Abstract
Objective
Acute pulmonary embolism (APE) is a serious complication after off-pump coronary artery bypass grafting (OPCABG). We aimed to analyze the risk factors for APE in patients with OPCABG.
Methods
In this retrospective, observational study, patients with OPCABG who were treated in our hospital from 1 January 2018 to 31 March 2020 were included. The basic characteristics of patients and results of preoperative laboratory examinations were collected and analyzed.
Results
A total of 707 patients with OPCABG were included and the incidence of APE was 3.21%. Left ventricular ejection fraction (LVEF), a history of smoking, number of bypass grafting, duration of surgery, and age were significant risk factors for APE in patients with OPCABG. The areas under the curves of LVEF, number of bypass grafting, duration of surgery, and age were 0.773, 0.759, 0.738, and 0.723, respectively. The cutoff values of LVEF, number of bypass grafting, duration of surgery, and age were 59.84, 3.18, 237.42, and 73.28, respectively.
Conclusions
LVEF, a history of smoking, number of bypass grafting, duration of surgery, and age may be risk factors for APE in patients with OPCABG. Early measures should be taken to target these risks to prevent APE.
Keywords: Acute pulmonary embolism, coronary artery bypass grafting, left ventricular ejection fraction, older age, smoking history, operation time
Background
Coronary heart disease is a common type of cardiovascular disease in the clinical setting. The pathological basis of coronary heart disease is that coronary artery lesions lead to reduced blood flow, myocardial cell ischemia, hypoxia, and metabolic disorders in the corresponding blood supply area.1,2 In the early stage, these patients may experience chest tightness, suffocation, tingling, and discomfort after being overworked or with emotional fluctuation.3 Early diagnosis and treatment of this condition are important for the prognosis of patients.4 Coronary artery bypass grafting (CABG) is often used to treat coronary heart disease in the surgical department. CABG can effectively relieve the symptoms of angina pectoris, improve myocardial ischemia, avoid occurrence of myocardial infarction, and improve the quality of life.5 CABG is one of the most effective methods for treating coronary heart disease-related myocardial ischemia.6
Off-pump coronary artery bypass grafting (OPCABG) is effective and safe.7 Compared with pump coronary artery bypass grafting, OPCABG can avoid mechanical damage of blood cells and reperfusion injury. OPCABG may also reduce kidney damage, and reduce resistance of the systemic circulation and pulmonary circulation, and intraoperative hemodynamics are relatively stable.8 However, OPCABG also has perioperative complications, of which acute pulmonary embolism (APE) is a serious or even fatal complication of OPCABG.9 With continuous improvement of surgical techniques, more patients with advanced age, poor cardiac function, and other serious underlying diseases are choosing OPCABG surgery for treatment. Therefore, the incidence of APE after surgery is gradually increasing. A total of 95% of APE occurs within 1 week after surgery in patients with OPCABG.10 The incidence of APE varies from 1.4% to 3.8%, and the mortality of APE can reach up to 38%, which seriously affects the quality of life and prognosis of patients.11 Therefore, this study aimed to investigate the risk factors for APE in patients with OPCABG to provide insight into the prophylaxis of APE after OPCABG.
Methods
Ethics
Our study was a retrospective analysis, and it was certified and approved by the ethics committee of The Affiliated Huai’an Hospital of Xuzhou Medical University (2018012855-c). Written informed consent was obtained from all of the included patients. Furthermore, we de-identified all of the patients’ personal details during data analyses.
Patients
Patients with coronary heart disease who underwent OPCABG in the Department of Cardiothoracic Surgery from 1 January 2018 to 31 March 2020 were identified as potential candidates. The inclusion criteria were as follows: (1) diagnosis of coronary heart disease was in accordance with the related guidelines;12 (2) the patients underwent OPCABG treatment; and (3) patients were well-informed and agreed to participate in this study. The exclusion criteria were as follows: (1) patients with severe liver and kidney injury, cardiomyopathy, lung infection, and other serious conditions; (2) patients with heart valve-related diseases; and (3) patients who disagreed to participate in the present study.
OPCABG treatment
We obtained the patients’ consent for treatment. Coronary artery bypass grafting under simple off-pump circulation was performed clinically. The operations were performed by the same group of surgeons. Before the operation, all patients stopped taking aspirin and clopidogrel, and were administered low-molecular-weight heparin calcium 60 million IU subcutaneously for 2 days. The patients continued to take oral statins, β-blockers, and angiotensin-converting enzyme inhibitor drugs whenever necessary. All patients were prohibited from smoking, drinking, and avoiding strong mood swings and strenuous exercise before surgery. All patients were routinely prepared before surgery, such as having routine blood tests, including measurement of levels of albumin, hemoglobin, blood sugar, creatinine, urea nitrogen, triiodothyronine, thyroxine, thyroid-stimulating hormone, free triiodothyronine, and free thyroxine, arterial oxygen tension, and arterial carbon dioxide tension. Imaging examinations, including computed tomography and ultrasound, were performed for all patients.
All patients underwent general anesthesia, conventional tracheal intubation, and a sternal incision, and an artificial heart–lung machine was used, but not prefilled. We freed the internal mammary artery and used a small dose of heparin (1 mg/kg) to maintain the activated whole blood clotting time for 300 s. We prepared a vascular bridge according to the conventional method. We adjusted the heart rate to approximately 60 minutes by controlling the depth of anesthesia or providing β-blockers. We then used a special sternum retractor to open the sternum and cut the pericardium longitudinally. We made two or three pericardial traction lines parallel to the left phrenic nerve, and sewed another pericardial traction line near the apex to lift the heart. A CTS stabilizer (CTS, Cupertino, CA, USA) was used to locally fix the coronary artery to be anastomosed to reduce the amplitude of the heartbeat and facilitate the operation. Under normal circumstances, left internal mammary artery–anterior descending artery anastomosis was performed first, and then anastomosis of the other target vessel was performed. We continuously monitored the patient’s electrocardiogram, blood pressure, heart rate, and oxygen saturation during the operation.
The patients were routinely returned to the cardiac intensive care unit (ICU) after surgery, and were provided comprehensive care, such as cardiosurgical special care, assisted respiration, comprehensive electrocardiographic monitoring, anti-infection, and nutritional myocardium (recombinant human brain natriuretic peptide [Xinhuosu; Xinhua Bioscience Co., Shanghai, China], 1.5 μg/kg/day; sodium creatine phosphate [Qingxi; Hengrui Co., Lianyungang, Jiangsu, China], 1 g/day). The patients were not on regular anticoagulation post-surgery. If patients experienced pain in the surgical incision postoperatively, painkillers were provided in a timely manner. If patients experienced nausea or vomiting, antiemetic and antispasmodic drugs were administered.
Diagnosis of APE
The diagnosis of APE was made in accordance with the related guidelines from the European Society of Cardiology (2019 update).13,14 All diagnoses of APE were verified by at least two senior doctors. Computed tomography pulmonary angiography was used for the diagnosis of APE with the following direct signs: there was a low-density filling defect in the pulmonary artery, which was partially or completely surrounded by the opaque blood flow (orbital sign), or it was a complete filling defect, and the distal blood vessels were not visible; or the lung field showed a wedge-shaped increased density, a banded high-density zone or discoid atelectasis, a dilated central pulmonary artery, and the distal vascular branches were decreased or disappeared.
Data collection
Two authors independently collected data on the basic characteristics of patients, including age, sex, body mass index, related complications, and a history of smoking. The results of preoperative laboratory examinations, such as albumin and hemoglobin levels, were also collected. Furthermore, details of surgery, such as the left ventricular ejection fraction (LVEF), number of bypass grafting, and duration of surgery, were evaluated and calculated.
Statistical analysis
All of the data were statistically analyzed using IBM SPSS, version 23 software (IBM Corp., Armonk, NY, USA). All continuous data are expressed as mean ± standard deviation, Comparison of binary data was conducted using the chi-square test and comparison of continuous data was performed using the t test. Furthermore, variables with statistical significance in univariate analyses were further included for logistics analyses. Logistics regression analyses were conducted to identify the relevant risk factors for APE. The receiver operating curve (ROC) was created and the area under the curve (AUC) was calculated to analyze its predictive value. A p value <0.05 was considered statistically significant.
Results
General information of the included patients
A total of 707 patients with OPCABG were included in the present study of whom 22 had an attack of APE. The incidence of APE in patients with OPCABG was 3.21%. Age was significantly older, the rates of hypertension, a history of smoking, and occurrence of concomitant deep vein thrombosis were higher, the LVEF was lower, the number of bypass grafting was higher, and the duration of surgery was longer in patients with APE than in those without APE (all p<0.05) (Table 1). There were no significant differences in sex, BMI, the rates of diabetes mellitus and hyperlipidemia, D-dimer levels, N-terminal pro-brain natriuretic peptide levels, high-sensitivity C-reactive protein levels, duration of surgery, length of ICU stay, length of hospital stay, and onset of mobilization after surgery between the two groups of patients.
Table 1.
Characteristics of included patients with off-pump coronary artery bypass grafting.
| Items | APE group (n=22) | Non-APE group (n=685) | t/χ2 | p |
|---|---|---|---|---|
| Age (years) | 75.4±5.46 | 70.4±5.11 | 12.095 | 0.028 |
| Male/female | 16/6 | 503/182 | 1.193 | 0.097 |
| BMI (kg/m2) | 22.5±3.45 | 22.4±2.99 | 1.830 | 0.061 |
| Hypertension | 17 (77.27%) | 208 (30.36%) | 1.126 | 0.005 |
| Diabetes mellitus | 6 (27.27%) | 179 (26.13%) | 1.390 | 0.097 |
| Hyperlipidemia | 4 (18.18%) | 122 (17.81%) | 2.359 | 0.160 |
| History of smoking | 15 (68.18%) | 274 (40%) | 1.104 | 0.031 |
| LVEF (%) | 57.4±3.28 | 86.9±5.06 | 5.344 | 0.006 |
| D-dimer (mg/L) | 4.70±1.73 | 4.59±1.55 | 1.097 | 0.081 |
| NT-proBNP (pg/ml) | 743.43±103.42 | 702.33±94.28 | 24.129 | 0.055 |
| hs-CRP (mg/L) | 10.27±1.33 | 9.12±1.48 | 1.294 | 0.071 |
| Number of bypass grafting | 3.8±1.01 | 2.1±0.74 | 1.102 | 0.036 |
| Duration of surgery (minutes) | 246.5±55.39 | 210.2±49.81 | 9.174 | 0.022 |
| Length of ICU stay (days) | 2.19±1.02 | 2.11±1.14 | 1.277 | 0.084 |
| Length of hospital stay (days) | 7.03±2.29 | 7.10±2.18 | 2.114 | 0.071 |
| Onset of mobilization after surgery (days) | 1.74±0.81 | 1.66±0.42 | 1.022 | 0.094 |
| Occurrence of concomitant deep vein thrombosis | 4 (18.18%) | 12 (1.75%) | 1.125 | 0.017 |
Data are expressed as mean ± standard deviation or n (%).
APE, acute pulmonary embolism; BMI, body mass index; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; hs-CRP, high-sensitivity C-reactive protein; ICU, intensive care unit.
Comparison of preoperative laboratory examination results
There were no significant differences in levels of albumin, hemoglobin, blood sugar, creatinine, urea nitrogen, triiodothyronine, thyroxine, thyroid-stimulating hormone, free triiodothyronine, and free thyroxine, arterial oxygen tension, and arterial carbon dioxide tension between the two groups (Table 2).
Table 2.
Patients’ preoperative laboratory examination results.
| Items | APE group (n=22) | Non-APE group (n=685) | t/χ2 | p |
|---|---|---|---|---|
| Albumin (g/L) | 45.2±5.18 | 44.39±4.96 | 8.197 | 0.059 |
| Hemoglobin (g/L) | 121.4±14.37 | 121.2±14.26 | 7.280 | 0.105 |
| Blood sugar (mmol/L) | 7.7±1.29 | 7.9±1.13 | 1.244 | 0.218 |
| Creatinine (μmol/L) | 76.2±9.06 | 75.5±9.13 | 3.207 | 0.143 |
| Urea nitrogen (mmol/L) | 7.7±1.24 | 7.6±1.27 | 2.128 | 0.095 |
| T3 (mU/L) | 1.3±0.13 | 1.3±0.16 | 1.034 | 0.221 |
| T4 (mU/L) | 95.4±17.36 | 96.3±16.09 | 7.483 | 0.107 |
| TSH (mU/L) | 2.6±0.59 | 2.5±0.74 | 1.228 | 0.085 |
| FT3 (mU/L) | 3.1±0.45 | 3.2±0.50 | 1.830 | 0.191 |
| FT4 (mU/L) | 16.3±2.27 | 16.2±2.19 | 1.122 | 0.106 |
| PaO2 (mmHg) | 84.3±12.11 | 84.7±13.03 | 8.253 | 0.141 |
| PaCO2 (mmHg) | 32.4±5.05 | 31.6±4.94 | 2.153 | 0.930 |
Data are expressed as mean ± standard deviation.
T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone, FT3, free triiodothyronine; FT4, free thyroxine; PaO2, arterial oxygen tension; arterial carbon dioxide tension.
Logistic regression analysis
Logistic regression analysis showed that LVEF, a history of smoking, number of bypass grafting, duration of surgery, and age were significant risk factors for APE in patients with OPCABG (all p<0.05) (Table 3).
Table 3.
Logistic regression analysis on the risk factors for acute pulmonary embolism in patients with off-pump coronary artery bypass grafting.
| Items | β | SE | OR | 95% CI | p |
|---|---|---|---|---|---|
| LVEF | 0.414 | 0.109 | 4.382 | 2.534–5.507 | 0.018 |
| History of smoking | 0.312 | 0.125 | 1.252 | 1.097–1.529 | 0.033 |
| Number of bypass grafting | 0.227 | 0.304 | 1.205 | 0.927–1.603 | 0.024 |
| Duration of surgery | 0.385 | 0.258 | 1.238 | 0.852–1.722 | 0.043 |
| Age | 0.271 | 0.119 | 1.077 | 0.736–1.487 | 0.047 |
SE, standard error; OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection fraction.
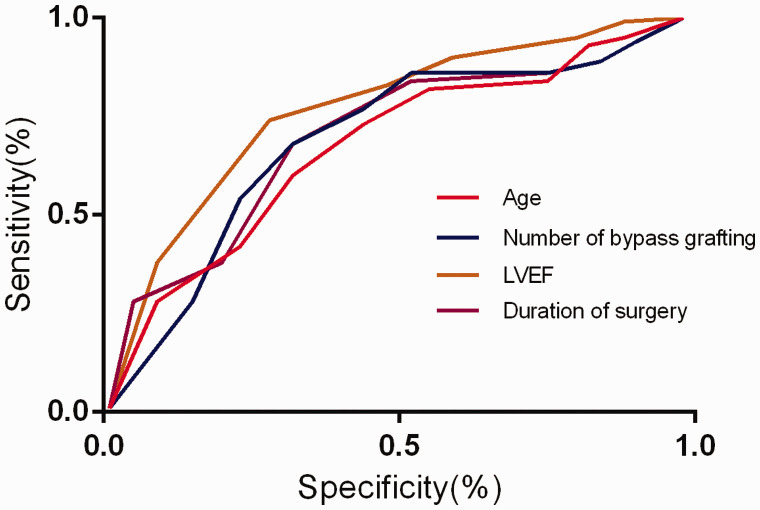
ROC analysis of related factors
The AUCs of LVEF, number of bypass grafting, duration of surgery, and age were 0.773, 0.759, 0.738, and 0.723, respectively (Figure 1 and Table 4). The cutoff values indicated that patients with an LVEF ≤59.84%, number of bypass grafting ≥3.18, duration of surgery ≥237.42 minutes, and age ≥73.28 years had a higher risk for APE after OPCABG.
Figure 1.
Receiver operating curve of relevant risk factors for acute pulmonary embolism in patients with off-pump coronary artery bypass grafting. LVEF, left ventricular ejection fraction.
Table 4.
Predictive value of factors related to acute pulmonary embolism.
| Variables | Cutoff | AUC | 95% CI | SEM |
|---|---|---|---|---|
| LVEF | 59.84 | 0.773 | 0.714–0.843 | 0.082 |
| Number of bypass grafting | 3.18 | 0.759 | 0.628–0.818 | 0.047 |
| Duration of surgery | 237.42 | 0.738 | 0.638–0.794 | 0.033 |
| Age | 73.28 | 0.723 | 0.674–0.787 | 0.048 |
AUC, area under the curve; CI, confidence interval; SEM, standard error of the mean; LVEF, left ventricular ejection fraction.
Discussion
Coronary heart disease, which is a common clinical disease, seriously endangers human health, and its morbidity and mortality have been increasing yearly.15 The main treatment methods for coronary heart disease include conservative medical treatment, percutaneous coronary intervention, and surgical treatment. In patients with surgical treatment, the mid- and long-term effects of this treatment are more obvious, the probability of disease recurrence is lower, and the risk of early and long-term death is relatively reduced compared with patients with conservative medical treatment and percutaneous coronary intervention.16 In recent years, OPCABG has been widely adopted worldwide and continues to develop and improve. OPCABG provides a wide range of ideas and options for treating coronary heart disease.17 OPCABG effectively reduces the adverse effects of cardiopulmonary bypass on various organs and systems of the human body and related complications during and after surgery. Therefore, patients with OPCABG can avoid injury to themselves under nonphysiological conditions while undergoing surgical treatment.18 The effect of OPCABG on the coagulation system causing hypercoagulability must be considered. APE is a serious complication in patients with OPCABG. The incidence of APE in patients with OPCABG in our study was 3.21%, which is similar to previous reports.19 Once APE occurs after OPCABG, patient may have a faster heart rate, shortness of breath, increased cardiac work, decreased oxygen uptake, and the possibility of pulmonary infection increases.20 Finally, APE can increase the risk of hypoxemia and pulmonary infection. If the above-mentioned symptoms and lesions cannot be improved in a timely and effective manner, they can lead to irreversible serious damage to cardiopulmonary function. Additionally, this situation can prolong the patient’s stay in the ICU and increase the financial burden, and a small number of patients with severe hypoxemia may even die.21 Therefore, prevention and avoidance of APE after OPCABG have important practical and clinical significance for patients. The present study showed that patients with an LVEF ≤59.84%, number of bypass grafting ≥3.18, duration of surgery ≥237.42 minutes, and age ≥73.28 years had a higher risk for APE after OPCABG. Therefore, early interventions targeted for these risk factors need to be adopted to improve the prognosis of patients with OPCABG.
Our study showed that an LVEF ≤59.84% was the main risk factor for APE in patients with OPCABG. Previous studies22,23 have shown that there is a certain relationship between the degree of preoperative cardiac dysfunction and formation of venous thrombosis. In patients with insufficiency of cardiac function, the heart is often accompanied by hypertrophy.24 For patients with preoperative cardiac insufficiency, cardiac function should be improved before surgical treatment, which may greatly reduce the incidence and mortality of postoperative APE.
People with a high incidence of coronary heart disease are relatively old, and age is closely related to disorder of the body’s coagulation function.25 Our study showed that older patients had a higher probability of APE after surgery, which is consistent with the results of previous studies.26,27 In elderly people aged older than 70 years, the physiological reserves of various organs are reduced, such as decreased heart, liver, and lung function.28 Elderly people are prone to disturbance of the cardiovascular system. With development of society and improvement of people’s living standards, an increasing amount of elderly patients are undergoing coronary artery bypass surgery.29,30 Special attention should be paid to patients with an older age and adequate preoperative preparations should be made to prevent the occurrence of postoperative APE.
Several previous studies31,32 have shown that smoking is closely related to atherosclerosis and thrombosis. Long-term heavy smoking can damage alveolar epithelial cells, resulting in a reduction in the amount of oxygen diffused. At the same time, this damages tracheal and bronchial epithelial cells, making them prone to bronchospasm, which ultimately leads to thrombosis.33 Previous studies34,35 have shown that patients who smoke have more postoperative sputum, which is thick and difficult to cough up, and easily blocks the airway, compared with those who do not smoke. At the same time, the risk of lung infection is significantly increased in smokers.36 The current study showed that smoking was an important risk factor for APE after OPCABG. Therefore, these patients should be instructed to quit smoking as early as possible to reduce postoperative APE.
Previous studies37–39 have shown that occurrence of APE is positively correlated with the operation time. Our study showed that the possibility of APE occurring increased as the operation time increased, which is consistent with related study results.37–39 A prolonged operation time increases stimulation to the body, and activates and aggregates neutrophils on the surface of the lungs through a series of inflammatory reactions. This then causes damage to endothelial factors of the lungs, leading to formation of thrombi.40 Therefore, to effectively reduce the occurrence of APE, surgeons need to reduce the operation time as much as possible.
There are several limitations in this study. First, we did not include myocardial infarction for consideration owing to the limited collected data. Therefore, further investigation on associations of severity of myocardial infarction and APE in patients with OPCABG are required. Second, several previous studies27,41,42 showed that BMI and diabetes were risk factors for pulmonary embolism. The sample size of our study was small, and it might not have had sufficient power to detect potential related risk factors. Therefore, more studies on risk factors for APE in patients with OPCABG are required in the future. Finally, we only followed up patients for 30 days and no patients died. However, long-term follow-up on the mortality rate of these patients is required.
Conclusions
The LVEF, a history of smoking, number of bypass grafting, duration of surgery, and age may be risk factors for APE in patients with OPCABG. For patients with OPCABG, improvement of cardiac function, quitting smoking as soon as possible, and shortening the operation time during the perioperative period might have an important role in the prophylaxis of postoperative APE.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Suhong Jia https://orcid.org/0000-0003-4323-2461
References
- 1.Peltzer S, Hellstern M, Genske A, et al. Health literacy in persons at risk of and patients with coronary heart disease: A systematic review. Soc Sci Med 2020; 245: 112711. [DOI] [PubMed] [Google Scholar]
- 2.Shao C, Wang J, Tian J, et al. Coronary Artery Disease: From Mechanism to Clinical Practice. Adv Exp Med Biol 2020; 1177: 1–36. [DOI] [PubMed] [Google Scholar]
- 3.Lacey MJ, Raza S, Rehman H, et al. Coronary Embolism: A Systematic Review. Cardiovasc Revasc Med 2020; 21: 367–374. [DOI] [PubMed] [Google Scholar]
- 4.Su JJ, Yu DSF, Paguio JT. Effect of eHealth cardiac rehabilitation on health outcomes of coronary heart disease patients: A systematic review and meta-analysis. J Adv Nurs 2020; 76: 754–772. [DOI] [PubMed] [Google Scholar]
- 5.Khan FM, Hameed I, Milojevic M, et al. Quality metrics in coronary artery bypass grafting. Int J Surg 2019; 65: 7–12. [DOI] [PubMed] [Google Scholar]
- 6.Arsalan M, Mack MJ. Coronary Artery Bypass Grafting Is Currently Underutilized. Circulation 2016; 133: 1036–1045. [DOI] [PubMed] [Google Scholar]
- 7.Gaudino M, Angelini GD, Antoniades C, et al. Off-Pump Coronary Artery Bypass Grafting: 30 Years of Debate. J Am Heart Assoc 2018; 7: e009934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel V, Unai S, Gaudino M, et al. Current Readings on Outcomes After Off-Pump Coronary Artery Bypass Grafting. Semin Thorac Cardiovasc Surg 2019; 31: 726–733. [DOI] [PubMed] [Google Scholar]
- 9.Khan H, Uzzaman M, Benedetto U, et al. On- or off-pump coronary artery bypass grafting for octogenarians: A meta-analysis of comparative studies involving 27,623 patients. Int J Surg 2017; 47: 42–51. [DOI] [PubMed] [Google Scholar]
- 10.Takagi H, Ando T, Mitta S, et al. Meta-Analysis Comparing ≥10-Year Mortality of Off-Pump Versus On-Pump Coronary Artery Bypass Grafting. Am J Cardiol 2017; 120: 1933–1938. [DOI] [PubMed] [Google Scholar]
- 11.Guida GA, Chivasso P, Fudulu D, et al. Off-pump coronary artery bypass grafting in high-risk patients: a review. J Thorac Dis 2016; 8: S795–S798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016; 134: e123–e155. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019; 54: 1901647. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z. Interpretation of the 2019 European Society of Cardiology's Guidelines for the Diagnosis and Treatment of Acute Pulmonary Embolism. Chinese Circulation Journal 2019; 34: 1155–1157. [Google Scholar]
- 15.Albus C, Barkhausen J, Fleck E, et al. The Diagnosis of Chronic Coronary Heart Disease. Dtsch Arztebl Int 2017; 114: 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph J, Velasco A, Hage FG, et al. Guidelines in review: Comparison of ESC and ACC/AHA guidelines for the diagnosis and management of patients with stable coronary artery disease. J Nucl Cardiol 2018; 25: 509–515. [DOI] [PubMed] [Google Scholar]
- 17.Voudris KV, Kavinsky CJ. Advances in Management of Stable Coronary Artery Disease: the Role of Revascularization? Curr Treat Options Cardiovasc Med 2019; 21: 15. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Sung J, Lee JH, et al. Clinical Practice Guideline for Cardiac Rehabilitation in Korea: Recommendations for Cardiac Rehabilitation and Secondary Prevention after Acute Coronary Syndrome. Korean Circ J 2019; 49: 1066–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue X, Sista AK. Catheter-Directed Thrombolysis for Pulmonary Embolism: The State of Practice. Tech Vasc Interv Radiol 2018; 21: 78–84. [DOI] [PubMed] [Google Scholar]
- 20.Pinsky MR. The right ventricle: interaction with the pulmonary circulation. Crit Care 2016; 20: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo HH, Queluz TH. El Dib R: Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev 2016; 1: CD010222. [DOI] [PubMed] [Google Scholar]
- 22.Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 1158–1192. [DOI] [PubMed] [Google Scholar]
- 23.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012; 379: 1835–1846. [DOI] [PubMed] [Google Scholar]
- 24.Koupenova M, Kehrel BE, Corkrey HA, et al. Thrombosis and platelets: an update. Eur Heart J 2017; 38: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van De Hoef TP, Echavarria-Pinto M, Meuwissen M, et al. Contribution of Age-Related Microvascular Dysfunction to Abnormal Coronary: Hemodynamics in Patients With Ischemic Heart Disease. JACC Cardiovasc Interv 2020; 13: 20–29. [DOI] [PubMed] [Google Scholar]
- 26.Mehilli J, Presbitero P. Coronary artery disease and acute coronary syndrome in women. Heart 2020; 106: 487–492. [DOI] [PubMed] [Google Scholar]
- 27.Gregson J, Kaptoge S, Bolton T, et al. Cardiovascular Risk Factors Associated With Venous Thromboembolism. JAMA Cardiol 2019; 4: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lever V, Erdini F, Ghimenton C, et al. Pulmonary Fat Embolism and Coronary Amyloidosis. Am J Case Rep 2018; 19: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Protopapas AD, Baig K, Mukherjee D, et al. Pulmonary embolism following coronary artery bypass grafting. J Card Surg 2011; 26: 181–188. [DOI] [PubMed] [Google Scholar]
- 30.Lee CK, Kim YM, Shim DJ, et al. The detection of pulmonary embolisms after a coronary artery bypass graft surgery by the use of 64-slice multidetector CT. Int J Cardiovasc Imaging 2011; 27: 639–645. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi T, Kato M, Ueda S, et al. Prevalence and significance of clinically unsuspected pulmonary embolism: detection using coronary computed tomography angiography. J Card Surg 2015; 30: 301–306. [DOI] [PubMed] [Google Scholar]
- 32.Falcetta G, Scioti G, Barzaghi C, et al. Pulmonary and paradoxical coronary embolism with a patent foramen ovale. Asian Cardiovasc Thorac Ann 2018; 26: 413–415. [DOI] [PubMed] [Google Scholar]
- 33.Spiropoulou A, Zareifopoulos N, Bellou A, et al. Review of the association between periodontitis and chronic obstructive pulmonary disease in smokers. Monaldi Arch Chest Dis 2019; 89. [DOI] [PubMed] [Google Scholar]
- 34.Jain S, Buttar HS, Chintameneni M, et al. Prevention of Cardiovascular Diseases with Anti-Inflammatory and Anti-Oxidant Nutraceuticals and Herbal Products: An Overview of Pre-Clinical and Clinical Studies. Recent Pat Inflamm Allergy Drug Discov 2018; 12: 145–157. [DOI] [PubMed] [Google Scholar]
- 35.Popovic B, Agrinier N, Bouchahda N, et al. Coronary Embolism Among ST-Segment-Elevation Myocardial Infarction Patients: Mechanisms and Management. Circ Cardiovasc Interv 2018; 11: e005587. [DOI] [PubMed] [Google Scholar]
- 36.Berti A, Matteson EL, Crowson CS, et al. Risk of Cardiovascular Disease and Venous Thromboembolism Among Patients With Incident ANCA-Associated Vasculitis: A 20-Year Population-Based Cohort Study. Mayo Clin Proc 2018; 93: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byard RW. Deep venous thrombosis, pulmonary embolism and long-distance flights. Forensic Sci Med Pathol 2019; 15: 122–124. [DOI] [PubMed] [Google Scholar]
- 39.Beck KS, Cho EK, Moon MH, et al. Incidental Pulmonary Embolism After Coronary Artery Bypass Surgery: Long-Term Clinical Follow-Up. AJR Am J Roentgenol 2018; 210: 52–57. [DOI] [PubMed] [Google Scholar]
- 40.Viana VB, Melo ER, Terra-Filho M, et al. Frequency of Deep Vein Thrombosis and/or Pulmonary Embolism After Coronary Artery Bypass Grafting Investigation Regardless of Clinical Suspicion. Am J Cardiol 2017; 119: 237–242. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Zhou HX, Hu YH, et al. [ Risk factors of pulmonary embolism in senile and non-senile inpatients and the predictive value of Caprini risk assessment model in these two populations]. Zhonghua Yi Xue Za Zhi 2017; 97: 755–760. [DOI] [PubMed] [Google Scholar]
- 42.Nicolay RW, Selley RS, Terry MA, et al. Body Mass Index as a Risk Factor for 30-Day Postoperative Complications in Knee, Hip, and Shoulder Arthroscopy. Arthroscopy 2019; 35: 874–882.e3. [DOI] [PubMed] [Google Scholar]