure 4.

2D-heat maps for the average of degree of conversion (%) as a function of the depth (mm) for experimental composites containing different silanized fillers with the aromatic (BDI) or aliphatic (HDDI) thiourethane oligomers. The averages and standard deviations for all tested resin composites at different depths are presented in the table. Values followed by different letters indicate statistically significant differences among the groups at the same depth (p ≤ 0.05).
Light transmission results are shown in Figure 5. Generally, the materials formulated with BDI-based thiourethane had lower light transmission through the 2 mm specimen than the other groups, as denoted by the maximum and final irradiances values (p<0.001 for both). The only exception was the 1% concentration, for which HDDI and BDI had similar results. All compositions showed decreased light transmission with time. The percentage reduction in the light transmission at the beginning compared to the one registered at the end of the experiment ranged from 1.5-7.6% for the TU-containing groups and for the negative control (unsilanized particles). The methacrylate silane (positive control) had a 23.8% decrease in light transmission with time.
Figure 5.

Left: Power Density (mW/cm2) as a function of time (s) for all formulations composed of thiourethane-functionalized filled particles. The power density corresponds to the total power per unit of area reaching the bottom sensor through 2 mm thick specimens. Right: Maximum irradiance and final irradiance values (mW/cm2) in table format. Values followed by the same lower case letter within the same column are statistically similar (α=0.05). For the groups marked with * only, the maximum value was not registered in the beginning of the readings.
Representative SEM micrographs for all tested composites at 5000x, 7500x, and 10,000x magnifications are presented in Figure 6. The filler particles groups with a higher starting silane concentration appear to be covered by a thicker layer of organic matter, though the differences are subtle.
Figure 6.
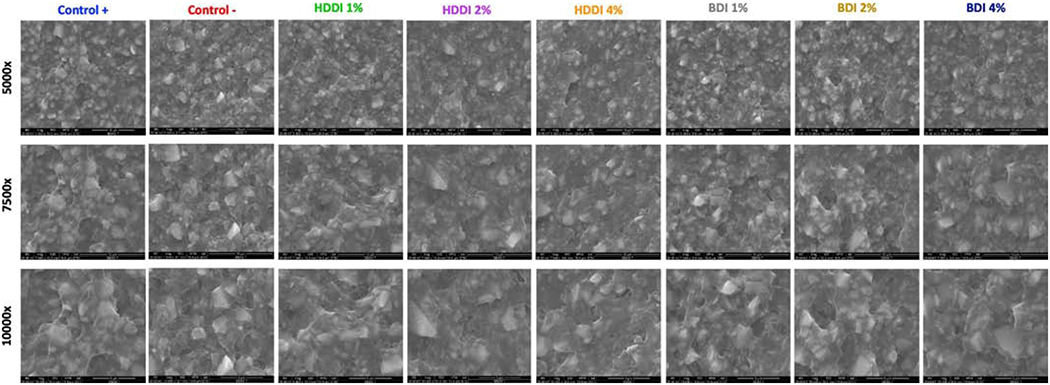
Representative scanning electron micrographs for all experimental composites containing different silanized fillers with the aromatic (BDI) or aliphatic (HDDI) thiourethane oligomers. The initial concentrations of silane used in the synthesis procedure varied from 1-4 wt% of the mass of solvents used. The magnification of each frame is shown on the left hand side.
Discussion
The use of different concentrations of thiourethane oligomer during the silanization process led to a monotonic increase in the masses of additive coupled to the filler, as expected. The mass loss was similar for either type of thiourethane (BDI- or HDDI-containing) within each concentration group and varied from about 5 to 13 wt%. The resulting composites presented different polymerization kinetics, degree of conversion, fracture toughness and polymerization stress. In general, higher fracture toughness values and lower polymerization stress were obtained with increasing concentrations of thiourethane, all statistically different from the unsilanized and methacrylate-silanized filler groups (negative and positive control, respectively). The degree of conversion was statistically higher for thiourethane-containing materials compared to the controls, but similar amongst thiourethane types and concentrations. Therefore, the null hypothesis of this study was rejected.
Silane coupling agents chemically bind the resin matrix and the inorganic filler in dental composites, improving mechanical properties (Söderholm 1981; Zanchi et al. 2015) by hampering crack propagation along the filler/matrix interface (Condon and Ferracane 1998). Without silane coupling agents, the particles essentially act as voids, as demonstrated previously in a study evaluating the fracture toughness of unsilanized fillers (Condon and Ferracane 1998). In addition, within the same filler type, as is the case in this study, unsilanized fillers are more hydrophilic than their silanized counterparts, thus leading to higher water sorption and hydrolytic degradation (Karmaker et al. 2007; Zanchi et al. 2015). The organic modification of silica surfaces has been characterized in detail, and a previous study has shown an increment in γ-MPS silane bonding with increasing concentrations in the synthesis solution, though concentrations higher than 5% do not seem to additionally increase filler surface coverage (Sideridou and Karabela 2009). Higher concentrations of silane may change the molecular orientation of silane at the filler surface and create multi-layered depositions instead of monolayers (Sideridou and Karabela 2009). In addition, the initial filler particle surface area influences bonding by the available silanol groups. In this study, since the silanizing agent was comprised of a high molecular weight species (Bacchi et al. 2014), different concentrations of silane were evaluated to allow for the systematic evaluation of the effect of potentially thicker coatings on the properties of composites. Indeed, filler particle functionalization increased with increased concentrations of thiourethane silane in the silanization solution during synthesis, as demonstrated by the mass loss results obtained with thermogravimetric analysis. It is assumed that the materials presenting the greater mass loss by TGA contained a thicker layer of silane, since it is unlikely that a multi-functional silane compound such as the ones used here led to formation of a monolayer.
In other words, rather than increasing the overall surface coverage, the thiourethane oligomers likely led to the formation of a crosslinked silane layer, with the same oligomer being capable of attachment at multiple points in the surface. TGA results showed a twofold increase in the mass loss for fillers silanized with 2 wt% thiourethane (initial concentration in the silanization solution) as compared to the methacrylate silane control. As already mentioned, rather than increased particle surface coverage by the silane, this result can be attributed to the higher molecular weight of thiourethane molecules in comparison to conventional methacrylate silanes, and therefore, to a thicker silane layer. We postulate that the crosslinking within the silane layer may have contribute to increasing the thickness of the “shock absorbing” layer around the filler, which helps explain some of the mechanical properties results, as explained in detail later in the text. Adding evidence to this thicker layer hypothesis, there was an observable difference in the handling characteristics of the composite pastes formulated with fillers silanized with thiourethane compared with the control. Because of the likely modified interaction of the silanized filler particles with the resin matrix and within particles, the apparent viscosity of those materials was greater compared to those formulated with methacrylate silanes. High concentrations of silane may lead to interphase degradation caused by hydrolysis of the oxane bonds between different silane molecules (Zanchi et al. 2015). The effect of degradation was not tested in this study, as only immediate properties were tested. However, the thiourethanes present much higher molecular weight, multiple points of attachment to the surface, and are crosslinked by nature, as already mentioned, so the potential for degradation of the silane coating is greatly diminished (Amdjadi et al. 2017). Future studies will focus on not only identifying the long-term degradation effects of systematically varied silane coating thickness, but also on characterizing the surface of the silanized filler particles with imaging techniques.
Novel fillers functionalized with thiourethane silanes were able to increase the final degree of conversion of resin composites in this study. This conversion increase was already reported in other methacrylate-based materials modified with thiourethanes, when added directly to the resin matrix (Bacchi et al. 2014; Bacchi and Pfeifer 2016). The mechanism for increased conversion in these materials relates to the chain-transfer reactions from the pendant thiols to the polymerizing methacrylate, as demonstrated previously (Hoyle and Bowman 2010; Senyurt et al. 2007a). Chain transfer is a chain-breaking mechanism, which allows for higher conversion to be achieved before diffusion limitations start to prevent further polymerization (Berchtold et al. 2002; Pfeifer et al. 2011). This translates to delayed gelation and vitrification, and ultimately, to higher conversion and lower stress (Li et al. 2007). In this study, the thiourethanes tethered to the filler surface showed the same behavior previously demonstrated in materials where it is added directly to the resin matrix (Bacchi and Pfeifer 2016; Bacchi et al. 2018). This increase in conversion was somewhat independent on both the type and concentration of the thiourethane on the filler, with a few exceptions. The group with BDI 4% presented the highest conversion at rate maximum (used as an estimation of the onset of vitrification), indicating that this silane was able to delay vitrification to higher conversions compared to the methacrylate control. Moreover, unsilanized fillers led to higher conversions in comparison to silanized fillers. This corroborates previous findings where lower concentrations of silane on the filler surface resulted in higher conversions (Condon and Ferracane 1998).
The delayed vitrification caused by chain transfer reactions not only affects conversion, but also reduces polymerization stress. This stress reduction is related to stress relief by chain rearrangement and flow as the network forms. Both thiourethane silanes tested in this study presented significant stress reduction. However, the aromatic (BDI) thiourethane stress reduction was significantly greater compared to the aliphatic (HDDI) oligomer, in accordance with previous findings (Bacchi et al. 2018). BDI 4% presented the lowest polymerization stress for all tested materials, about half of the stress shown by the methacrylate control. The 2% lower conversion achieved in this group in comparison to the other thiourethanes, although statistically significant, only partially explains the reduction in stress. However, the degree of conversion of this group was still significantly higher than the positive control. This highlights the potential of these materials as stress-relieving agents, achieved at conversions that are higher than the methacrylate silane controls.
Mechanical properties such as fracture toughness, which essentially defines the largest amount of stress that a material can bear prior to failure (Mese et al. 2016), are affected by filler silanization and degree of conversion of the composite. It has been previously demonstrated and corroborated in this study that filler silanization increases fracture toughness in composite materials. As already mentioned, un-treated particles essentially work as “voids” in the material, and facilitate crack propagation, whereas material in which the inorganic fillers are bonded to the resin matrix through an organofunctional silane cause crack arrest or deflection, decreasing its propagation energy (Condon and Ferracane 1998). The rationale for the use of thiourethanes as silanes was to localize the toughening and stress-reducing mechanisms exactly at the interface filler-matrix, where stress concentration is greater (Amdjadi et al. 2017). The toughening of materials with the use of thiourethanes and thiol-isocyanate materials in general has already been demonstrated for applications requiring high impact strength (Li et al. 2007). The thiocarbamate bonds are flexible and able to accommodate part of the stress and act as “energy sinks” for propagating cracks (Hoyle and Bowman 2010; Li et al. 2007; Senyurt et al. 2007a). In the case of the thiourethane silanes, this attribute is localized in the critical point in the composite. Indeed, composites where the fillers were silanized with thiourethanes showed an upward trend in terms of fracture toughness values in relation to the control, with a direct relationship with the increased silane concentration on the surface. However, only one group (HDDI 4%) presented statistically higher fracture toughness in relation to the control. This lack of statistical difference in the fracture toughness results also matches the only subtle differences in the SEM images among the groups. It needs to be pointed out that fractographic analysis is composites is much more complex than, for example, ceramics due to the less brittle nature of the crack propagation. In previous studies, where fracture toughness increase was observed with thiourethanes added to the matrix, the overall thiourethane concentration was 6 wt% (Bacchi and Pfeifer 2016; Bacchi et al. 2018). In this study, the maximum overall concentration of thiourethane ranged from 2 to 6 wt% (for 1 and 4% initial thiourethane concentrations, respectively). A relatively large particle was used here (1.5 μm average size), at a relatively low overall filler concentration (50 wt%). Still, the effect of increased fracture toughness was similar to the reports where the oligomer was added directly to the matrix in much higher filler loadings (Bacchi et al. 2015; Bacchi et al. 2014; Bacchi and Pfeifer 2016; Bacchi et al. 2018).
The same filler particle size and distribution was used for all groups, avoiding the potential effect of the increased surface area in smaller particles compared to the same volume of larger particles. It is also important to highlight that the filler loading was calculated as a percentage of the mass of the composite. Therefore, in the groups where the silane coating was thicker, the overall inorganic content was slightly lower. The effect of filler size and concentration on properties of materials containing thiourethane-functionalized particles will be the object of future studies. Finally, the structure of the thiourethane influenced the results, with the aliphatic version (HDDI) showing more significant improvement of the fracture toughness than the aromatic oligomer (BDI). This result is in agreement with another study where a trifunctional thiol was combined with an aliphatic isocyanate structure to produce a thiourethane oligomer which resulted in higher fracture toughness than the aromatic version (Bacchi et al. 2018). This was explained by the increased flexibility of the aliphatic structure compared to the more rigid aromatic conformation, which has been correlated with increased toughness (Senyurt et al. 2007b).
Regarding the degree of conversion in depth, thiourethane-containing formulations presented similar or higher results compared with the traditional methacrylate control for all depths analyzed, which agrees with previous reports (Faria-e-Silva and Pfeifer 2017; Fugolin et al. 2019). This can be ascribed to the increased refractive index of thiourethane (Faria-e-Silva and Pfeifer 2017), which in turn decreases the mismatch between the organic and inorganic phase and minimizes the light scattering and, ultimately, maximizes the light penetration through the depth of the material (Chen et al. 2005). This effect is well-documented for sulfur-containing molecules, due to its highly polarizable nature atom, facilitating light transmission (Bhagat et al. 2012). Interestingly, while the increase in the aliphatic thiourethane concentration was translated into higher degrees of conversion, the increase in the concentration of the aromatic version had the opposite effect. This was somewhat unexpected since, in general, aromatic molecules have strong polarizability anisotropy due to the delocalization of the π electrons (Zhang et al. 2017), and therefore, present higher refractive index, which in turn, was hypothesized to reduce the mismatch between the organic matrix and the inorganic filler, as already discussed. However, as previously demonstrated (Bacchi et al. 2014), the aromatic TU has higher Tg due to the introduction of the rigid aromatic core. This may have decreased the relative mobility of the polymerizing medium, leading to decreased conversion. One additional factor that may explain these results is the possibility that aliphatic polymers may facilitate light propagation due to lower intermolecular and intramolecular charge transfer interactions (Cosutchi et al. 2012). To test this assumption, the light transmission was assessed for 2 mm thick specimens in real-time with polymerization. It is important to note that this was measured in 2 mm specimens, and even at that thickness, the aliphatic HDDI formulations had greater light transmission in comparison with the aromatic BDI composites at the higher TU-silane concentrations (2 and 4%). In fact, as the concentration increased, the discrepancy between aliphatic and aromatic compositions increased in terms of light transmission. This was true from the start of the recording period, and the difference was maintained throughout the experiment (60 s). This helps explain the results found on the degree of conversion mapping (Fig.6), as both data sets follow similar trends in terms of material ranking.
Therefore, it appears that the greater density of the HDDI-based oligomers influenced light transmission to a greater extent than the refractive index mismatch compared to the BDI-based compositions, showing that the increased polymerization in depth obtained with TU oligomers in general is composition-dependent. It is interesting to note that both control groups showed lower light transmission at the end of the experiment than at the beginning, which was not expected based on the increase in refractive index of the organic matrix as the material polymerizes, reducing the mismatch with the filler particles (Howard et al. 2010). One possible explanation is void formation or increase in size of existing porosity with polymerization, which can be speculated to have been greater for the materials presenting higher stress results (control +) or with fillers not covalently attached to the organic matrix (control −). However, shrinkage was not assessed here, and the SEM images do not present evidence to confirm this speculation. This will be further investigated in a separate study.
Conclusion
Novel filler functionalization with thiourethane silanes is a potential alternative for improving dental composites properties by significantly increasing the degree of conversion, fracture toughness and reducing the polymerization stress. This study showed promising results for improving important material properties through localization of the toughening effect imparted by the thiourethane on a critical region of the composite: the filler/matrix interface. Even though benchmarking this material to other commercial examples was not within the scope of this study, future investigations will concentrate on optimizing the overall composition for potential commercialization.
Supplementary Material
Highlights.
Thiourethane-silanes present a concentration-dependent effect on properties, including polymerization stress, fracture toughness and depth of cure.
Signficant stress reduction and fracture toughness enhancement were observed when 4% thiourethane was used in the silanization solution
Depth of cure increased significantly for the aliphatic thiourethane material.
Acknowledgements
The authors thank NIH-NIDCR for financial support (R15-DE023211; U01-DE023756; K02-DE025280). Dr. Parag Shah and Dr. Jeffrey Stansbury at University of Colorado are also acknowledged for the invaluable contribution with the TGA measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Amdjadi P, Ghasemi A, Najafi F, Nojehdehian H. 2017. Pivotal role of filler/matrix interface in dental composites: Review. Biomedical Research (India). 28(3):1054–1065. [Google Scholar]
- ASTM. 1997. E399-90: Standard test method for plane-strain fracture toughness of metallic materials. Philadelphia, PA: American Society for Testing Materials. [Google Scholar]
- Bacchi A, Consani RL, Martim GC, Pfeifer CS. 2015. Thio-urethane oligomers improve the properties of light-cured resin cements. Dental Materials. 31(5):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi A, Dobson A, Ferracane JL, Consani R, Pfeifer CS. 2014. Thio-urethanes improve properties of dual-cured composite cements. Journal of Dental Research. 93(12):1320–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi A, Pfeifer CS. 2016. Rheological and mechanical properties and interfacial stress development of composite cements modified with thio-urethane oligomers. Dental Materials. 32(8):978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi A, Yih JA, Platta J, Knight J, Pfeifer CS. 2018. Shrinkage / stress reduction and mechanical properties improvement in restorative composites formulated with thio-urethane oligomers. Journal of the Mechanical Behavior of Biomedical Materials. 78:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold KA, Hacioglu B, Lovell L, Nie J, Bowman CN. 2001. Using changes in initiation and chain transfer rates to probe the kinetics of cross-linking photopolymerizations: Effects of chain length dependent termination. Macromolecules. 34(15):5103–5111. [Google Scholar]
- Berchtold KA, Lovestead TM, Bowman CN. 2002. Coupling chain length dependent and reaction diffusion controlled termination in the free radical polymerization of multivinyl (meth)acrylates. Macromolecules. 35(21):7968–7975. [Google Scholar]
- Bhagat SD, Chatterjee J, Chen B, Stiegman AE. 2012. High refractive index polymers based on thiol-ene cross-linking using polarizable inorganic/organic monomers. Macromolecules. 45(3):1174–1181. [Google Scholar]
- Chen YC, Ferracane JL, Prahl SA. 2005. A pilot study of a simple photon migration model for predicting depth of cure in dental composite. Dental Materials. 21(11):1075–1086. [DOI] [PubMed] [Google Scholar]
- Condon JR, Ferracane JL. 1998. Reduction of composite contraction stress through non-bonded microfiller particles. Dental Materials. 14(4):256–260. [DOI] [PubMed] [Google Scholar]
- Cosutchi Al, Nica SL, Hulubei C, Homocianu M, loan S. 2012. Effects of the aliphatic/aromatic structure on the miscibility, thermal, optical, and rheological properties of some polyimide blends. Polymer Engineering and Science. 52(7):1429–1439. [Google Scholar]
- Da Rosa Rodolpho PA, Donassollo TA, Cenci MS, Loguercio AD, Moraes RR, Bronkhorst EM, Opdam NJM, Demarco FF. 2011. 22-year clinical evaluation of the performance of two posterior composites with different filler characteristics. Dental Materials. 27(10):955–963. [DOI] [PubMed] [Google Scholar]
- Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJM. 2012. Longevity of posterior composite restorations: Not only a matter of materials. Dental Materials. 28(1):87–101. [DOI] [PubMed] [Google Scholar]
- Faria-e-Silva AL, dos Santos A, Tang A, Girotto EM, Pfeifer CS. 2018. Effect of thio-urethane filler surface functionalization on stress, conversion and mechanical properties of restorative dental composites. Dental Materials. 34(9):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria-e-Silva AL, Pfeifer CS. 2017. Impact of thio-urethane additive and filler type on light-transmission and depth of polymerization of dental composites. Dental Materials. 33(11):1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracane JL, Berge HX. 1995. Fracture toughness of experimental dental composites aged in ethanol. Journal of Dental Research. 74(7):1418–1423. [DOI] [PubMed] [Google Scholar]
- Fu SY, Feng XQ, Lauke B, Mai YW. 2008. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate-polymer composites. Composites Part B: Engineering. 39(6):933–961. [Google Scholar]
- Fugolin AP, Sundfeld D, Ferracane JL, Pfeifer CS. 2019. Toughening of dental composites with thiourethane-modified filler interfaces. Scientific Reports. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib E, Wang R, Wang Y, Zhu M, Zhu XX. 2016. Inorganic fillers for dental resin composites: Present and future. ACS Biomaterials Science and Engineering. 2(1):1–11. [DOI] [PubMed] [Google Scholar]
- Howard B, Wilson ND, Newman SM, Pfeifer CS, Stansbury JW. 2010. Relationships between conversion, temperature and optical properties during composite photopolymerization. Acta Biomaterialia. 6(6):2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CE, Bowman CN. 2010. Thiol-ene click chemistry. Angewandte Chemie - International Edition. 49(9):1540–1573. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Stafford CM. 2010. Effect of interfacial adhesion on viscoelastic relaxation processes in thin polymer film indentation. ACS Applied Materials and Interfaces. 2(7):2108–2115. [Google Scholar]
- Karmaker A, Prasad A, Sarkar NK. 2007. Characterization of adsorbed silane on fillers used in dental composite restoratives and its effect on composite properties. Journal of Materials Science: Materials in Medicine. 18(6):1157–1162. [DOI] [PubMed] [Google Scholar]
- Kubát J, Maurer FHJ, Rigdahl M, Welander M. 1988. Interfacial effects on the ageing behavior of high density polyethylene filled with glass spheres. Colloid & Polymer Science. 266(11):990–998. [Google Scholar]
- Li Q, Zhou H, Wicks DA, Hoyle CE. 2007. Thiourethane-based thiol-ene high t g networks: Preparation, thermal, mechanical, and physical properties. Journal of Polymer Science, Part A: Polymer Chemistry. 45(22):5103–5111. [Google Scholar]
- Marghalani HY. 2010. Effect of filler particles on surface roughness of experimental composite series. Journal of Applied Oral Science. 18(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mese A, Palamara JEA, Bagheri R, Fani M, Burrow MF. 2016. Fracture toughness of seven resin composites evaluated by three methods of mode i fracture toughness (kic). Dental Materials Journal. 35(6):893–899. [DOI] [PubMed] [Google Scholar]
- Moraes RR, Garcia JW, Barros MD, Lewis SH, Pfeifer CS, Liu J, Stansbury JW. 2011. Control of polymerization shrinkage and stress in nanogel-modified monomer and composite materials. Dental Materials. 27(6):509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najeeb S, Khurshid Z, Agwan AS, Zafar MS, Alrahabi M, Qasim SB, Sefat F. 2016. Dental applications of nanodiamonds. Science of Advanced Materials. 8(ll):2064–2070. [Google Scholar]
- Park HY, Kloxin CJ, Fordney MF, Bowman CN. 2012. Stress relaxation of trithiocarbonate-dimethacrylate-based dental composites. Dental Materials. 28(8):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer CS. 2017. Polymer-based direct filling materials. Dental Clinics of North America. 61(4):733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. 2011. Delayed gelation through chain-transfer reactions: Mechanism for stress reduction in methacrylate networks. Polymer. 52(15):3295–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyurt AF, Hoyle CE, Wei H, Piland SG, Gould TE. 2007a. Thermal and mechanical properties of cross-linked photopolymers based on multifunctional thiol-urethane ene monomers. Macromolecules. 40(9):3174–3182. [Google Scholar]
- Senyurt AF, Wei H, Hoyle CE, Piland SG, Gould TE. 2007b. Ternary thiol-ene/acrylate photopolymers: Effect of acrylate structure on mechanical properties? Macromolecules. 40(14):4901–4909. [Google Scholar]
- Sideridou ID, Karabela MM. 2009. Effect of the amount of 3-methacyloxypropyltrimethoxysilane coupling agent on physical properties of dental resin nanocomposites. Dental Materials. 25(11):1315–1324. [DOI] [PubMed] [Google Scholar]
- Söderholm KJ. 1981. Degradation of glass filler in experimental composites. Journal of Dental Research. 60(11):1867–1875. [DOI] [PubMed] [Google Scholar]
- Sowan N, Cox LM, Shah PK, Song HB, Stansbury JW, Bowman CN. 2018. Dynamic covalent chemistry at interfaces: Development of tougher, healable composites through stress relaxation at the resin-silica nanoparticles interface. Advanced Materials Interfaces. 5(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbury JW, Dickens SH. 2001. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dental Materials. 17(1):71–79. [DOI] [PubMed] [Google Scholar]
- Üstündag E, Dragoi D, Clausen B, Brown D, Bourke MAM, Balch DK, Dunand DC. 2000. Internal stresses in bulk metallic glass matrix composites. MRS Proceedings. 644:L9.3. [Google Scholar]
- Zanchi CH, Ogliari FA, Marques e Silva R, Lund RG, Machado HH, Prati C, Carreño NLV, Piva E. 2015. Effect of the silane concentration on the selected properties of an experimental microfilled composite resin. Applied Adhesion Science. 3(1). [Google Scholar]
- Zhang C, Bell D, Harger M, Ren P. 2017. Polarizable multipole-based force field for aromatic molecules and nucleobases. Journal of Chemical Theory and Computation. 13(2):666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


