Abstract
Objective
This study aimed to examine a novel microRNA (miR-652-3p) biomarker to improve early diagnosis of acute kidney injury (AKI) in patients with acute decompensated heart failure (ADHF) and to evaluate the survival predictive value of miR-652-3p.
Methods
We retrospectively analyzed the data of 196 patients with ADHF, including 65 who developed AKI during hospitalization. Neutrophil gelatinase-associated lipocalin (NGAL) levels were measured in serum and urine samples. Real-time quantitative PCR was applied to evaluate miR-652-3p mRNA expression. The diagnostic performance of miR-652-3p was examined using receiver operating characteristic curve analysis. The prognostic value of miR-652-3p was also analyzed.
Results
Serum and urinary NGAL and miR-652-3p levels were elevated in patients with ADHF and AKI. Serum and urinary miR-652-3p expression had diagnostic value in predicting AKI onset in patients with ADHF, and it had improved diagnostic performance when used with NGAL. Patients with AKI and high miR-652-3p levels had a high failure rate of renal recovery and poor 180-day survival.
Conclusion
Serum and urinary miR-652-3p may be a candidate biomarker for early diagnosis of AKI in patients with ADHF and for predicting the prognosis of AKI. The combination of NGAL and miR-652-3p may accurately predict AKI onset in ADHF.
Keywords: Acute decompensated heart failure, acute kidney injury, microRNA-652-3p, neutrophil gelatinase-associated lipocalin, diagnostic biomarker, serum creatinine
Introduction
Acute decompensated heart failure (ADHF) is a clinical syndrome, which is characterized by dyspnea, and it suddenly occurs and rapidly peaks on the basis of cardiac dysfunction.1 More than 30% of patients with ADHF are accompanied by acute kidney injury (AKI) during hospitalization, leading to an increased mortality rate.2 Therefore, early prediction of occurrence of AKI is important for decisions on clinical management and improving clinical outcomes in patients with ADHF.3 Currently, the diagnosis of AKI mainly relies on some indicators of renal function, such as urine output and serum creatinine levels, but they show limited sensitivity and delayed results.4 Neutrophil gelatinase-associated lipocalin (NGAL) has been recently used as an early diagnostic biomarker of AKI, but its diagnostic accuracy is not ideal.5 Therefore, there is an urgent requirement to examine novel biomarkers to predict the occurrence of AKI in patients with ADHF.
Recent studies on biomarkers in various human diseases have highlighted the clinical significance of microRNAs (miRNAs) in diagnosis and prognosis of disease.6,7 These miRNAs are a series of small non-coding RNAs, and some members that are aberrantly expressed in human diseases have critical biological functions in the pathogenesis of disease.8,9 In progression of ADHF, some miRNAs have deregulated expression and functional roles.10 With regard to the diagnosis of AKI, some aberrantly expressed miRNAs, such as miR-192 and miR-26b, have been identified as potential diagnostic biomarkers.11,12 MicroRNA-652 (miR-652) has been investigated in several human diseases.13 Silencing of miR-652 exerts a protective effect against pathological remodeling of the heart and ameliorating cardiac function.14 Additionally, circulating miR-652 is a marker for predicting acute coronary syndrome.15 Importantly, Bruno et al.16 investigated miRNAs involved in early worsening of renal function in patients with acute heart failure, and found that miR-652-3p expression was significantly increased in patients with renal dysfunction. Therefore, we speculate that miR-652-3p might be involved in development of AKI in patients with ADHF.
In this study, the clinical significance of miR-652-3p in diagnosis and prognosis of AKI in patients with ADHF was evaluated by analyzing serum and urinary miR-652-3p mRNA expression. The diagnostic performance of NGAL and synthesis of miR-652-3p and NGAL were also evaluated. The 180-day survival outcomes were recorded and assessed to examine the relationship between miR-652-3p and prognosis. Our results may provide a novel insight into diagnosis of AKI and prediction of prognosis in patients with ADHF.
Materials and methods
Recruitment of patients
This study retrospectively analyzed data of patients with ADHF who were admitted to the Second Hospital of Tianjin Medical University between 2014 and 2019. The diagnosis of ADHF was performed by using the European Society of Cardiology Criteria.17 Specific manifestations of the patients were presentation of heart failure symptoms and signs, such as dyspnea, lung moist rales, hypotension, tissue perfusion, increased jugular pressure, peripheral edema, hepatomegaly, and congestion of the intestines, and a chest X-ray showed pulmonary congestion. The New York Heart Association heart function stages of patients with ADHF were stages III and IV, and the left ventricular ejection fraction was ≤50%. Patients who met the following criteria were excluded: 1) age <18 years or with pregnancy; 2) a preadmission estimated glomerular filtration rate < 30 mL/minute per 1.730 m2; 3) patients received nephrotoxin within 4 weeks before admission or during hospitalization; 4) obstruction or infection of the urinary tract; 5) a history of malignancy or multiple organ function syndrome; 6) the hospital stay was shorter than 24 hours; and 7) an incomplete electronic medical record.
During hospitalization, determination of AKI was performed according to guidance of diagnosis from the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for Acute Kidney Injury.18 AKI was defined as increased serum creatinine levels of ≥3 µg/mL or >1.5 times the basal value within 48 hours. Occurrence of this condition was confirmed or inferred within 7 days or AKI was defined as a urine output <0.5 mL/kg/hour for 6 hours. The patients with AKI were divided into three KDIGO stages on the basis of changes in serum creatinine and urine levels. The clinicopathological characteristics of the patients with ADHF are summarized in Table 1. Renal recovery of patients with AKI was defined as serum creatinine levels returning to those on the day of admission at the time of hospital discharge. The survival information of patients with AKI during a 180-day follow-up survey was recorded for analysis of prognosis.
Table 1.
Comparison of clinical characteristics in patients with ADHF with and without AKI.
| Characteristics |
Patients with ADHF (n = 196) |
P value | |
|---|---|---|---|
| Non-AKI (n = 131) | AKI (n = 65) | ||
| Age (years) | 62.7 ± 20.2 | 64.1 ± 21.6 | 0.663 |
| Sex (female/male) | 85/38 | 35/30 | 0.135 |
| Diabetes history (n, %) | 42 (32.1) | 27 (41.5) | 0.191 |
| Myocardial infarction history (n, %) | 13 (9.9) | 11 (16.9) | 0.159 |
| Heart failure history (n, %) | 68 (51.9) | 34 (52.3) | 0.958 |
| Coronary heart disease (n, %) | 30 (22.9) | 17 (26.2) | 0.616 |
| Hypertension (n, %) | 16 (12.2) | 11 (16.9) | 0.368 |
| Chronic kidney disease (n, %) | 29 (22.1) | 36 (55.4) | <0.001 |
| Primary cause of heart failure (n, %) | 0.341 | ||
| Ischemic heart disease | 70 (53.4) | 39 (60.0) | |
| Cardiomyopathy | 28 (21.4) | 7 (10.8) | |
| Rheumatic heart disease | 23 (17.6) | 13 (20.0 | |
| Hypertension | 10 (7.6) | 6 (9.2) | |
| LVEF (<45%/>45%) | 68/63 | 35/30 | 0.798 |
| NYHA stage IV (n, %) | 63 (48.1) | 35 (53.8) | 0.448 |
| NT-pro-BNP (pg/mL) | 6580 (3279–9064) | 8948 (3615–24956) | 0.063 |
| Serum albumin (µg/mL) | 33 ± 5.7 | 29.6 ± 6.8 | 0.011 |
| Scr (µM) | 126.3 ± 36.7 | 146.84 ± 27.7 | 0.008 |
| eGFR (mL/minute per 1.73 m2) | 65.3 ± 25.1 | 54.2 ± 20.9 | 0.002 |
| KDIGO stage (n, %) | – | ||
| Stage 1 | – | 45 (69.2) | |
| Stages 2–3 | – | 20 (30.8) | |
ADHF, acute decompensated heart failure; AKI, acute kidney injury; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes.
The protocol of this study was approved by the Ethics Committee of the Second Hospital of Tianjin Medical University (Approval no. TJMU-20140829). Signed written informed consent was obtained from each participant for use of samples and data analysis.
Collection of serum and urine samples
We collected venous blood and spot urine samples from patients with ADHF immediately after their admission. None of the patients had received any therapy before sampling. Serum samples were isolated from blood using centrifugation and urine samples were centrifuged to obtain supernatants for further use. All of the samples were kept at −80°C.
Enzyme-linked immunosorbent assay
NGAL levels are generally detected as a biomarker of AKI to reflect renal function. We evaluated NGAL levels in serum and urine samples using an enzyme-linked immunosorbent assay kit (Immuno-Biological Laboratories, Fujioka, Japan) by following the manufacturer’s protocols.
RNA extraction and real-time quantitative PCR
Total RNA was extracted from serum and urine samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA concentrations were evaluated using the NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Single-stranded cDNA was synthesized from RNA using the PrimeScript RT reagent kit (TaKaRa, Shiga, Japan) by following the instructions of the manufacturer. To measure miR-652-3p mRNA expression, real-time quantitative PCR was carried out using the SYBR green I Master Mix kit (Invitrogen) and the 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). U6 was used as an internal control and the final value of miR-652-3p expression was calculated using the 2−ΔΔCt method.19 The following primers were used: miR-652-3p forward, 5′-GCCGAGAATGGCGCCACTAG-3′; miR-652-3p reverse, 5′-CTCAACTGGTGTCGTGGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′; and U6 reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Statistical analysis
All data are expressed as mean ± standard deviation or median (interquartile range). Data were analyzed using IBM SPSS version 21.0 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA). The Student’s t test or Mann–Whitney U test was used to analyze continuous variables, and Pearson’s chi-square test was used to assess categorical variables. Logistic regression analysis was applied to evaluate the risk of miR-652-3p for predicting AKI onset or renal recovery in patients with ADHF. Clinical features that showed significant results from univariate analysis were applied in multivariate analysis to adjust the results of miR-652-3p. A receiver operating characteristic (ROC) curve was plotted to analyze the diagnostic performance of NGAL and miR-652-3p levels. The prognostic value of miR-652-3p for predicting the 180-day survival outcome in patients with AKI was assessed using the Kaplan–Meier method and Cox regression analysis. Differences with P <0.05 were considered statistically significant.
Results
Clinical characteristics of the patients
We analyzed 196 patients with ADHF and 65 of these patients developed AKI. We summarized and compared the clinical characteristics between patients with ADHF and AKI and those without AKI. There were no significant differences in age, sex, history of diabetes, myocardial infarction, and heart failure between the two groups (Table 1). There were also no differences in the incidence of coronary heart disease, hypertension, and cardiomyopathy, which represent risk factors of heart failure, between patients with AKI and those without AKI. Patients with AKI had with a higher incidence of chronic kidney disease compared with those without AKI (P <0.001). The primary causes of heart failure included ischemic heart disease, cardiomyopathy, rheumatic heart disease, and hypertension, and there was no difference in the cause of heart failure between the two groups. For cardiac function of the patients, there were no differences in the left ventricular ejection fraction, New York Heart Association stage, and N-terminal pro-brain natriuretic peptide levels between the two groups. Admission serum creatinine levels were significantly higher, while serum albumin levels and the preadmission estimated glomerular filtration rate were lower, in patients with AKI compared with those without AKI (all P <0.05). Among the 65 patients with AKI, 45 had KDIGO stage 1 and 20 had stages 2 to 3 based on the changes in serum creatinine and urine levels. Among these patients, 16 received renal replacement therapy.
Serum and urinary levels of NGAL and miR-652-3p in patients with ADHF
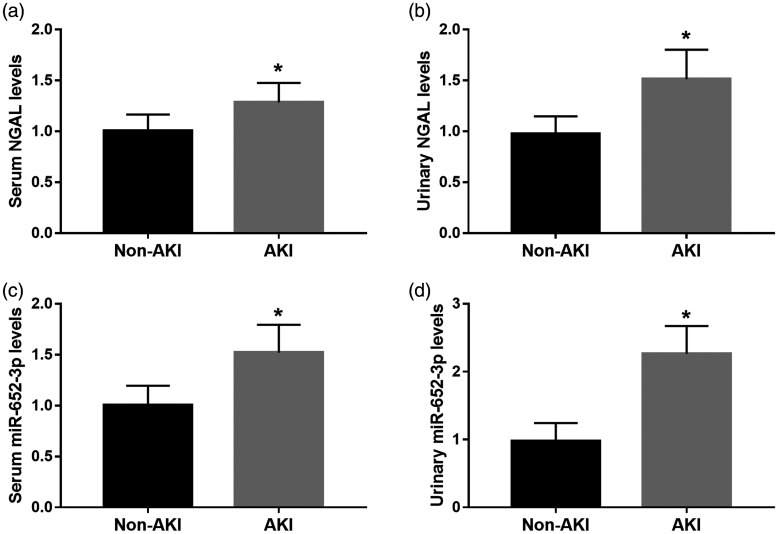
Serum and urinary levels of NGAL were significantly higher in patients with AKI compared with those without AKI (both P <0.05, Figure 1a and 1b). We also found that patients with AKI had significantly higher serum and urinary miR-652-3p expression levels than those without AKI (both P< 0.05, Figure 1c and 1d).
Figure 1.
Serum and urinary NGAL and miR-652-3p expression levels in patients with acute decompensated heart failure. (a) and (b). Serum and urinary NGAL levels in patients with AKI and those without AKI. (c) and (d) Serum and urinary miR-652-3p mRNA expression in patients with AKI and those without AKI. *P < 0.05.
AKI, acute kidney injury; NGAL, neutrophil gelatinase-associated lipocalin.
Association of miR-652-5p mRNA expression with occurrence of AKI in patients with ADHF
Logistic analysis was used to assess the associations of clinical features and miR-652-3p mRNA expression with the onset of AKI to investigate potential risk factors for occurrence of AKI in patients with ADHF. We found that serum miR-652-3p (odds ratio [OR] = 1.519, 95% confidence interval [CI] = 1.051–2.881, P = 0.042), urinary miR-652-3p (OR = 1.945, 95% CI = 1.569–4.010, P = 0.015), and NGAL (OR = 1.698, 95% CI = 1.084–2.964, P = 0.038) levels were independently associated with the onset of AKI (Table 2). These results suggest that these variables are risk factors for occurrence of AKI in patients with ADHF.
Table 2.
Risk factor analysis of acute kidney injury in patients with acute decompensated heart failure.
| Variables |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.003 | 0.989–1.018 | 0.661 | – | – | – |
| Sex | 0.631 | 0.345–1.157 | 0.137 | – | – | – |
| Diabetes history | 1.506 | 0.814–2.784 | 0.192 | – | – | – |
| Myocardial infarction history | 1.849 | 0.778–4.392 | 0.164 | – | – | – |
| Heart failure history | 0.444 | 0.183–1.080 | 0.063 | – | – | – |
| Coronary heart disease | 0.552 | 0.254–1.125 | 0.069 | – | – | – |
| Hypertension | 1.464 | 0.637–3.368 | 0.370 | – | – | – |
| Cardiomyopathy | 2.121 | 0.912–3.125 | 0.413 | – | – | – |
| LVEF | 1.192 | 0.600–2.370 | 0.616 | – | – | – |
| NT-pro-BNP | 1.213 | 0.824–2.859 | 0.123 | – | – | – |
| Serum albumin | 2.052 | 1.032–3.854 | 0.045 | 1.245 | 0.625–2.589 | 0.489 |
| Scr | 2.125 | 1.098–3.945 | 0.038 | 1.056 | 1.029–1.165 | 0.047 |
| eGFR | 1.899 | 1.021–3.588 | 0.048 | 1.789 | 0.785–4.268 | 0.175 |
| sNGAL | 1.525 | 0.789–2.975 | 0.110 | – | – | – |
| uNGAL | 1.956 | 1.125–4.129 | 0.026 | 1.698 | 1.084–2.964 | 0.038 |
| smiR-652-3p | 1.912 | 1.112–3.986 | 0.029 | 1.519 | 1.051–2.881 | 0.042 |
| umiR-652-3p | 2.135 | 1.369–4.398 | 0.006 | 1.945 | 1.569–4.010 | 0.015 |
OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; sNGAL, serum neutrophil gelatinase-associated lipocalin; uNGAL, urinary neutrophil gelatinase-associated lipocalin; smiR-652-3p, serum microRNA-652-3p; umiR-652-3p, urinary microRNA-652-3p.
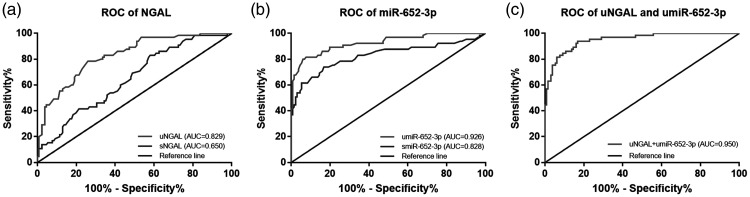
Diagnostic performance of miR-652-3p in patients with ADHF and AKI
We evaluated the diagnostic performance of serum and urinary NGAL levels and miR-652-3p mRNA expression for differentiation between AKI-positive cases and AKI-negative cases in patients with ADHF. The ROC curves based on NGAL levels shown in Figure 2a showed that the area under the curve (AUC) for serum NGAL levels was 0.650 and that for urinary NGAL levels was 0.829 (Figure 2a). The ROC curves for miR-652-3p showed that that the AUCs were 0.828 for serum miR-652-3p mRNA expression and 0.926 for urinary miR-652-3p mRNA expression (Figure 2b). From the data of the AUCs, diagnostic sensitivity and specificity values were better when we analyzed urinary NGAL or miR-652-3p levels compared with those analyzed using serum levels (Table 3).
Figure 2.
ROC curves based on serum and urinary NGAL and miR-652-3p levels in patients with acute decompensated heart failure. (a) ROC curves based on serum and urinary NGAL levels. (b) ROC curves based on serum and urinary miR-652-3p levels. (c) ROC curves based on the combination of urinary NGAL and miR-652-3p levels.
AUC, area under the curve. ROC, receiver operating characteristic; NGAL, neutrophil gelatinase-associated lipocalin; uNGAL, urinary neutrophil gelatinase-associated lipocalin; sNGAL, serum neutrophil gelatinase-associated lipocalin; umiR-652-3p, urinary microRNA-652-3p; smiR-652-3p, serum microRNA-652-3p.
Table 3.
Receiver operating characteristic curve analysis results for the diagnostic performance of NGAL and miR-652-3p levels.
| Variables | AUC | Cutoff value | Sensitivity | Specificity |
|---|---|---|---|---|
| sNGAL | 0.650 | 0.915 | 83.1% | 42.0% |
| uNGAL | 0.829 | 1.235 | 78.5% | 74.1% |
| smiR-652-3p | 0.828 | 1.165 | 73.9% | 83.2% |
| umiR-652-3p | 0.926 | 1.365 | 81.5% | 92.4% |
| uNGAL + umiR-652-3p | 0.950 | 0.219 | 83.1% | 94.0% |
NGAL, neutrophil gelatinase-associated lipocalin; AUC, area under the curve; sNGAL, serum neutrophil gelatinase-associated lipocalin; uNGAL, urinary neutrophil gelatinase-associated lipocalin; smiR-652-3p, serum microRNA-652-3p; umiR-652-3p, urinary microRNA-652-3p.
The combination of urinary NAGL and miR-652-3p levels in diagnosing AKI was further evaluated. The ROC curve showed that the AUC was 0.950, which indicated that the diagnostic performance of urinary NAGL levels might be improved when combined with urinary miR-652-3p levels (Figure 2c).
Prognostic value of miR-652-3p mRNA expression for predicting renal recovery and 180-day survival outcome in patients with AKI
Renal function recovered in 36 (55.4%) patients with AKI, and the predictive value of miR-652-3p mRNA expression for renal recovery was evaluated using logistic regression analysis. We found that serum and urinary miR-652-3p mRNA expression was independently associated with renal recovery in patients with AKI (OR = 2.400, 95% CI = 1.389–4.561, P =0.036 for serum miR-652-3p expression; OR = 2.716, 95% CI = 1.788–4.942, P =0.012 for urinary miR-652-3p expression) (Table 4).
Table 4.
Prediction of renal recovery in patients with acute kidney injury using logistic analysis.
| Variables |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 1.021 | 0.960–1.019 | 0.461 | – | – | – |
| Sex | 1.500 | 0.400–5.621 | 0.547 | – | – | – |
| Diabetes history | 1.510 | 0.364–3.267 | 0.571 | – | – | – |
| Myocardial infarction history | 1.869 | 0.460–4.630 | 0.154 | – | – | – |
| Heart failure history | 1.992 | 0.596–4.812 | 0.357 | – | – | – |
| Coronary heart disease | 1.582 | 0.621–3.120 | 0.285 | – | – | – |
| Hypertension | 2.720 | 0.845–5.631 | 0.107 | – | – | – |
| Cardiomyopathy | 1.852 | 0.689–3.523 | 0.551 | – | – | – |
| LVEF | 1.498 | 0.397–5.652 | 0.314 | – | – | – |
| NT-pro-BNP | 2.542 | 0.712–6.255 | 0.116 | – | – | – |
| Serum albumin | 1.522 | 0.516–4.491 | 0.126 | – | – | – |
| Scr | 1.856 | 1.096–3.842 | 0.035 | 1.756 | 1.066–3.415 | 0.044 |
| eGFR | 1.725 | 1.059–3.446 | 0.045 | 1.525 | 0.952–2.854 | 0.068 |
| sNGAL | 2.526 | 0.559–5.814 | 0.059 | – | – | – |
| uNGAL | 2.421 | 1.452–4.621 | 0.022 | 2.202 | 1.217–4.116 | 0.038 |
| smiR-652-3p | 2.589 | 1.528–4.777 | 0.019 | 2.400 | 1.389–4.561 | 0.036 |
| umiR-652-3p | 2.964 | 1.965–5.196 | 0.002 | 2.716 | 1.788–4.942 | 0.012 |
OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; sNGAL, serum neutrophil gelatinase-associated lipocalin; uNGAL, urinary neutrophil gelatinase-associated lipocalin; smiR-652-3p, serum microRNA-652-3p; umiR-652-3p, urinary microRNA-652-3p.
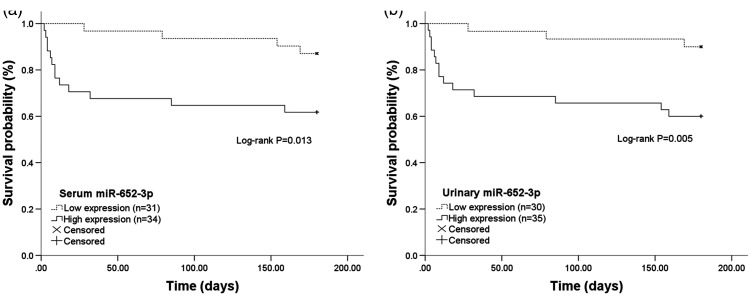
For 180-day follow-up survival, miR-652-3p mRNA expression was divided into low and high expression groups on the basis of its median value, and Kaplan–Meier survival analysis was performed. Survival curves show that patients with high serum (Figure 3a) or urinary (Figure 3b) miR-652-3p mRNA expression had a shorter survival time compared with those with low miR-652-3p mRNA expression (both P <0.05). Furthermore, clinical features and miR-652-3p mRNA expression were included in multivariate Cox regression analysis. This analysis showed that serum and urinary miR-652-3p expression independently predicted survival of patients with AKI (hazard ratio = 2.903, 95% CI =1.475–6.714, P = 0.041 for serum miR-652-3p expression; hazard ratio = 3.418, 95% CI = 1.889–7.969, P = 0.013 for urinary miR-652-3p expression; Table 5).
Figure 3.
Kaplan–Meier survival curves based on 180-day survival in patients with different serum and urinary miR-652-3p levels. (a) High serum miR-652-3p levels were associated with a shorter survival time (log-rank P = 0.013). (b) High urinary miR-652-3p levels predicted poor survival in patients with acute kidney injury (log-rank P = 0.005).
Table 5.
Cox regression analysis in patients with acute decompensated heart failure and acute kidney injury.
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age | 0.989 | 0.959–1.020 | 0.489 |
| Sex | 1.005 | 0.990–1.020 | 0.545 |
| Diabetes history | 1.798 | 0.498–6.489 | 0.370 |
| Myocardial infarction history | 1.775 | 0.091–14.407 | 0.705 |
| Heart failure history | 1.583 | 0.450–5.576 | 0.474 |
| Coronary heart disease | 1.193 | 0.338–4.208 | 0.783 |
| Hypertension | 2.347 | 0.620–5.891 | 0.202 |
| Cardiomyopathy | 0.473 | 0.084–2.653 | 0.395 |
| LVEF | 0.465 | 0.032–6.814 | 0.576 |
| NT-pro-BNP | 0.398 | 0.011–14.407 | 0.615 |
| Serum albumin | 0.726 | 0.276–1.911 | 0.517 |
| Scr | 0.443 | 0.133–1.471 | 0.184 |
| eGFR | 2.745 | 0.822–6.657 | 0.113 |
| sNGAL | 2.815 | 0.954–6.851 | 0.075 |
| uNGAL | 2.941 | 1.527–7.088 | 0.034 |
| smiR-652-3p | 2.903 | 1.475–6.714 | 0.041 |
| umiR-652-3p | 3.418 | 1.889–7.969 | 0.013 |
HR, hazard ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; sNGAL, serum neutrophil gelatinase-associated lipocalin; uNGAL, urinary neutrophil gelatinase-associated lipocalin; smiR-652-3p, serum microRNA-652-3p; umiR-652-3p, urinary microRNA-652-3p.
Discussion
During hospitalization, patients with ADHF have a high risk of developing AKI, which contributes to a poor prognosis and high mortality. Therefore, one challenge for improving the clinical outcomes of patients with ADHF is investigation of novel biomarkers to accurately predict the occurrence of AKI in patients with ADHF.3 Serum creatinine levels are an established biomarker for diagnosing AKI from patients with ADHF, but its clinical use is limited by its delayed results.20 NGAL has recently been used as a classic biomarker of AKI, which is closely related to renal function.21 Our study also showed that serum and urinary NGAL levels were significantly upregulated in patients with ADHF and AKI. Additionally, elevated urinary NGAL levels could be used to predict AKI in patients with ADHF and were associated with the overall survival outcome in patients with AKI. The diagnostic and prognostic value of urinary NGAL levels were better than that for serum NAGL levels. This may be because urinary NGAL levels are more likely reflect injury in the kidney under the setting of ADHF.22 Despite NGAL being generally used to predict AKI, its modest performance in clinical practices leads to an urgent requirement to investigate novel efficient biomarkers to achieve a more accurate early diagnosis of AKI in patients with ADHF.
Accumulated studies have reported that aberrantly expressed miRNAs serve pivotal roles in development and progression of various human diseases, including ADFH and AKI.10,23 The miRNAs can be easily detected from body fluid, such as serum and urine, making them good diagnostic and prognostic tools in human diseases.24 Serum miR-150 expression was found to be decreased in patients with post-acute myocardial infarction and heart failure, and was useful as a biomarker for predicting onset of heart failure.25 Another study by Wang et al.26 provided evidence for elevated circulating miR-21 levels as a diagnostic biomarker of type 2 cardiorenal syndrome in patients with chronic heart failure. With regard to the relationship between miRNAs and AKI, aberrant miR-494 expression was found to predict the prognosis of AKI in children after cardiac surgery.27 A previous study showed that urinary miR-26b levels were elevated in patients with sepsis-associated AKI, and they could serve as a candidate biomarker for occurrence of AKI in patients with sepsis.11 However, whether miRNAs are related to the onset of AKI in patients with ADHF is still unclear.
A previous study by Bernardo et al.14 showed that silencing of miR-652 ameliorated heart function under pathological hypertrophy and cardiac dysfunction. Circulating miR-652 levels are a biomarker of acute coronary syndrome.15 Notably, a study by Bruno et al.16 provided evidence for miR-652-3p as an aberrantly expressed miRNA that was related to worsening of renal function in patients with acute heart failure. However, the clinical significance of miR-652-3p in the relationship with AKI in patients with ADHF remains elusive. In our study, serum and urinary miR-652-3p mRNA expression was higher in patients with ADHF and AKI compared with those without AKI, and served as a risk factor for occurrence of AKI. This finding indicates the potential role of miR-652-3p in determining development of AKI. To determine the clinical value of NGAL in diagnosis of AKI, this study evaluated and compared the diagnostic performance of NAGL and miR-652-3p levels. We found that serum and urinary miR-652-3p levels had a relatively high diagnostic accuracy in differentiating patients with AKI from those without AKI. Additionally, a better diagnostic performance was observed when using urinary miR-652-3p levels than serum levels, which also might be because urinary samples may directly indicate injury in the kidney. Furthermore, this study showed that the combination of urinary NGAL and miR-652-3p levels had a better diagnostic performance compared with any indicator alone. This finding implies that clinical use of urinary NGAL levels for prediction of AKI in patients with ADHF may be improved by considering urinary miR-652-3p levels.
In addition to the diagnostic value, the prognostic value of miR-652-3p was also evaluated by analyzing renal recovery and 180-day survival. We found that high serum and urinary miR-652-3p levels were associated with failure of renal recovery and a poor prognosis in patients with ADHF and AKI. Multivariate analysis showed that miR-652-3p was an independent prognostic factor for predicting renal recovery and survival outcomes in patients with AKI. Although this study provided evidence for the clinical significance of aberrant miR-652-3p levels in patient with ADsHF and AKI, the biological function of miR-652-3p in progression of AKI remains unclear. The regulatory effect of miR-652-3p on cell viability has been reported in endometrial cancer,13 endothelial repair28 and trophoblast cells.29 Whether miR-652-3p also plays a regulatory role in renal cell viability during AKI development is unclear. Therefore, further investigation of this possibility is required.
This study has some limitations. One limitation is the limited sample size, especially the small sample size of patients with AKI. Additionally, the mechanisms underlying aberrantly expressed miR-652-3p acting in development of AKI are unknown. Therefore, further investigations are necessary to confirm the clinical and functional role of miR-652-3p in development and progression of AKI in patients with ADHF.
Taken together, our results show that serum and urinary miR-652-3p levels are elevated in patients with ADHF and AKI, and could serve as a candidate biomarker to predict occurrence of AKI in patients with ADHF. The combination of urinary NGAL and miR-652-3p levels has an improved predictive performance for AKI onset. Additionally, upregulated serum and urinary miR-652-3p levels predict a poor 180-day survival in patients with AKI, and this finding can be used to develop a potential prognostic biomarker. The findings of this study suggest a novel biomarker for diagnosis and prognosis of AKI in patients with ADHF.
Footnotes
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Aili Jiang https://orcid.org/0000-0003-4828-7429
References
- 1.Abdo AS. Hospital Management of Acute Decompensated Heart Failure. Am J Med Sci 2017; 353: 265–274. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C, Bellasi A, Di Lullo L. Implication of Acute Kidney Injury in Heart Failure. Heart Fail Clin 2019; 15: 463–476. [DOI] [PubMed] [Google Scholar]
- 3.Gudsoorkar PS, Thakar CV. Acute Kidney Injury, Heart Failure, and Health Outcomes. Cardiol Clin 2019; 37: 297–305. [DOI] [PubMed] [Google Scholar]
- 4.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019; 394: 1949–1964. [DOI] [PubMed] [Google Scholar]
- 5.Bellos I, Fitrou G, Daskalakis G, et al. Neutrophil gelatinase-associated lipocalin as predictor of acute kidney injury in neonates with perinatal asphyxia: a systematic review and meta-analysis. Eur J Pediatr 2018; 177: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 6.Bertoli G, Cava C, Castiglioni I. MicroRNAs: New Biomarkers for Diagnosis, Prognosis, Therapy Prediction and Therapeutic Tools for Breast Cancer. Theranostics 2015; 5: 1122–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirzaei H, Momeni F, Saadatpour L, et al. MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. J Cell Physiol 2018; 233: 856–865. [DOI] [PubMed] [Google Scholar]
- 8.Fan PC, Chen CC, Chen YC, et al. MicroRNAs in acute kidney injury. Hum Genomics 2016; 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vegter EL, Van Der Meer P, De Windt LJ, et al. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 2016; 18: 457–468. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Chen Y, Du Y, et al. Circulating exosomal miR-92b-5p is a promising diagnostic biomarker of heart failure with reduced ejection fraction patients hospitalized for acute heart failure. J Thorac Dis 2018; 10: 6211–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Wang CJ, Tang XM, et al. Urinary miR-26b as a potential biomarker for patients with sepsis-associated acute kidney injury: a Chinese population-based study. Eur Rev Med Pharmacol Sci 2018; 22: 4604–4610. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Xu Y, Xue S, et al. Implications of dynamic changes in miR-192 expression in ischemic acute kidney injury. Int Urol Nephrol 2017; 49: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Dongol S, Qiu C, et al. miR-652 Promotes Tumor Proliferation and Metastasis by Targeting RORA in Endometrial Cancer. Mol Cancer Res 2018; 16: 1927–1939. [DOI] [PubMed] [Google Scholar]
- 14.Bernardo BC, Nguyen SS, Winbanks CE, et al. Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. FASEB J 2014; 28: 5097–5110. [DOI] [PubMed] [Google Scholar]
- 15.Pilbrow AP, Cordeddu L, Cameron VA, et al. Circulating miR-323-3p and miR-652: candidate markers for the presence and progression of acute coronary syndromes. Int J Cardiol 2014; 176: 375–385. [DOI] [PubMed] [Google Scholar]
- 16.Bruno N, Ter Maaten JM, Ovchinnikova ES, et al. MicroRNAs relate to early worsening of renal function in patients with acute heart failure. Int J Cardiol 2016; 203: 564–569. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen MS, Bohm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 26: 384–416. [DOI] [PubMed] [Google Scholar]
- 18.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013; 61: 649–672. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 20.Moledina DG, Parikh CR. Phenotyping of Acute Kidney Injury: Beyond Serum Creatinine. Semin Nephrol 2018; 38: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaiao SM, Paiva J. Biomarkers of renal recovery after acute kidney injury. Rev Bras Ter Intensiva 2017; 29: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrestha K, Shao Z, Singh D, et al. Relation of systemic and urinary neutrophil gelatinase-associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol 2012; 110: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilflingseder J, Jelencsics K, Bergmeister H, et al. miR-182-5p Inhibition Ameliorates Ischemic Acute Kidney Injury. Am J Pathol 2017; 187: 70–79. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Zhong H. The diagnosis, prognosis, and therapeutic application of MicroRNAs in haematological malignancies. Hematology 2016; 21: 263–271. [DOI] [PubMed] [Google Scholar]
- 25.Lin X, Zhang S, Huo Z. Serum Circulating miR-150 is a Predictor of Post-Acute Myocardial Infarction Heart Failure. Int Heart J 2019; 60: 280–286. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Liang Y, Zhao W, et al. Circulating miRNA-21 as a diagnostic biomarker in elderly patients with type 2 cardiorenal syndrome. Sci Rep 2020; 10: 4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R, Wu Y, Yang L, et al. [Value of serum level of microRNA-494 in predicting prognosis of acute renal injury after cardiac surgery in children]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019; 31: 1469–1473. [DOI] [PubMed] [Google Scholar]
- 28.Huang R, Hu Z, Cao Y, et al. MiR-652-3p inhibition enhances endothelial repair and reduces atherosclerosis by promoting Cyclin D2 expression. EBioMedicine 2019; 40: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Z, Liu B, Li Y, et al. MicroRNA-652-3p promotes the proliferation and invasion of the trophoblast HTR-8/SVneo cell line by targeting homeobox A9 to modulate the expression of ephrin receptor B4. Clin Exp Pharmacol Physiol 2019; 46: 587–596. [DOI] [PubMed] [Google Scholar]