Abstract
While the most common symptom associated with endometriosis is pelvic pain, the systemic manifestations of the disease and the accompanying adverse psychological, emotional, social, familial, sexual, educational and workplace effects are increasingly recognized. Elagolix is an oral gonadotropin-releasing hormone receptor antagonist that is approved for the management of moderate to severe pain associated with endometriosis. However, the benefits of elagolix extend beyond reducing pain symptoms. This article reviews the non-pain systemic manifestations associated with endometriosis and summarizes the beneficial effects of elagolix on non-pain outcomes. This includes improvements in quality of life, reductions in fatigue and improvements in workplace and household productivity. These results indicate that elagolix provides non-pain benefits in women with endometriosis and improves outcomes that are clinically meaningful to patients.
Keywords: elagolix, endometriosis, fatigue, phamacoeconomics, quality of life
Introduction
Endometriosis is a chronic, debilitating, gynaecological condition affecting approximately 6–10% of women of reproductive age.1,2 The disease is characterized by the presence of endometrial tissue outside the uterine cavity where the implanted cells secrete prostaglandin E2 and multiple cytokines that elicit an inflammatory response.2,3 The most common clinical symptoms/sequelae of endometriosis are chronic pelvic pain (i.e. dysmenorrhoea, non-menstrual pelvic pain, dyspareunia) and infertility.2,3 However, endometriosis is also increasingly recognized as a systemic condition with many non-pain-related manifestations that extend beyond the pelvis and peritoneum into multiple organ systems.4,5 These systemic effects are characterized by altered inflammatory, immunologic and metabolic functions that can produce non-pain-related symptoms such as fatigue, bowel dysfunction, weight loss, allergies and systemic inflammation.4 As reviewed by Alderman and colleagues,4 several comorbidities have been associated with endometriosis, including cardiovascular disease, allergy, autoimmune disease and cancers. Although the mechanism of these non-pelvic systemic manifestations is not well understood, increased circulating inflammatory cytokines, growth factors, micro-RNAs and excreted endometrial cells may be involved.4 The pathogenesis of endometriosis is complex involving multiple processes and is currently an active area of research.6–8 A complete understanding of the pathophysiology of endometriosis may lead to the development of novel therapies for this disabling condition.
The non-pelvic effects of endometriosis result in numerous non-pain outcomes that can impact patients’ lives. Fatigue is a common symptom in patients with endometriosis,9–13 and patients experience many adverse psychological, emotional, social, familial, sexual, educational and workplace effects, all of which can result in decreased health-related quality-of-life (HRQoL).14–19
In addition, endometriosis is associated with a substantial economic burden, including reduced workplace and household productivity.14,20–22 Thus, it is important that endometriosis be considered more than just a disease of the pelvis and that a more holistic approach to disease management should be considered.
Endometriosis-related pelvic pain can be treated with medical therapy or surgery.23–28 First-line medical treatments include non-steroidal anti-inflammatory drugs and hormonal contraceptives.23–26 Over time, women may progress to second-line therapy, which includes progestogens and anti-progestogens, gonadotropin-releasing hormone receptor agonists and antagonists, and aromatase inhibitors.23–26 Because of the complex nature of endometriosis, treatment is often individualized and based on the impact of the disease and the effect of the treatment on HRQoL. Elagolix, an oral gonadotropin-releasing hormone receptor antagonist, was approved in July 2018 by the United States Food and Drug Administration (FDA) for management of moderate to severe pain associated with endometriosis. This article reviews the benefits of this new therapy on non-pain outcomes, including HRQoL, fatigue and productivity in women with moderate to severe endometriosis.
Elagolix in endometriosis
Elagolix is a novel, non-peptide, orally active gonadotropin-releasing hormone receptor antagonist that produces dose-dependent suppression of the pituitary-ovarian axis.16 The safety and efficacy of elagolix in the treatment of endometriosis have been evaluated in four phase 2 trials,16,29–31 two phase 3 trials and two phase 3 extension studies.32,33 The initial phase 2 trials suggested that elagolix was effective for endometriosis-related pain and associated with an adequate safety profile.16,29–31 Based on the positive results obtained in the phase 2 studies, two phase 3 trials (Elaris Endometriosis I and II (EM-I and EM-II)) were conducted to further evaluate the efficacy and safety of elagolix in women with moderate to severe endometriosis-associated pain.33
EM-I (N = 872) and EM-II (N = 817) were of a very similar design, evaluating the effects of two doses of elagolix (150 mg once daily or 200 mg twice daily) versus placebo over 6 months.33 The studies included premenopausal women aged 18 to 49 years with a surgical diagnosis of endometriosis within the previous 10 years who were experiencing moderate to severe endometriosis-related pain.33 Baseline demographic and clinical characteristics of these patients are summarized in Table 1.33 There were two primary efficacy endpoints in these studies: (1) the proportion of women who had clinical response to dysmenorrhoea, and (2) the proportion who had clinical response with respect to non-menstrual pelvic pain at 3 months, as assessed by a reduction in pain scores and decreased/stable use of rescue analgesic agents.33 In both EM-I and EM-II, elagolix treatment was associated with significantly lower scores for dysmenorrhoea and non-menstrual pelvic pain compared with placebo in each dosage group at 3 and 6 months.33 In addition, elagolix (200 mg twice daily group) was also superior to placebo with respect to the use of rescue analgesic use, dyspareunia score and rescue opioid use. The most common adverse events were hot flushes, headache and nausea, with 25–30%, 20–22% and 12–18% of patients in the 150 mg once daily group and 52–55%, 25–29% and 15–25% of patients in the 200 mg twice daily group experiencing these symptoms, respectively.32 The majority of hot flushes were mild to moderate with discontinuation rates for hot flushes ranging from <1% for the lower dose group to <3% for the higher dose.33 Positive efficacy results obtained in the pivotal trials led to the approval of elagolix for the management of moderate to severe pain associated with endometriosis.34
Table 1.
Demographic and clinical characteristics of patients in EM-I and EM-II.33
| Characteristic | EM-I |
EM-II |
||||
|---|---|---|---|---|---|---|
| Placebo (n = 374) | Elagolix 150 mg Once daily (n = 249) |
Elagolix 200 mg Twice daily (n = 248) |
Placebo (n = 360) | Elagolix 150 mg Once daily (n = 226) |
Elagolix 200 mg Twice daily (n = 229) |
|
| Age (years), median (range) | 31 (18–49) | 32 (19–48) | 31 (18–47) | 33 (18–49) | 33 (20–49) | 34 (18–47) |
| Race, % | ||||||
| White | 86 | 89 | 87 | 89 | 88 | 90 |
| Black | 9 | 8 | 10 | 8 | 11 | 8 |
| Othera | 5 | 4 | 4 | 3 | 1 | 2 |
| BMI (kg/m2), mean ± SD | 28 ± 6 | 28 ± 6 | 28 ± 6 | 27 ± 6 | 27 ± 7 | 27 ± 7 |
| Months since diagnosis, mean ± SD | 45 ± 30 | 41 ± 29 | 40 ± 27 | 46 ± 39 | 42 ± 36 | 52 ± 41 |
BMI, body mass index; EM-I, Elaris Endometriosis I; EM-II, Elaris Endometriosis II; SD, standard deviation.
Other includes Asian, multiracial, American Indian or Alaskan native, and native Hawaiian or other Pacific Islander.
Effect of elagolix on non-pain outcomes
In addition to the primary endpoints of dysmenorrhoea and non-menstrual pelvic pain, several non-pain outcomes were evaluated in EM-I and EM-II, either as secondary endpoints or in post hoc analyses. These include elagolix effects on HRQoL and fatigue as well as the economic impact of elagolix therapy, particularly on workplace and household productivity. These effects are reviewed in detail in the following sections.
Health-related quality of life
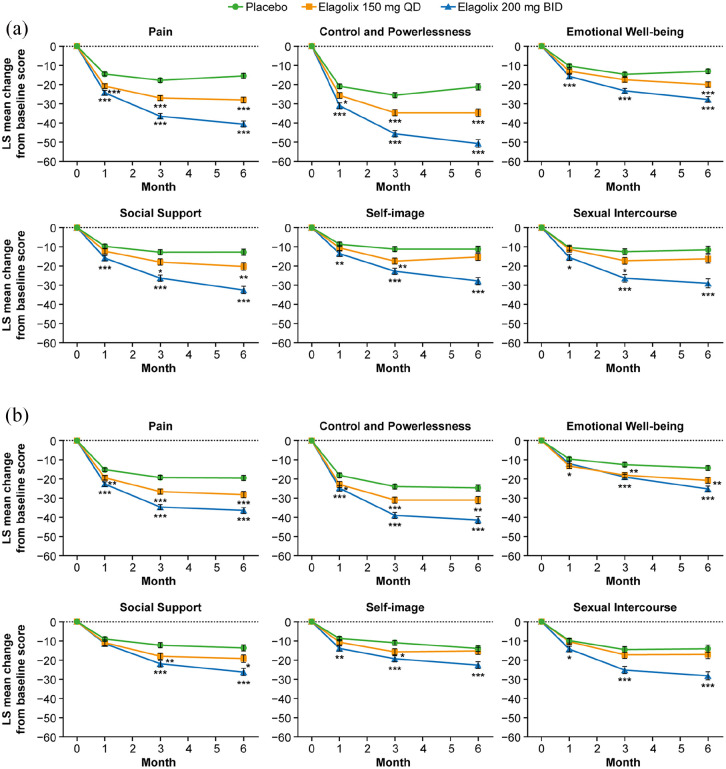
HRQoL was assessed in the EM-I and EM-II trials using the self-administered 30-item Endometriosis Health Profile (EHP-30) with assessments at 1, 3 and 6 months.33 The EHP-30 is an endometriosis-specific instrument that assesses five core domains: pain (11 questions), control and powerlessness (six questions), emotional well-being (six questions), social support (four questions) and self-image (three questions). The EHP-30 is the most extensively validated HRQoL instrument for women with endometriosis.35 A modular questionnaire assessing sexual intercourse (five questions) was also included, making a total of six domains assessing HRQoL. Responses to the EHP-30 and sexual intercourse questionnaires were coded as 0 (never), 1 (rarely), 2 (sometimes), 3 (often) and 4 (always), and were normalized to a scale of 0 (best health) to 100 (worst health) for each domain. Lower scores indicate better HRQoL.33
Baseline EHP-30 scores for patients in EM-I and EM-II are summarized in Table 2.36 While all domains showed impairment, the control and powerlessness and sexual relationship domains showed the greatest impairment. Treatment with elagolix in these studies was associated with dose-dependent improvements in all six domains of the EHP-30 for the elagolix 150 mg once daily and 200 mg twice daily doses versus placebo in both EM-I and EM-II (Figure 1) with significant differences from placebo evident as early as 1 month. For elagolix 150 mg/day, significant differences from placebo were seen at 3 and 6 months in three of six domains in EM-I (pain, control and powerlessness, social support) and in four of six domains in EM-II (pain, control and powerlessness, emotional well-being, social support). Improvements in the elagolix 200 mg twice daily group were significantly greater than for placebo in all six EHP-30 domains at months 1, 3 and 6 in both EM-I and EM-II with one exception (social support at month 1 in EM-II). For all domains, improvements in the elagolix 200 mg twice daily group were progressively greater at each assessment time point (i.e. 1, 3 and 6 months). Furthermore, improvements in EHP-30 scores were greater for the elagolix 200 mg twice daily group than for the lower dose group at all time points.
Table 2.
Mean baseline EHP-30 scores among patients with endometriosis in the EM-I and EM-II trials.36
| EHP-30 domain | EM-I
(N = 871) |
EM-II
(N = 815) |
||
|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |
| Pain | 858 | 58.2 ± 14.3 | 807 | 55.3 ± 16.2 |
| Control and powerlessness | 863 | 69.8 ± 19.4 | 809 | 62.4 ± 23.2 |
| Emotional well-being | 864 | 49.2 ± 19.9 | 810 | 46.2 ± 20.8 |
| Social support | 866 | 54.8 ± 25.6 | 812 | 50.5 ± 25.8 |
| Self-image | 864 | 51.0 ± 27.6 | 811 | 45.6 ± 28.3 |
| Sexual relationship | 668 | 64.5 ± 24.7 | 639 | 58.2 ± 26.1 |
EHP-30, 30-item Endometriosis Health Profile; EM-I, Elaris Endometriosis I; EM-II, Elaris Endometriosis II; SD, standard deviation.
Figure 1.
Mean change from baseline to month 6 in the EHP-30 scores in patients receiving elagolix 150 mg once daily or 200 mg twice daily or placebo in (a) EM-I and (b) EM-II.33 Each domain had multiple questions. Each question was scored from 0 (never) to 4 (always) and normalized to a scale of 0–100 for each domain. Lower scores indicate better quality of life. Statistical significance was based on contrasts within a one-way ANCOVA with treatment as the main effect and baseline value as a covariate. The mean difference from placebo is indicated for two-sided p value; p < 0.05 (*), p < 0.01 (**), p < 0.001 (***). Error bars respresent standard error and month 0 refers to baseline.
Source: From Taylor and colleagues,33 Copyright © 2017 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.33
ANCOVA, analysis of covariance; BID, twice daily; EHP-30, 30-item Endometriosis Health Profile; LS, least squares; QD, once daily.
A post hoc analysis used pooled data from the EM-I and EM-II trials to apply responder definitions for EHP-30 subscales in order to determine whether EHP-30 improvements were clinically meaningful.37 Responders in the various domains of the EHP-30 were defined as follows: a 35-point reduction in control and powerlessness, a 30-point reduction in pain and 30-point reductions in emotional well-being, social support, self-image and sexual intercourse. At month 6, patients receiving elagolix 200 mg twice daily were significantly more likely to meet the EHP-30 definition of response for all six domains compared with placebo with responder rates of 65% versus 24% for pain, 66% versus 28% for control and powerlessness, 62% versus 36% for emotional well-being, 59% versus 33% for social support, 53% versus 30% for self-image and 62% versus 38% for sexual intercourse, respectively, for the elagolix and placebo groups (p < 0.0001 for all comparisons). For patients receiving elagolix 150 mg/day, response rates were somewhat lower than for the higher dose group, but these patients were significantly more likely than placebo-treated patients to meet the definition of responder for all domains except sexual intercourse.
Another post hoc analysis of the EM-I and EM-II trials evaluated the relationship between endometriosis pain symptoms and improvements in HRQoL produced by elagolix, as measured by the EHP-30.38 Patients who were characterized as achieving a clinical response for dysmenorrhoea or non-menstrual pelvic pain were found to achieve improvements in all domains of the EHP-30. Mean changes in EHP-30 domain scores were above the thresholds of clinical meaningfulness among patients who were categorized as being either a dysmenorrhoea responder or a non-menstrual pelvic pain responder at 3 months following treatment initiation. Among dysmenorrhoea responders, mean decreases in EHP-30 domain scores ranged from 24 points (self-image) to 48 points (control and powerlessness) in EM-I and from 21 points (self-image) to 40 points (control and powerlessness) in EM-II. The proportions of dysmenorrhoea responders who met the EHP-30 domain threshold for a clinically meaningful EHP-30 response ranged from 53% (self-image) to 70% (control and powerlessness) in EM-I and from 48% (self-image) to 60% (pain) for patients in EM-II. Similar improvements in EHP-30 domains were also seen among non-menstrual pelvic pain responders. In contrast, patients who did not achieve a pain response (either dysmenorrhoea or non-menstrual pelvic pain) had smaller improvements in EHP-30 domains. The EHP-30 domain response ranged from 19% for the pain domain in EM-I to 37% for the sexual relationship domain for both dysmenorrhoea non-responders and non-menstrual pelvic pain non-responders.36
Non-pain symptoms
Fatigue was measured in the EM-I trial using the Patient-Reported Outcomes Measurement Information System (PROMIS) Fatigue Short Form 6a questionnaire.39 This instrument is composed of six questions related to the severity of fatigue within the last 7 days with patients answering the question as ‘not at all’, ‘a little bit’, ‘somewhat’, ‘quite a bit’ or ‘very much’, with the responses assigned a raw score of 1 through 5, respectively. Raw scores were converted to a standardized T-score with a mean of 50 that represents the average for the US general population.39,40 Higher T-scores indicate worse fatigue.
Among the 860 patients included in the fatigue analysis in EM-I, the mean baseline T-score ranged from 62 to 64 among the three treatment groups.39 More than 54% of respondents reported having fatigue-related issues ‘quite a bit’ or ‘very much’. All three primary symptoms associated with endometriosis (non-menstrual pelvic pain, dyspareunia, dysmenorrhoea) were independently associated with an increased fatigue score at baseline.
Treatment with elagolix was associated with significant dose-dependent improvements in fatigue scores versus placebo in this analysis with improvements evident as early as 1 month and maintained over 6 months (Figure 2). Differences between elagolix groups and placebo continued to increase over time, particularly in the elagolix 200 mg twice daily group. At 1 month, the mean changes from baseline in T-score were −3.27, −4.28 and −5.54, respectively, in the placebo, elagolix 150 mg/day and elagolix 200 mg twice daily groups with the difference versus placebo reaching statistical significance in the elagolix high-dose group (p < 0.001). By month 6, mean changes from baseline increased to −4.53, −6.73 and −10.42, respectively (p = 0.008 and p < 0.001 versus placebo, respectively). The proportion of patients who had responses of ‘quite a bit’ or ‘very much’ in the six individual items on PROMIS decreased from 58–79% to 29–43% at 6 months in the elagolix 150 mg/day group and from 56–79% to 14–29% in the elagolix 200 mg twice daily group. By comparison, the percentage of patients in the placebo group with responses of ‘quite a bit’ or ‘very much’ in the six individual items decreased from 49–68% at baseline to 35–50% at 6 months.39 The reductions in fatigue scores were maintained through 12 months. There was a strong relationship between the achievement of clinical responses and fatigue improvement in patients who achieved a clinically meaningful response in dysmenorrhoea and non-menstrual pelvic pain compared with non-responders.39
Figure 2.
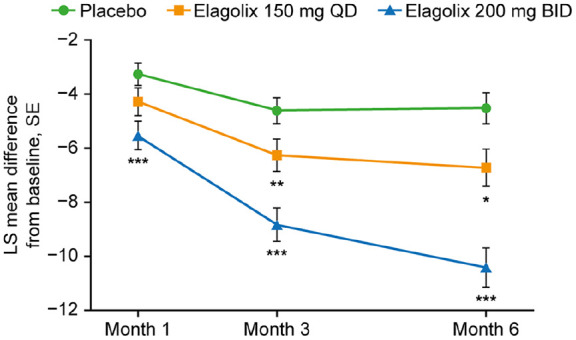
Mean change from baseline to month 6 in the PROMIS Fatigue Questionnaire T-scores in patients receiving elagolix 150 mg once daily or 200 mg twice daily or placebo in EM-I.39 N values (placebo/elagolix 150 mg once daily/elagolix 200 mg twice daily): month 1: 348/224/219; month 3: 314/216/198; month 6: 246/170/161. The mean difference from placebo is indicated for p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
Source: Reprinted from Surrey and colleagues,39 Copyright © 2019, with permission from Elsevier.
BID, twice daily; LS, least squares; PROMIS, Patient-Reported Outcomes Measurement Information System, QD, once daily; SE, standard error.
Long-term outcomes with elagolix
EM-II and EM-IV (Elaris Endometriosis IV) were extension studies32 that enrolled women who completed the EM-I or EM-II studies. Women were excluded if after the first 6 months of elagolix treatment in EM-I/EM-II they had a bone mineral density decrease from baseline of 8% or greater in the spine, femoral neck or total hip or if they had a clinically significant condition detected in EM-I or EM-II. EM-III (Elaris Endometriosis III) and EM-IV consisted of two periods: a 6-month treatment period and a post-treatment follow-up period of up to 12 months. Women who enrolled in the extension studies received the same elagolix dose that was taken in EM-I and EM-II for an additional 6 months. Of 952 women who initiated treatment with elagolix in EM-I and EM-II,33 569 participated in the extension studies. Patients discontinued the original studies for a variety of reasons, including adverse events, non-compliance and withdrawal of consent, and the percentage of patients who discontinued for each reason was similar across the placebo and elagolix groups.33 Treatment with elagolix for 12 months resulted in sustained reductions in dysmenorrhoea, non-menstrual pelvic pain and dyspareunia.32
Long-term elagolix treatment also had anticipated changes as a result of decreased oestradiol levels, including hot flushes, reduced bone mineral density and increased lipid levels.32 The incidence of hot flush over 12 months of treatment was 30–55% (across EM-III/EM-IV and both doses). Bone mineral density loss was dose dependent and increased with duration of treatment. After 6 months of treatment, 2–4%, 16–21% and 1–2% of patients experienced more than 5% loss in bone mineral density in the 150 mg once daily, 200 mg twice daily and placebo groups, respectively.33 In the extension studies, 1 patient in the 150 mg once daily group and 10 patients in the 200 mg twice daily group discontinued treatment (as required by the study protocol) because they experienced a greater than 8% decrease in bone mineral density from baseline.32 The observed decreases in bone mineral density may not be completely reversible, and the impact of these losses on long-term bone health and risk of future fractures is unknown.34 Phase 3 trials (NCT03213457, NCT03343067) are currently being conducted to assess the efficacy and safety of elagolix in combination with low-dose, add-back hormonal therapy in women with endometriosis-related pain.
From baseline to 6 months (EM-I and EM-II), significantly greater increases in total cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides were observed in both elagolix dose groups compared with placebo.33 No further increases in lipid levels were observed during the extension studies (EM-III and EM-IV), and the lipid levels returned to pretreatment levels within the first month of the post-treatment follow-up period.32 The impact of these changes in lipid levels on long-term cardiovascular risk is unknown.
The FDA prescribing information instructs physicians to counsel patients on signs and symptoms of liver injury, advise patients to seek immediate medical attention if they experience depression, warn patients that use of elagolix may reduce their ability to recognize pregnancy and use the lowest effective dose of elagolix, considering the severity of symptoms and treatment objectives of individual patients.34
Economic impacts
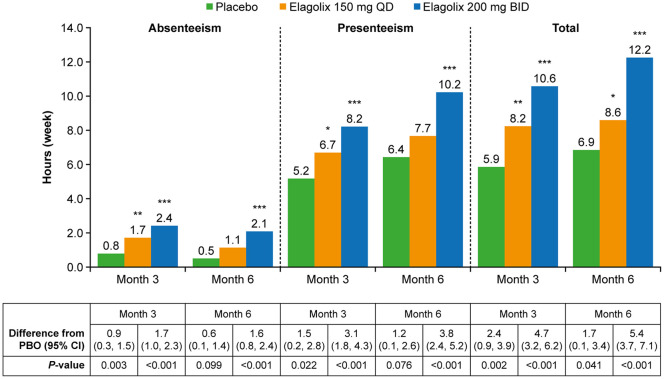
Workplace and household productivity in the populations of the EM-I and EM-II trials were assessed in a post hoc analysis.41,42 The analysis of workplace productivity included 1270 women who were employed full- or part-time, while the household analysis included 1565 women. Productivity was assessed using the Health-Related Productivity Questionnaire, a nine-item instrument that assesses the ability to perform employment- and household-related activities. Captured outcomes included absenteeism (work time missed) and presenteeism (reduced work effectiveness) due to endometriosis in the workplace and household.43 The changes from baseline over 6 months in workplace and household productivity are illustrated in Figures 3 and 4, respectively.42 Both doses of elagolix were associated with significant improvements in productive workplace hours, but the effect was greatest for the 200 mg twice daily group. Relative to placebo, women treated with elagolix 150 mg/day gained 2.4 productive workplace hours per week at 3 months (p = 0.002) and 1.7 workplace hours per week at 6 months (p = 0.041). Even greater improvements in workplace productivity versus placebo were seen among women receiving elagolix 200 mg twice daily with gains of 4.7 hours per week at 3 months (p < 0.001) and 5.4 hours per week at 6 months (p < 0.001). Reduced presenteeism accounted for approximately two-thirds of the gains relative to placebo. Elagolix 150 mg/day and 200 mg twice daily were also associated with significantly greater gains than placebo for scheduled employment-based hours actually worked with increases of 6.6% and 11.6%, respectively, at 3 months and 5.2% and 14.6%, respectively, at 6 months relative to placebo.
Figure 3.
Gains in productive workplace hours per week over time in pooled data from EM-I and EM-II.42 Mean hours gained in workplace productivity, defined as −1 × LS mean change from baseline in hours of workplace productivity lost due to absenteeism, presenteeism and total hours lost (absenteeism + presenteeism). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
Source: With kind permission from Springer Science + Business Media: Surrey and colleagues,42 Copyright © 2019, Springer Nature.
BID, twice daily; CI, confidence interval; EM-I, Elaris Endometriosis I; EM-II, Elaris Endometriosis II; LS, least squares; PBO, placebo; QD, once daily.
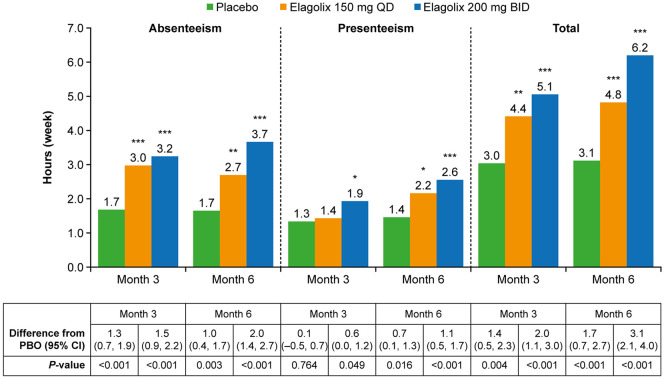
Figure 4.
Gains in household productivity per week over time in pooled data from EM-I and EM-II.42 Mean hours gained in household productivity, defined as −1 × LS mean change from baseline in hours of household productivity lost due to absenteeism, presenteeism and total hours lost (absenteeism + presenteeism). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
Source: With kind permission from Springer Science + Business Media: Surrey and colleagues,42 Copyright © 2019, Springer Nature.
BID, twice daily; CI, confidence interval; EM-I, Elaris Endometriosis I; EM-II, Elaris Endometriosis II; LS, least squares; PBO, placebo; QD, once daily.
Household productivity was also significantly increased in both elagolix dosage groups at months 3 and 6. Gains of 1.4 and 2.0 hours per week, respectively, were reported for the elagolix 150 mg/day and 200 mg twice daily groups at 3 months relative to placebo (p = 0.004 and p < 0.001, respectively). These gains in productive household hours increased to 1.7 and 3.1 hours per week at 6 months in the two elagolix dosage groups (p < 0.001 for both).
Cost savings associated with the improvements in absenteeism and presenteeism were calculated by multiplying the number of hours gained with treatment by the average hourly employer cost in the United States in 2018.41 For the 150 mg/day dose, the average cost savings were >US$1500 at 6 months, >US$3100 after 12 months of treatment and >US$6200 for 24 months of treatment. For the 200 mg twice daily dose, the cost savings were >US$3300 over 6 months of treatment.41
In another study, a Markov model was developed using data from the EM-I and EM-II clinical trials to assess the cost-effectiveness of elagolix versus leuprolide acetate for the management of moderate to severe endometriosis pain over 1- and 2-year time frames.44 Both doses of elagolix were associated with a positive net monetary benefit (NMB; i.e. more quality-adjusted life years for less costs) relative to leuprolide acetate over both 1- and 2-year time frames. Over a 1-year horizon, elagolix 150 mg/day was associated with an NMB of US$5660 while elagolix 200 mg twice daily was associated with an NMB of US$6443. Over 2 years, both doses were also associated with a positive NMB relative to leuprolide acetate (US$2374 and US$1342, respectively).44 The results were robust to sensitivity analyses.
Key messages
Pain is the most common manifestation of endometriosis; however, the disease is complex with multiple systemic manifestations.4,5 As noted previously, endometriosis is associated with several comorbidities such as autoimmune disease, allergy, cancers and cardiovascular disease, suggesting that the disease affects inflammatory, immunologic and metabolic functions.4 Given that endometriosis has such widespread effects, a selective focus on pain-related outcomes will underestimate the full disease burden on women with endometriosis. A more complete assessment of disease burden should include non-pain symptoms as well as the impact on HRQoL and economic outcomes. Indeed, the importance of patient-reported outcomes such as the EHP-30 is increasingly recognized and included in interventional clinical trials in endometriosis.35
Numerous studies have demonstrated that endometriosis is associated with negative physical, psychological, emotional, social, familial, sexual and educational workplace effects that adversely impact HRQoL.14,15,17–19,21,45,46 In particular, women with endometriosis often report experiencing a sense of powerlessness, a loss of control over one’s life and increases in anxiety and depression.18,45–48 The EHP-30 is a reliable and valid HRQoL instrument that was designed as a disease-specific tool for the evaluation of HRQoL in women with endometriosis47,49 and has been shown to perform well in the assessment of HRQoL of endometriosis.35 A meta-analysis of studies assessing the impact of endometriosis found that the disease has a moderate to high negative effect on all core domains of the EHP-30 with the domains of control and powerlessness, emotional well-being and social support having the most negative effects on HRQoL.50
An analysis of data from the EM-I and EM-II trials showed that elagolix was associated with dose-dependent improvements in all six domains of the EHP-30 with significant differences from placebo evident as early as 1 month.33 This indicates that elagolix produces improvements in all aspects of HRQoL that are clinically meaningful to patients. A detailed analysis of HRQoL data from EM-I and EM-II allowed a determination of EHP-30 thresholds for clinically meaningful change.38 Using these thresholds, it was shown that elagolix-treated patients who demonstrated a clinical response in dysmenorrhoea or non-menstrual pelvic pain also achieved clinically meaningful improvements in all EHP-30 domains.36 This suggests that the EHP-30 may be useful in the clinic, providing a way to evaluate and monitor outcomes and treatment progress in areas that are important to individual patients. Such a focus on patient-centred outcomes is likely to be well received by patients and can allow clinicians to tailor treatment toward achieving each individual’s goals.38
Fatigue is a well-recognized symptom of endometriosis that is associated with significant physical, psychological and HRQoL impacts.9,11,13 In the EM-I and EM-II trials, fatigue was commonly reported at baseline, with 17% of patients having a severe level of fatigue and 57% having a moderate level of fatigue at baseline.39 When patients were asked how fatigued they were, how bothersome the fatigue was and how rundown they felt, more than 60% of patients responded ‘quite a bit’ or ‘very much’. Fatigue was associated with a difficulty in initiating activities and an interference in physical functioning in more than half of patients.39 Data from the EM-I trial demonstrated that fatigue was improved with elagolix, beginning as early as 1 month and maintained throughout the 6-month study period.39 The benefit was dose dependent with improvements greatest for the elagolix twice daily regimen although both doses produced significantly greater improvements versus placebo. Given the high prevalence of fatigue in the women with endometriosis, these benefits are likely to be clinically important.
Finally, the economic burden of endometriosis is also substantial. It has been estimated that the annual costs associated with endometriosis are similar to that of diabetes mellitus, Crohn’s disease and rheumatoid arthritis.21 The lost work and reduced productivity associated with endometriosis is a very important concern for women with endometriosis.14,45 For example, a survey of women with endometriosis found that work productivity was extremely affected in 48% of respondents.39 Pelvic pain and disease severity appear to be the major factors that drive losses of productivity.14,43
Results from EM-I and EM-II demonstrated that elagolix 150 mg/day and 200 mg twice daily were associated with significant improvements in workplace and household productivity relative to placebo after 3 and 6 months of treatment.42 The estimated economic benefits of these improvements in productivity were substantial, with cost savings ranging from >US$1500 to >US$3300 over 6 months of treatment.41 Furthermore, a cost-effectiveness analysis found that elagolix was dominant over leuprolide acetate in the treatment of moderate to severe endometriosis (i.e. clinically superior and cost saving). These economic benefits were robust to extensive sensitivity analyses.
Given the systemic nature of endometriosis and the negative effect that endometriosis-related symptoms have on physical, psychological, emotional and social well-being; familial and sexual relationships; and workplace productivity, it seems reasonable to take a holistic approach to relieve the burden of endometriosis. Such an approach would include not only pain management but also emotional and social support, and services to assist with work issues. Future clinical trials evaluating endometriosis treatments need to include outcomes that assess the effect of treatment regimens on fatigue, depression, anxiety, emotional distress and various aspects of HRQoL in order to address the individual needs of women suffering from endometriosis.
Conclusion
In summary, it is increasingly recognized that the clinical impact of endometriosis extends beyond pelvic pain with multiple systemic effects that can produce non-pain symptoms and adversely impact psychological and social function, workplace and household productivity, and ultimately HRQoL. Thus, it is important to assess the effect of treatment regimens on these non-pain outcomes that are very meaningful to the lives of women with endometriosis. Elagolix is a gonadotropin-releasing hormone receptor antagonist that is not only effective for the treatment of dysmenorrhoea and non-menstrual pelvic pain but also positively impacts HRQoL, reduces fatigue and improves work/household productivity.
Acknowledgments
Medical writing assistance was provided by Bret Fulton (JK Associates, Inc., member of Fishawack Health, Conshohocken, PA) and was funded by AbbVie, Inc., North Chicago, IL.
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.F.A. is a consultant to AbbVie, Agile Therapeutics, Exeltis, National Institute of Child Health and Human Development (NICHD), ObsEva and TherapeuticsMD, and receives grants/has contracts for clinical research from AbbVie, NICHD, Myovant, ObsEva and TherapeuticsMD. A.M.S. is an employee of AbbVie, Inc., and has stock/stock options. S.K.A. has served as a consultant for AbbVie and has received research support from AbbVie and DOBI. H.S.T. receives no personal funds from Pharma. Yale University received a grant to support his research from AbbVie.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The elagolix trials, which are summarized in this article, were funded by AbbVie, Inc. AbbVie sponsored the trials; contributed to the design; participated in collection, analysis and interpretation of data; and in writing, reviewing and approval of the final version of this review.
ORCID iD: Sanjay K. Agarwal  https://orcid.org/0000-0002-8046-9807
https://orcid.org/0000-0002-8046-9807
Contributor Information
David F. Archer, CONRAD Clinical Research Center, Department of Obstetrics and Gynecology, Eastern Virginia Medical School, 601 Colley Avenue, Suite 241, Norfolk, VA 23507, USA.
Ahmed M. Soliman, Health Economics and Outcomes Research, AbbVie, Inc., North Chicago, IL, USA
Sanjay K. Agarwal, Center for Endometriosis Research and Treatment, University of California, San Diego, CA, USA
Hugh S. Taylor, Department of Obstetrics and Gynecology and Reproductive Sciences, Yale School of Medicine, New Haven, CT, USA
References
- 1. Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Invest 2017; 82: 453–461. [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010; 362: 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bulun SE. Endometriosis. N Engl J Med 2009; 360: 268–279. [DOI] [PubMed] [Google Scholar]
- 4. Alderman MH, 3rd, Yoder N, Taylor HS. The systemic effects of endometriosis. Semin Reprod Med 2017; 35: 263–270. [DOI] [PubMed] [Google Scholar]
- 5. Taylor HS. Endometriosis: a complex systemic disease with multiple manifestations. Fertil Steril 2019; 112: 235–236. [DOI] [PubMed] [Google Scholar]
- 6. Laganà AS, Garzon S, Götte M, et al. The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci 2019; 20: 5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laganà AS, Vitale SG, Salmeri FM, et al. Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses 2017; 103: 10–20. [DOI] [PubMed] [Google Scholar]
- 8. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012; 98: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen KE, Kesmodel US, Baldursson EB. et al. The influence of endometriosis-related symptoms on work life and work ability: a study of Danish endometriosis patients in employment. Eur J Obstet Gynecol Reprod Biol 2013; 169: 331–339. [DOI] [PubMed] [Google Scholar]
- 10. Lemaire GS. More than just menstrual cramps: symptoms and uncertainty among women with endometriosis. J Obstet Gynecol Neonatal Nurs 2004; 33: 71–79. [DOI] [PubMed] [Google Scholar]
- 11. Ramin-Wright A, Schwartz ASK, Geraedts K, et al. Fatigue – a symptom in endometriosis. Hum Reprod 2018; 33: 1459–1465. [DOI] [PubMed] [Google Scholar]
- 12. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod 2002; 17: 2715–2724. [DOI] [PubMed] [Google Scholar]
- 13. Touboul C, Amate P, Ballester M, et al. Quality of life assessment using EuroQOL EQ-5D questionnaire in patients with deep infiltrating endometriosis: the relation with symptoms and locations. Int J Chronic Dis 2013; 2013: 452134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011; 96: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vercellini P, Meana M, Hummelshoj L, et al. Priorities for endometriosis research: a proposed focus on deep dyspareunia. Reprod Sci 2011; 18: 114–118. [DOI] [PubMed] [Google Scholar]
- 16. Acs N, O’Brien C, Jiang P, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist: results from a Phase II, randomized controlled study. J Endometr Pelvic Pain Disord 2015; 7: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod 2013; 28: 2677–2685. [DOI] [PubMed] [Google Scholar]
- 18. Facchin F, Barbara G, Saita E, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol 2015; 36: 135–141. [DOI] [PubMed] [Google Scholar]
- 19. Gao X, Yeh YC, Outley J, et al. Health-related quality of life burden of women with endometriosis: a literature review. Curr Med Res Opin 2006; 22: 1787–1797. [DOI] [PubMed] [Google Scholar]
- 20. Gao X, Outley J, Botteman M, et al. Economic burden of endometriosis. Fertil Steril 2006; 86: 1561–1572. [DOI] [PubMed] [Google Scholar]
- 21. Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012; 27: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 22. Soliman AM, Yang H, Du EX, et al. The direct and indirect costs associated with endometriosis: a systematic literature review. Hum Reprod 2016; 31: 712–722. [DOI] [PubMed] [Google Scholar]
- 23. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014; 29: 400–412. [DOI] [PubMed] [Google Scholar]
- 24. Practice bulletin no. 114: management of endometriosis. Obstet Gynecol 2010; 116: 223–236. [DOI] [PubMed] [Google Scholar]
- 25. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril 2014; 101: 927–935. [DOI] [PubMed] [Google Scholar]
- 26. Ferrero S, Remorgida V, Venturini PL. Current pharmacotherapy for endometriosis. Expert Opin Pharmacother 2010; 11: 1123–1134. [DOI] [PubMed] [Google Scholar]
- 27. Raffaelli R, Garzon S, Baggio S, et al. Mesenteric vascular and nerve sparing surgery in laparoscopic segmental intestinal resection for deep infiltrating endometriosis. Eur J Obstet Gynecol Reprod Biol 2018; 231: 214–219. [DOI] [PubMed] [Google Scholar]
- 28. Laganà AS, Vitale SG, Trovato MA, et al. Full-thickness excision versus shaving by laparoscopy for intestinal deep infiltrating endometriosis: rationale and potential treatment options. Biomed Res Int 2016; 2016: 3617179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carr B, Dmowski WP, O’Brien C, et al. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: effects on bone mineral density. Reprod Sci 2014; 21: 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carr B, Giudice L, Dmowski WP, et al. Elagolix, an oral GnRH antagonist for endometriosis-associated pain: a randomized controlled study. J Endometr Pelvic Pain Disord 2013; 5: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diamond MP, Carr B, Dmowski WP, et al. Elagolix treatment for endometriosis-associated pain: results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod Sci 2014; 21: 363–371. [DOI] [PubMed] [Google Scholar]
- 32. Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol 2018; 132: 147–160. [DOI] [PubMed] [Google Scholar]
- 33. Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med 2017; 377: 28–40. [DOI] [PubMed] [Google Scholar]
- 34. Orlissa (Elagolix). Prescribing information. North Chicago, IL: AbbVie, Inc, 2019. [Google Scholar]
- 35. Bourdel N, Chauvet P, Billone V, et al. Systematic review of quality of life measures in patients with endometriosis. PLoS ONE 2019; 14: e0208464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pokrzywinski RM, Soliman AM, Chen J, et al. Achieving clinically meaningful response in endometriosis pain symptoms is associated with improvements in health-related quality of life and work productivity: analysis of 2 phase III clinical trials. Am J Obstet Gynecol 2020; 222: 592.e1–592.e10. [DOI] [PubMed] [Google Scholar]
- 37. Taylor HS, Soliman AM, Johns B, et al. Clinically meaningful health-related quality of life improvements with elagolix in endometriosis. Obstet Gynecol 2020; 136: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pokrzywinski R, Soliman AM, Chen J, et al. Responsiveness evaluation and recommendation for responder thresholds for Endometriosis Health Profile-30: analysis of two phase III clinical trials. J Womens Health 2020; 29: 253–261. [DOI] [PubMed] [Google Scholar]
- 39. Surrey ES, Soliman AM, Agarwal SK, et al. Impact of elagolix treatment on fatigue experienced by women with moderate to severe pain associated with endometriosis. Fertil Steril 2019; 112: 298–304. [DOI] [PubMed] [Google Scholar]
- 40. Healthmeasure. A brief guide to the PROMIS fatigue instruments, http://www.healthmeasures.net/administrator/components/com_instruments/uploads/PROMIS%20Fatigue%20Scoring%20Manual%2011042016.pdf (2019, accessed 19 February 2020).
- 41. Pokrzywinski RM, Soliman AM, Chen J, et al. Impact of elagolix on work loss due to endometriosis-associated pain: estimates based on the results of two phase III clinical trials. Fertil Steril 2019; 112: 545–551. [DOI] [PubMed] [Google Scholar]
- 42. Surrey ES, Soliman AM, Palac HL, et al. Impact of elagolix on workplace and household productivity among women with moderate to severe pain associated with endometriosis: a pooled analysis of two phase III trials. Patient 2019; 12: 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soliman AM, Coyne KS, Gries KS, et al. The effect of endometriosis symptoms on absenteeism and presenteeism in the workplace and at home. J Manag Care Spec Pharm 2017; 23: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang S-T, Johnson SJ, Mitchell D, et al. Cost-effectiveness of elagolix versus leuprolide acetate for treating moderate-to-severe endometriosis pain in the USA. J Comp Eff Res 2019; 8: 337–355. [DOI] [PubMed] [Google Scholar]
- 45. Fourquet J, Baez L, Figueroa M, et al. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril 2011; 96: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vitale SG, La Rosa VL, Rapisarda AMC, et al. Impact of endometriosis on quality of life and psychological well-being. J Psychosom Obstet Gynaecol 2017; 38: 317–319. [DOI] [PubMed] [Google Scholar]
- 47. Jones G, Kennedy S, Barnard A, et al. Development of an endometriosis quality-of-life instrument: the Endometriosis Health Profile-30. Obstet Gynecol 2001; 98: 258–264. [DOI] [PubMed] [Google Scholar]
- 48. Laganà AS, La Rosa VL, Rapisarda AMC, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health 2017; 9: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones G, Jenkinson C, Taylor N, et al. Measuring quality of life in women with endometriosis: tests of data quality, score reliability, response rate and scaling assumptions of the Endometriosis Health Profile Questionnaire. Hum Reprod 2006; 21: 2686–2693. [DOI] [PubMed] [Google Scholar]
- 50. Chaman-Ara K, Bahrami MA, Moosazadeh M, et al. Quality of life in women with endometriosis: a systematic review and meta-analysis. World Cancer Res J 2017; 4: e839. [Google Scholar]