Abstract
Aims:
The aim of the study was to evaluate the efficacy of endoscopic rhizotomy (ER) for denervation of lumbar facet joints in patients with chronic low back pain (LBP) due to facet joint syndrome (FJS).
Methods:
A total of 50 consecutive patients suffering from chronic LBP due to facet joints were screened to be treated with ER. The patients participating in the study had a 2-year follow up. Numeric Rating Scale (NRS) and Oswestry Disability Index (ODI) were assessed in the preoperative and postoperative period. To evaluate secondary endpoints, patients were divided into groups. One group included the patients previously treated with percutaneous radiofrequency (RF). The other group comprised patients at their first interventional treatment. We also compared patients dividing them by age and by number of joints treated, trying to elucidate if these parameters could be predictive of effectiveness of the procedure.
Results:
All patients had a reduction in NRS and an improvement in ODI. NRS was reduced significantly after 1 month and remained the same until the end of the study. ODI was significantly improved from T1 (1 month after surgery) up to T7 (end of the study). The improvements did not differ whether already treated with percutaneous rhizotomy or not. Patients less than 60 years or with 1–2 joints treated had better improvement compared with the others.
Conclusion:
The results obtained demonstrate that ER for denervation of the facet joint is an effective treatment in patients with chronic LBP, with consistent and stable results at 2-year follow up. The technique has a rapid learning curve and no major complications occurred. Moreover, the previous percutaneous RF treatment had no influence on the results obtained with endoscopic technique. There is evidence that best results are obtained in younger patients and/or in patients with 1–2 joints treated.
Lay summary
Low-back pain has facet joints inflammation or degeneration as pain generator in 20–40% of cases. Nervous lesion of the dorsal ramus innervating the facet joints has been shown as an efficacious treatment to obtain good analgesia. Percutaneous techniques have provided short term results for several reasons. This research aimed to see whether endoscopic denervation, which guarantees a more precise approach to anatomical structure, would result in more durable results. The study conducted on 40 patients has made it clear that this approach gives significant analgesia for at least 2 years, which was the time of patient follow up.
Keywords: endoscopic radiofrequency, facet joint syndrome, low back pain, medial branch radiofrequency, radiofrequency neurolesion
Introduction
Chronic low back pain (CLBP) represents one of the most important and widespread pathological conditions. Many anatomical structures are involved in generating pain in the spine, such as ligaments, fascia, muscles, nerve root, dura, intervertebral discs, muscles, and sacroiliac joints. CLBP has a prevalence of 3–10% in the general population and is associated with a great number of co-morbidities, such as depression, immobilization, and inability to work, all of which has a great impact on health costs.1–4
The incidence of CLBP for different reasons, e.g. chronic neuroinflammatory processes,5 increases with age. Hence, due to the increasing number of elderly people, the prevalence of this pathology is increasing.6 These patients frequently do not have easy access to specialist pain management.7 Also, following the most advanced guidelines, pharmacological treatment is not easy.8 In fact, besides the usual pharmacological approach with non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, many authors have suggested different add-on therapies,9,10 or even neuro-behavioral and biopsychosocial rehabilitation approaches.11,12
Inflammatory or degenerative processes affecting lumbar facet (zygapophyseal) joints (FJs) are a frequent reason for CLBP. FJ inflammation is responsible for 27–40% of all cases of CLBP.13 Peri-articular or intra-articular infiltration of steroids and local anesthetics is a common widespread therapeutic practice. In general, this treatment provides short-term pain relief, but does not guarantee long-term benefits.14,15 The same technique is used commonly for diagnostic block to determine if the facet is the true pain generator. A positive diagnostic block is suggested as a significant indicator to have good results both with percutaneous and/or endoscopic rhizotomy.16–18
In recent years, percutaneous denervation of the FJ has been suggested as the “gold standard” in treatment of this painful condition.19 A recent meta-analysis suggests that radiofrequency (RF) has good efficacy at 12 months in reducing low back pain (LBP) due to FJ inflammation, and that these data are related strictly to previous diagnostic block with local anesthetics, which has a statistically relevant impact on the outcome of these patients.20
Endoscopic rhizotomy (ER) is an evolution of percutaneous needle RF ablation of the dorsal ramus.21 This technique has the same target, but it guarantees a direct visualization of anatomical structures. Also, it allows easy nerve detection and stable and long-lasting pain relief due to the more complete and wide denervation of the branch. Endoscopic neurotomy associated with rhizotomy has the advantage of direct visualization of anatomical structures such as nerve root and articular capsule. It allows a more accurate neurotomy and, if necessary, capsulotomy. Only a few clinical trials have studied the efficacy of endoscopic approaches to zygapophysial lumbar joint and other nervous structures; all report a good efficacy of the technique with long-term pain relief and few side effects.22–24
This study aimed to establish the long-term (2 years) efficacy of endoscopic dorsal ramous ablation in patients with LBP due to chronic FJ inflammation. As a secondary endpoint, we compared the different results between patients that were or were not previously treated with percutaneous RF. Moreover, the influence of patient age and number of denervated facets was studied.
Materials and methods
This was a prospective cohort study, including the first 50 patients screened for dorsal ramus rhizotomy in the period between 1 January 2017 and 1 January 2018. The clinical trial was conducted in a single Pain Center. The protocol was approved by the Institutional Ethics Committee. All patients screened for the study were informed exhaustively on the surgical procedure and on the whole study. All signed written consent, both for the procedure and for publication of data. They were also instructed on the use of the Numeric Rating Scale (NRS) and Oswestry Disability Index (ODI).
Inclusion criteria were CLBP, with a NRS score at the beginning of the study ⩾7; failure of conservative treatments, e.g., pharmacological or physiotherapeutic, for at least 2 months; positivity to diagnostic FJ block performed with lidocaine 2% 1.5 ml every painful level, with reduction of NRS > 50% after 1 h and lasting for at least 3 h.
Exclusion criteria were major depression, use of anticoagulants, uncontrolled hepatic, cardiovascular, hematologic, renal diseases or other chronic severe comorbid diseases. Complex regional pain syndromes (CRPS), drug addiction, previous lumbar spine surgery were also excluding criteria. Patients with definite lumbar instability prompting surgical stabilization, prominent radiating leg pain, history of concomitant scoliosis of more than 15 degrees, sagittal misalignment requiring surgery, metabolic bone disease, vertebral fractures, or tumors were also excluded.
Patients were submitted to the NRS and ODI tests at admission (T0). They were divided into two groups depending if they had (A) or had not (B) received a previous treatment with percutaneous RF. Patients were also divided by age (<60 years old, ⩾60 years), considering the reported change of incidence of LBP at this age limit.25 Moreover, they were grouped based on the number of treated joints (1–2 joints, ⩾3 joints).
Postoperatively, patients were mobilized after 6 h without restrictions. Analgesic medicines were prescribed for 48 h (paracetamol or paracetamol + codeine), as rescue medications, if necessary. Patients were encouraged to strengthen their spinal and abdominal muscles, and, if necessary, to lose weight, in order to prevent further progression of degenerative processes at the lumbar spine.
Surgery
All procedures were performed in the operating room, with the support of fluoroscopy. Preoperatively, patients received intravenous (i.v.) midazolam (1–2 mg). They also had an infiltration of local anesthetics (5 ml of lidocaine 1% + 5 ml ropivacaine 0.75% for each level treated). Sedation was obtained using propofol 1 mg/kg/h i.v., if necessary. The target was identified under C-Arm.
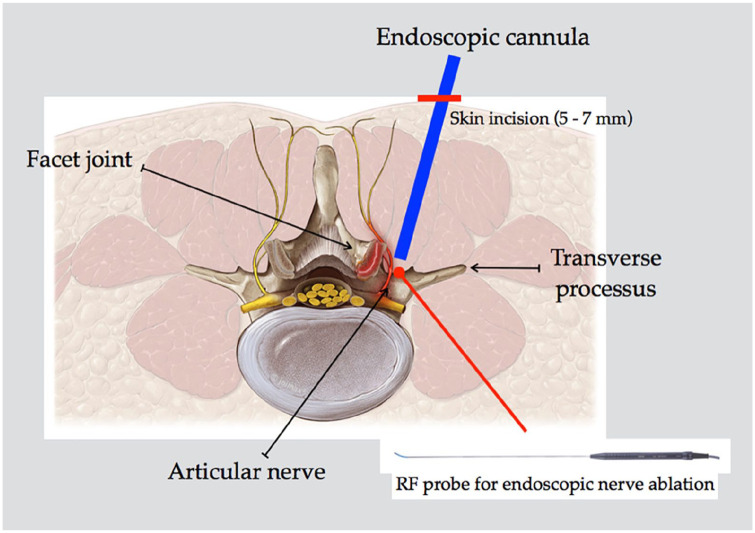
Procedure
FJs are innervated by the medial branch of dorsal ramous at the same level and one level above it. Therefore, to successfully treat pain arising from a FJ, the medial branch one level above the target shall to be ablated as well. Dorsal ramous also gives off lateral branch and sometimes the intermediate branch, and while they do not primarily innervate the FJs, they provide iliolumbar musculature and cutaneous innervation, and may contribute to generating back pain. The target point for ablation is the junction of the transverse process and the base of the superior articular process (SAP) (Figure 1). With C-arm in oblique position to check needle trajectory and position, an 18 G 15 cm spinal needle was inserted through the sterilized skin and docked onto the target point, represented by the medial part of transverse process on the painful level. A guidewire was placed in the needle, a 5-mm incision was made beside the needle, and the needle was removed. Sequential dilators were placed over the guidewire until the final beveled working cannula (7.9 mm diameter) was placed. The Joimax working channel endoscope was placed in the working cannula, and its position was confirmed by anteroposterior (AP) and lateral fluoroscopy. Under endoscopic visualization, keeping the longer part of the scope in contact with transverse process of the vertebra, the dorsal ramous was identified under the mammillary-transverse ligament (Figure 2). The Joimax Legato monopolar or Vaporflex (Joimax, Karlsruhe, Germany) bipolar RF probes were then introduced through the working channel endoscope and used to ablate the nerve and coagulate small vessels, if necessary. Saline solution was used as irrigation. The end point of the procedure was considered when the dorsal ramous was no longer detectable under endoscopic view. Upon extraction of the endoscope, the skin was sutured. Surgical sutures were removed 10 days after surgery.
Figure 1.
Schematic representation of endoscopic approach to dorsal ramus. Of note, the nerve shall be ablated when lying on the medial part of transverse process before reaching articular joint.
RF, radiofrequency.
Figure 2.
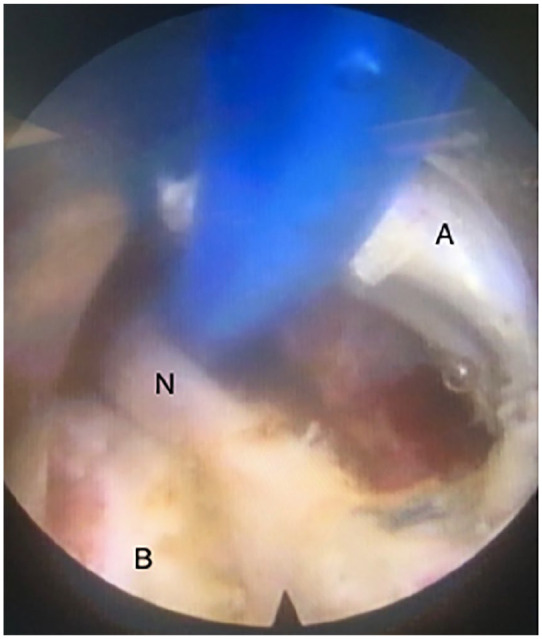
Endoscopic view of dorsal ramous (N) in the center of the screen detected and isolated by the coagulator probe (blue). In the medial part of the screen (right side, A) it is possible to see the articular capsule. In the lower part of the screen (B) it is possible to visualize the bone structure, a part of transverse process.
Efficacy of the intervention was evaluated using the NRS and ODI at 1 month (T1), 3 months (T2), 6 months (T3), 9 months (T4), 12 months (T5), 18 months (T6), and 24 months (T7). At the same times, all eventual side effects, complications, and necessity of rescue analgesia were reported.
Statistics
All the statistical analysis was performed using SPSS software. NRS and ODI values from T0 to T7 between patients, between Group A and Group B, and between patients divided by age (39–60/61–80 years) and number of joints treated (1–2/>3) were analyzed using an unpaired t test; p value < 0.05 was considered statistically significant.
A paired non-parametric test (Wilcoxon test) was used for the comparison between groups. The sub-group of age (39–60 versus 61–81 years old), and the number of treated joints (1–2 versus ⩾3) were compared using the paired t test.
Results
In the study period, 40 out of the 50 screened patients met the inclusion and exclusion criteria or accepted to enter the study; hence, they were included in the study protocol. Their demography and clinical features are shown in Table 1. Their mean age was 61.8 years (min 39, max 81) and the mean NRS value and ODI score at the beginning of the study (before surgery, T0) were 7.18 and 57.95, respectively. Mean surgery time was 48 min. No severe complications were registered. None of the patients enrolled in the clinical trial had to leave the study.
Table 1.
Demographic and clinical information of study patients.
| Characteristics | Mean (SD) |
|---|---|
| Patients excluded from the study | |
| Refused consent | 1 |
| Had exclusion criteria | 9 |
| Gender | |
| Male | 17 (42.5%) |
| Female | 23 (57.5%) |
| Body weight (kg) | 73.7 (SD 8.54) |
| Age (years) | 61.8 (SD 11) |
| Preoperative symptoms | |
| NRS | 7.18 (SD 0.8) |
| ODI | 57.95 (SD 15.77) |
| Age range | |
| 39–60 years | 18 (45%) patients |
| 61–81 years | 22 (55%) patients |
| Previous percutaneous RF treatment | |
| YES | 20 patients (50%) |
| NO | 20 patients (50%) |
| Number of joints treated | |
| 1–2 | 20 patients (50%) |
| ⩾3 | 20 patients (50%) |
NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; RF, radiofrequency; SD, standard deviation.
NRS improved significantly after the intervention. Its variations during the study times are shown in Figure 3. ODI improved as well from T0 up to the end of the study (Figure 4).
Figure 3.
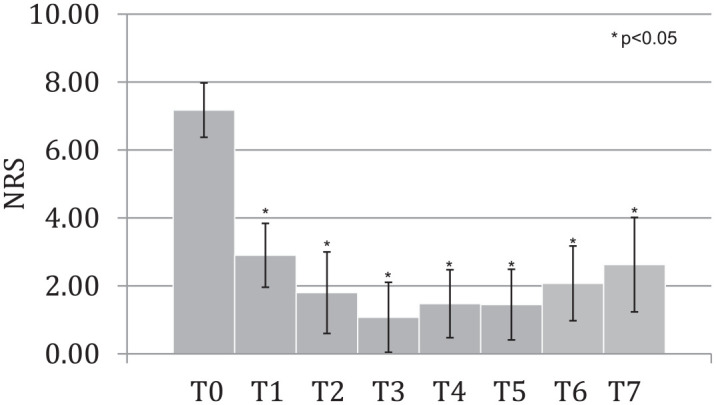
NRS at different times of the study.
NRS, Numeric Rating Scale.
Figure 4.
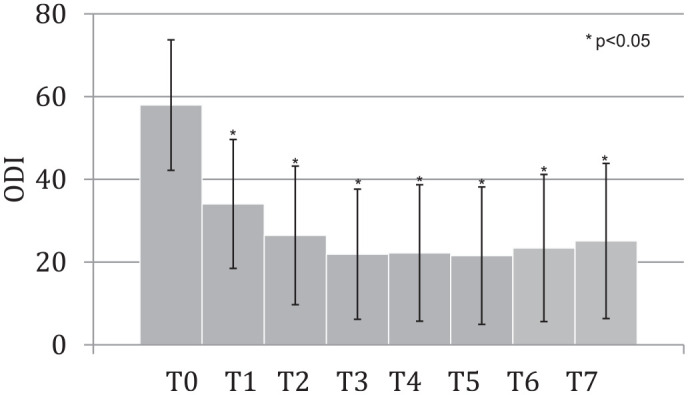
ODI values at different times of the study.
ODI, Oswestry Disability Index.
Analysis of the secondary endpoints revealed that there were no significant differences in NRS score and ODI index between groups previously treated or not with percutaneous rhizotomy (Tables 2 and 3). Moreover, we also analyzed the results dividing the patients by age (39–60 versus 61–81 years old). The results showed that there is a significant difference in NRS and ODI (p < 0.005), showing that younger people have lower NRS levels and better ODI improvements (Tables 4 and 5). Lastly, we analyzed the results dividing patients by number of joints treated (1–2 versus ⩾3). Even in this case, the differences were significant, in terms of NRS reduction and ODI improvement (Tables 6 and 7). This make clear that patients treated with endoscopic rhizotomy on 1–2 joints have better results.
Table 2.
Comparison of NRS and ODI values between patients of Group A (previous percutaneous treatment) and Group B (no previous percutaneous treatment). No statistical differences were observed between groups.
| NRS | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| Group A | 7.05 (SD: ±0.739) | 2.85 (SD: ±1.013) | 1.75 (SD: ±1.259) | 1.05 (SD: ±0.920) | 1.45 (SD: ±1.023) | 1.40 (SD: ±0.969) | 2.05 (SD: ±1.116) | 2.55 (SD: ±1.116) |
| Group B | 7.30 (SD: ±0.842) | 2.95 (SD: ±0.864) | 1.85 (SD: ±1.152) | 1.10 (SD: ±1.135) | 1.51 (SD: ±0.974) | 1.49 (SD: ±1.118) | 2.10 (SD: ±1.090) | 2.72 (SD: ±1.615) |
| p values | p = 0.38 | p = 0.77 | p = 0.80 | p = 0.90 | p = 0.90 | p = 0.80 | p = 0.90 | p = 0.75 |
| ODI | ||||||||
| Group A | 55.80 (SD: ±15.657) | 31.50 (SD: ±13.665) | 24.60 (SD: ±15.480) | 21.10 (SD: ±14.247) | 21.50 (SD: ±15.256) | 21.70 (SD: ±15.430) | 23.60 (SD: ±17.024) | 24.80 (SD: ±17.022) |
| Group B | 60.41 (SD: ±15.600) | 36.97 (SD: ±16.912) | 28.72 (SD: ±17.745) | 23.08 (SD: ±17.056) | 23.28 (SD: ±17.623) | 21.69 (SD: ±17.721) | 23.54 (SD: ±18.323) | 25.74 (SD: ±20.338) |
| p values | p = 0.48 | p = 0.37 | p = 0.54 | p = 0.77 | p = 0.82 | p = 0.96 | p = 0.95 | p = 0.92 |
NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; SD, standard deviation.
Table 3.
Comparison of NRS and ODI values between patients divided by age: 39–60 versus 61–81 yo (*p < 0.005).
| NRS | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| 39–60 yo | 7.00 (SD: ±0.745) | 2.50 (SD: ±0.763) | 1.17 (SD: ±0.897) | 0.39 (SD: ±0.590) | 0.83 (SD: ±0.600) | 0.89 (SD: ±0.874) | 1.33 (SD: ±0.745) | 1.67 (SD: ±0.606) |
| 61–81 yo | 7.32 (SD: ±0.819) | 3.23 (SD: ±0.950) | 2.32 (SD: ±1.182) | 1.64 (SD: ±0.979) | 2.00 (SD: ±0.953) | 1.91 (SD: ±0.949) | 2.68 (SD: ±0.971) | 3.41 (SD: ±1.336) |
| p values | p = 0.22 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| ODI | ||||||||
| 39–60 yo | 44.67 (SD: ±7.972) | 22.00 (SD: ±5.656) | 13.22 (SD: ±5.380) | 9.11 (SD: ±5.086) | 8.56 (SD: ±5.155) | 8.00 (SD: ±4.898) | 8.67 (SD: ±5.537) | 9.78 (SD: ±5.573) |
| 61–81 yo | 68.82 (SD: ±11.749) | 43.91 (SD: ±14.122) | 37.27 (SD: ±15.045) | 32.36 (SD: ±13.626) | 33.36 (SD: ±14.005) | 32.64 (SD: ±14.464) | 35.45 (SD: ±14.859) | 37.64 (SD: ±16.227) |
| p values | p = 0.29 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
NRS, Numeric Rating Scale; ODI, Oswestry Disability Index; SD, standard deviation; yo, years old.
Table 4.
Comparison of NRS and ODI values between patients divided by number of joint treated: 1–2 versus ⩾3 joints (*p < 0.005).
| NRS | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| 1–2 joints | 7.05 | 2.60* | 1.10* | 0.35* | 0.95* | 1.05* | 1.30* | 1.70* |
| ⩾3 joints | 7.30 | 3.20 | 2.50 | 1.80 | 2.03 | 1.90 | 2.90 | 3.59 |
| ODI | ||||||||
| 1–2 joints | 44.70 | 23.80* | 15.20* | 11.30* | 11.40* | 10.40* | 11.20* | 12.30* |
| ⩾3 joints | 68.97 | 44.92 | 38.36 | 33.23 | 33.69 | 33.38 | 36.36 | 38.72 |
NRS, Numeric Rating Scale; ODI, Oswestry Disability Index.
Table 5.
Comparison of ODI values between patients divided by age: 39–60 versus 61–81 yo. (*p < 0.005).
| ODI | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| 39–60 yo | 44.67 | 22.00* | 13.22* | 9.11* | 8.56* | 8.00* | 8.67* | 9.78* |
| 61–81 yo | 68.82 | 43.91 | 37.27 | 32.36 | 33.36 | 32.64 | 35.45 | 37.64 |
ODI, Oswestry Disability Index; yo, years old.
Table 6.
Comparison of NRS values between patients divided by number of joint treated: 1–2 versus ⩾3 joints. (*p < 0.005).
| NRS | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| 1–2 joints | 7.05 | 2.60* | 1.10* | 0.35* | 0.95* | 1.05* | 1.30* | 1.70* |
| ⩾3 joints | 7.30 | 3.20 | 2.50 | 1.80 | 2.03 | 1.90 | 2.90 | 3.59 |
NRS, Numeric Rating Scale.
Table 7.
Comparison of ODI values between patients divided by number of joint treated: 1–2 versus ⩾3 joints. (*p < 0.005).
| ODI | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| 1–2 joints | 44.70 | 23.80* | 15.20* | 11.30* | 11.40* | 10.40* | 11.20* | 12.30* |
| ⩾3 joints | 68.97 | 44.92 | 38.36 | 33.23 | 33.69 | 33.38 | 36.36 | 38.72 |
ODI, Oswestry Disability Index.
Discussion
This study has shown that endoscopic rhizotomy is useful for long-term results when a lumbar facet denervation is indicated. CLBP is one of the most common painful conditions and can be due to FJ arthritis in many cases.13,26 Its treatment includes different options, but with no long-term pain reduction. The most common therapies include intra-articular injections, medial branch blocks, and RF ablation of targeted nerve root.26 FJ denervation with RF percutaneous needle was initially described in 1975,27 and is a well-established treatment modality. Its efficacy has been proven to provide significant and satisfactory pain relief in patients with CLBP that are refractory to more conservative treatment options.16,28
RF can be considered a good and safe therapeutic option for faceto-genic LBP, but some studies suggest that pain relief is only temporary, lasting less than 12 months.29–31 These data, in general, are confirmed by all pain physicians that use percutaneous RF daily. One of the reasons for the short-lasting efficacy can be the regeneration of dorsal ramous, which is not completely interrupted by the tip of the needle. Also, it can depend on incorrect positioning of the tip of the needle. We know that the extent of the effect of RF stimuli on the target is a few millimeters near the tip, and non-perfect positioning can lead to a complete failure of the procedure. This problem can be eliminated using the endoscopic technique, which allows direct visualization of the nerve. In this way, the target nerve can be detected when it lays over the transverse process, where it is bigger and well recognizable. This technique has already provided good results.21,32,33 Moreover, traditional RF ablation allows ablation of the target medial branch in only one point. Endoscopic guided RF ablation allows the operator to interrupt the target medial branch at multiple sites, just following the nerve course with the support of the endoscope and RF bipolar probe.
The use of the endoscope was first described by Bogduk with the nerve-entrapped technique.34 As already noted, endoscopy allows direct visualization of the structures and exact detection of the dorsal ramous. In this way, ablation can be more accurate and nerve root injury rate is reduced. Consequently, incidence of sensory loss or analgesia of skin are reduced, and nerve regeneration is rare after extensive ablation.35 Moreover, the endoscopic approach allows the operator to dissect the articular capsule, with a more distal denervation and wash out of the articular space, if necessary.36
In this study, we investigated the efficacy of endoscopic technique in patients that did not have a previous lumbar spine surgery in their clinical history. The choice was made to reduce possible bias to our results, even though the endoscopic technique has been used in patients with failed back surgery syndrome (FBSS).37 Also, the study included patients treated only with ablation of the dorsal ramous, without articular capsulotomy because we wanted to be sure that pain reduction was due only to neuroablation and not to capsulotomy. Superiority of endoscopic technique compared with the percutaneous approach, as shown in this study, was also reported in a recent randomized controlled trial (RCT), especially in terms of safety.38
For analyses of the secondary endpoints, we divided patients into different groups to see if the procedure gives better results in some specific group of patients. In particular we wanted to detect if a previous RF percutaneous treatment could affect the results. In our study, no significant differences were obtained in patients that had, or had not had, a RF treatment in their clinical history, showing that a previous percutaneous neuroablation has no influence on a good response to the endoscopic treatment. This is probably explainable considering the “target” of the two procedures, the dorsal ramus, that is achieved in two different positions depending on the technique, more “distal” from the foramen with needle techniques (the famous “eye of Scottie dog”) and more proximal with the endoscopic approach (when the nerve lays on transverse process). We also considered age as a parameter that could affect the efficacy of the endoscopic approach. The results showed that younger patients (<60 years) have better results compared with older patients. This might be due to the central sensitization of chronic pain, more frequent in people with a long history of chronic pain.5 Lastly, we analyzed if the number of joints treated in each patient may be responsible for better results. The patients whose neuroablation was limited to 1–2 joints had significant better results than patients with three or more joints treated. This may obviously be explained by the better conditions of patients requiring only the denervation of less than three FJs. In any case, this point should be better studied, and a deeper analysis should be made to see whether, when more than two facets are involved, the association with capsulotomy may increase the efficacy. Of course, this would need a more specific study protocol.
Study limitations
This study would have been more complete if the same protocol had been used in different Pain Centers, with different groups of pain physicians and a higher number of patients treated. A multicenter study would make clear the influence of operator skill, and how a larger number of cases may influence the results.
Conclusion
Endoscopic FJ denervation can be considered an evolution of percutaneous RF denervation by needle. This technique can be considered more surgical than percutaneous RF. It requires endoscopic instrumentation and skillfulness with the endoscope and the on-screen view, but the learning curve is not that long, and it has proved safer than the percutaneous technique.36 Previous percutaneous RF treatments does not influence the results of the endoscopic procedure, even if the two techniques have different targets. Some pain physicians prefer to first use the percutaneous approach, and, in case of relapse, use the endoscopic technique. In our Pain Center we used to start always with the percutaneous technique first. Now we always start with endoscopy, also because, in our study, previous percutaneous RF treatments did not change the outcome. Compared with percutaneous RF ablation, the endoscopic technique also has disadvantages, such as longer duration of the procedure, lengthened recovery time, and increased costs. But the advantages of the procedure in terms of longer and better analgesia are unquestionable. The endoscopic approach is always effective in reducing pain in all patients observed, and even better results are obtained in younger patients or in patients with less than 3 joints treated.
Footnotes
Conflict of interest statement: GV is member of the Editorial Board of this journal. The other authors declare that they do not have any conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The editing of manuscript and proofs has been generously supported by the Paolo Procacci Foundation.
ORCID iD: Giustino Varrassi  https://orcid.org/0000-0002-3822-2923
https://orcid.org/0000-0002-3822-2923
Contributor Information
Stefano Meloncelli, Valle Giulia Hospital, Roma, Lazio, Italy.
Giorgio Germani, Valle Giulia Hospital, Roma, Lazio, Italy.
Ignazio Urti, Valle Giulia Hospital, Roma, Lazio, Italy.
Marco Divizia, Valle Giulia Hospital, Roma, Lazio, Italy.
Maria Rosciano, S.A.M.O. Pain Management Center, Roma, Italy.
Filomena Puntillo, Department of Emergency and Organ Transplants, University “Aldo Moro” of Bari, Bari, Puglia, Italy.
Antonella Paladini, Department of MESVA, University of L’Aquila, L’Aquila, Abruzzo, Italy.
Giustino Varrassi, Paolo Procacci Foundation, Via Tacito 7, Roma, 00193, Italy.
References
- 1. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med 2009; 169: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Juniper M, Le TK, Mladsi D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opin Pharmacother 2009; 10: 2581–2592. [DOI] [PubMed] [Google Scholar]
- 3. Langley P, Müller-Schwefe G, Nicolaou A, et al. The societal impact of pain in the European Union: health-related quality of life and healthcare resource utilization. J Med Econ 2010; 13: 571–581. [DOI] [PubMed] [Google Scholar]
- 4. Langley P, Müller-Schwefe G, Nicolaou A, et al. The impact of pain on labor force participation, absenteeism and presenteeism in the European Union. J Med Econ 2010; 13: 662–672. [DOI] [PubMed] [Google Scholar]
- 5. Fusco M, Skaper SD, Coaccioli S, et al. Degenerative joint diseases and neuroinflammation. Pain Pract 2017; 17: 522–532. [DOI] [PubMed] [Google Scholar]
- 6. Manchikanti L, Boswell MV, Singh V, et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord 2004; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Giorno R, Frumento P, Varrassi G, et al. Assessment of chronic pain and access to pain therapy: a cross-sectional population-based study. J Pain Res 2017; 10: 2577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence. Best evidence for a better back (BE FABB) - a triage, assessment and education service for patients with low back pain with or without sciatica, https://www.nice.org.uk/sharedlearning/best-evidence-for-a-better-back-be-fabb-a-triage-assessment-and-education-service-for-patients-with-low-back-pain-with-or-without-sciatica (accessed 3 May 2020).
- 9. Paladini A, Fusco M, Cenacchi T, et al. Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: a pooled data meta-analysis. Pain Physician 2016; 19: 11–24. [PubMed] [Google Scholar]
- 10. Paladini A, Varrassi G, Bentivegna G, et al. Palmitoylethanolamide in the treatment of failed back surgery syndrome. Pain Res Treat 2017; 2017: 1486010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vitoula K, Venneri A, Varrassi G, et al. Behavioral therapy approaches for the management of low back pain: an up-to-date systematic review. Pain Ther 2018; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ 2015; 350: h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Filippiadis DK, Kelekis A. A review of percutaneous techniques for low back pain and neuralgia: current trends in epidural infiltrations, intervertebral disk and facet joint therapies. Br J Radiol 2016; 89: 20150357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manchikanti L, Singh V, Falco FJ, et al. Evaluation of lumbar facet joint nerve blocks in managing chronic low back pain: a randomized, double-blind, controlled trial with a 2-year follow-up. Int J Med Sci 2010; 7: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu T, Zhao WH, Dong Y, et al. Effectiveness of ultrasound-guided versus fluoroscopy or computed tomography scanning guidance in lumbar facet joint injections in adults with facet joint syndrome: a meta-analysis of controlled trials. Arch Phys Med Rehabil 2016; 97: 1558–1563. [DOI] [PubMed] [Google Scholar]
- 16. Boswell MV, Colson JD, Sehgal N, et al. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician 2007; 10: 229–253. [PubMed] [Google Scholar]
- 17. Hammer M, Meneese W. Principles and practice of radiofrequency neurolysis. Curr Rev Pain 1998; 2: 267–278. [Google Scholar]
- 18. Sluijter ME, van Kleef M. Pulsed radiofrequency. Pain Medicine 2007; 8: 388–389. [DOI] [PubMed] [Google Scholar]
- 19. Cohen SP, Bhaskar A, Bhatia A, et al. Consensus practice guidelines on interventions for lumbar facet joint pain from a multispecialty, international working group. Reg Anesth Pain Med. Epub ahead of print 3 April 2020. DOI: 10.1136/rapm-2019-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee CH, Chung CK, Kim CH. The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: a meta-analysis of randomized controlled trials. Spine J. Epub ahead of print 30 May 2017. DOI: 10.1016/j.spinee.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 21. Li Z-Z, Hou S-X, Shang W-L, et al. Evaluation of endoscopic dorsal ramus rhizotomy in managing facetogenic chronic low back pain. Clin Neurol Neurosurg 2014; 126: 11–17. [DOI] [PubMed] [Google Scholar]
- 22. Jeong SY, Kim JS, Choi WS, et al. The effectiveness of endoscopic radiofrequency denervation of medial branch for treatment of chronic low back pain. J Korean Neurosurg Soc 2014; 56: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeung A, Gore S. Endoscopically guided foraminal and dorsal rhizotomy for chronic axial back pain based on cadaver and endoscopically visualized anatomic study. Int J Spine Surg 2014; 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Divizia M, Germani G, Urti I, et al. Endoscopic neuromodulation of suprascapular nerve in chronic shoulder pain: a case report. Anesth Pain Med 2020; 10: 103624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoy D, Brooks P, Blyth F, et al. The epidemiology of low back pain. Best Pract Res Clin Rheumatol 2010; 24: 769–781. [DOI] [PubMed] [Google Scholar]
- 26. Lakemeier S, Lind M, Schultz W, et al. A comparison of intraarticular lumbar facet joint steroid injections and lumbar facet joint radiofrequency denervation in the treatment of low back pain: a randomized, controlled, double-blind trial. Anesth Analg 2013; 117: 228–235. [DOI] [PubMed] [Google Scholar]
- 27. Shealy CN. Facet denervation in the management of back and sciatic pain. Clin Orthop Relat Res 1976; 115: 157–164. [PubMed] [Google Scholar]
- 28. Boswell MV, Trescot AM, Datta S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician 2007; 10: 7–111. [PubMed] [Google Scholar]
- 29. Leclaire R, Fortin L, Lambert R, et al. Radiofrequency facet joint denervation in the treatment of low back pain: a placebo-controlled clinical trial to assess efficacy. Spine (Phila Pa 1976) 2002; 27: 556–557. [DOI] [PubMed] [Google Scholar]
- 30. van Wijk RM, Geurts JW, Wynne HJ, et al. Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain: a randomized, double-blind, sham lesion-controlled trial. Clin J Pain 2005; 21: 335–344. [DOI] [PubMed] [Google Scholar]
- 31. Kim MH, Kim SW, Ju CI, et al. Effectiveness of repeated radiofrequency neurotomy for facet joint syndrome after microscopic discectomy. Korean J Spine 2014; 11: 232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walter SG, Schildberg FA, Rommelspacher Y. Endoscopic sacrolumbar fect joint denervation in osteoarthritic and degenerated zygoapophyseal joint. Arthrosc Tech 2018; 7: e1275–e1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walter SG, Struwe C, Scheidt S, et al. Encoscopic facet joint denervation for treatment of chronic lower back pain. Clin Neurol Neurosurg 2020; 195: 105904. [DOI] [PubMed] [Google Scholar]
- 34. Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine (Phila Pa 1976) 1982; 7: 319–330. [DOI] [PubMed] [Google Scholar]
- 35. Manchikanti L, Kaye AD, Boswell MV, et al. A systematic review and best evidence synthesis of the effectiveness of therapeutic facet joint interventions in managing chronic spinal pain. Pain Physician 2015; 18: E535–E582. [PubMed] [Google Scholar]
- 36. Haufe SM, Mork AR. Endoscopic facet debridement for the treatment of facet arthritic pain—a novel new technique. Int J Med Sci 2010; 7: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ibrahim R, Telfeian AE, Gohlke K, et al. Endoscopic radiofrequency treatment of the sacroiliac joint complex for low back pain: a prospective study with a 2-year follow-up. Pain Physician 2019; 22: E111–E118. [PubMed] [Google Scholar]
- 38. Xue Y, Ding T, Wang D, et al. Endoscopic rhizotomy for chronic lumbar zygapophysial joint pain. J Orthop Surg Res 2020; 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]