Abstract
Objective
Waardenburg syndrome type 2 (WS2) is an autosomal dominant syndrome, characterized by bright blue eyes, hearing loss, and depigmented patches of hair and skin. It exhibits high phenotypic and genetic heterogeneity. We explored the molecular etiology in a Chinese family with WS2.
Methods
We recruited a three-generation family with three affected members. Medical history was obtained from all family members who underwent detailed physical examinations and audiology tests. Genomic DNA was extracted from peripheral blood of each individual, and 139 candidate genes associated with hearing loss were sequenced using Illumina HiSeq 2000 (Illumina Inc., San Diego, CA, USA) and verified by Sanger sequencing.
Results
Genetic evaluation revealed a novel nonsense heterozygous variant, NM_006941.4: c.342G>A (p.Trp114Ter) in exon 2 of the SOX10 gene in the three affected patients; no unaffected family member carried the variation. We did not detect the variation in 500 Chinese individuals with normal hearing or in 122 unrelated Chinese families with hearing loss, suggesting that it was specific to our patients.
Conclusions
We identified a novel heterozygous nonsense variation in a family with syndromic hearing loss and WS2. Our findings expand the pathogenic spectrum and strengthen the clinical diagnostic role of SOX10 in patients with WS2.
Keywords: Hereditary hearing loss, Waardenburg syndrome, gene variation, next-generation sequencing, SOX10 gene, nonsense variant
Introduction
Waardenburg syndrome (WS) is characterized by pigmentation abnormalities, including depigmented patches of the skin and hair, vivid blue eyes or heterochromia iridis, and sensorineural hearing loss.1 It is an autosomal dominant inherited disorder involving the neural crest cells and the processes of proliferation, survival, migration, and differentiation.2 Seventy-one percent of patients with WS have hearing loss and it is predominantly bilateral and sensorineural.3 The diagnostic criteria for WS recommended by the Waardenburg Association are as follows: (1) congenital sensorineural deafness; (2) abnormal distribution of the pigment in the iris; that is, complete or partial heterochromia iridis; (3) hair hypopigmentation, mainly presenting as white forehead hair; and (4) inner canthus dislocation. The W index is commonly used for the evaluation of patients with WS. A W index value >1.95 indicates a diagnosis of WS type 1 (WS1), which is different from WS2. (5) First-degree relatives are affected (parents, siblings, children). The secondary diagnostic criteria include the following: (1) hereditary leukoderma; (2) thick and straight eyebrows; (3) broad nose; (4) alae nasi dysplasia; and (5) development of gray hair before the age of 30 years. Patients must satisfy two main diagnostic criteria or one main diagnostic criterion plus two secondary diagnostic criteria to be diagnosed with WSl. The diagnosis of the other three types of WS is made on the basis of the diagnosis of WSl.4,5 Among the different types, WS1 mainly manifests as abnormalities in the frontal area; the difference between WS2 and WS1 is that WS2 is not characterized by an abnormal inner canthal distance. Currently, the literature reports that WS1 and WS2 are the most common types observed in the clinical setting; most studies have shown that WS2 is more common than WS1, whereas WS3 and WS4 are relatively rare.6,7 WS2 with Hirschsprung disease is the criteria for WS4. WS2 can be further classified into five subtypes according to different pathogenic genes or variations: WS2A is caused by MITF gene variations on chromosome 3p13;8 WS2B is caused by a mutation in chromosome 1p21-p13.3;9 WS2C is caused by mutation in chromosome 8p23;10 WS2D is caused by mutation in chromosome 8q11.21;11 and WS2E is caused by mutation in the SOX10 gene on chromosome 22q13.12
Next-generation sequencing is a high-throughput DNA sequencing technology that is based on and developed from Sanger sequencing. Because of its ability to survey the whole exome and genome in an unbiased manner, next-generation sequencing is well suited to identifying the causative mutations of hereditary hearing loss. In this study, we discuss a case of WS2 in a three-generation Chinese family in which we used targeted next-generation sequencing of 139 genes related to deafness to discover new gene mutations that may underlie human congenital disorders that affect ear development.
Materials and methods
Ethics statement
This study was approved by the Committee of Medical Ethics of Lanzhou University Second Hospital. Written informed consent was obtained from all participants or their next of kin on the behalf of the minor or child participants involved in this study.
Participants
A three-generation Chinese family with syndromic hearing loss (SHL) presented to the Department of Otolaryngology and Head and Neck Surgery at Lanzhou University Second Hospital (Lanzhou, China). Clinical evaluations were completed by an otorhinolaryngologist, an ophthalmologist, and a clinical geneticist, and included otoscopic examination, visual reinforcement, audiometry, tympanometry, acoustic reflex, pure-tone audiometry or play audiometry, distortion-product evoked otoacoustic emissions, auditory brainstem responses, and auditory steady-state response. Air conduction (AC) thresholds were determined bilaterally at octave frequencies of 0.25 to 8.0 kHz. The AC average thresholds at conversational frequencies of 0.5, 1, 2, and 4 kHz were measured and used to define the severity of hearing loss. Hearing levels were designated subtle (16–25 dB), mild (26–40 dB), moderate (41–70 dB), severe (71–95 dB), or profound (95 dB).13 The inner canthus, outer canthus, pupillary distance, and W index were calculated as follows: X = [2 (0.2119C + 3.909)]/C; Y = [2A − (0.249B + 3.909)]/B; W = X + Y + (A/B), where A is the inner canthus, B is the pupillary distance, C is the outer canthus, and X and Y are indices. A value of W > 2.07 indicates inner canthus dislocation.14,15 High-resolution computed tomography (CT) and head magnetic resonance imaging (MRI) scans were also performed to verify whether the family members had complications other than hearing disorders.
Peripheral blood samples
After informed consent was obtained, blood samples (2 mL) were obtained from members of the whole pedigree, as well as from 500 healthy control individuals (263 males and 237 females, aged from 18 to 25 years) and 122 unrelated Chinese families with hearing loss.
Targeted gene capture and high-throughput sequencing
Genomic DNA from peripheral blood leukocytes of the participants was obtained using the phenol/chloroform method. The DNA was quantified using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, >3 µg of DNA was used to construct the indexed Illumina libraries (Illumina Inc., San Diego, CA, USA). A total of 3 µg of genomic DNA was fragmented using a Covaris-S220 ultrasonicator (Covaris, Woburn, MA, USA). An “A-tail” was ligated to the 3′ end of each DNA fragment, and Illumina adapters were ligated to the fragments. The target sample size was a 350- to 400-bp product, and the size-selected product was amplified by PCR as follows: Initial denaturation of 98°C for 1 minute, 9 cycles of denaturation at 98°C for 20 s, annealing at 65°C for 30 s, extension at 72°C for 30 s, and a final extension of 72°C for 5 minutes. All samples were checked with a Nanodrop 2000 or Qubit 4 fluorometer (Thermo Fisher Scientific) to determine whether they represented a qualifying captured library. DNA fragments between 350 and 450 bp and the oligonucleotides containing the adapter sequences were selected for the DNA libraries.16
Sanger sequencing
After filtering against multiple databases, all PCR amplified products were purified with a Montage PCR 96 Millipore plate (Millipore, Billerica, MA, USA), and then sequenced using an ABI 3730 Sequencer (Applied Biosystems, Foster City, CA, USA). The sequence data were analyzed and compared with reference sequences of SOX10 (NM_006941.3) using the DNAStar 5.0 (DNAStar, Madison, WI, USA) and BioEdit (Borland, CA, USA) software packages.
Results
Clinical phenotype analysis of WS2 patients
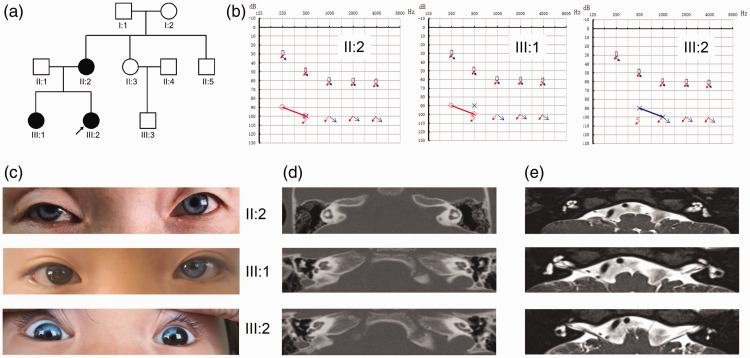
Ten individuals from a three-generation family were included in this study. Figure 1a shows the relationship and the incidence of WS2 within the pedigree. The pedigree included three patients (II-2, III-1, III-2), with III-2 being the proband, and seven healthy family members (I-1, I-2, II-1, II-3, II-4, II-5, and III-3). All three patients presented with bilateral severe sensorineural deafness (Figure 1b); in addition, the proband’s mother (II-2) had bilateral blue irises, and the older sister (III-1) had a blue left iris. Physical examination of the three patients showed that binocular vision, intelligence, digestive system, cardiovascular system, and nervous system were normal (Figure 1c). A full medical history showed that none of the three patients had a history of ear trauma, otitis media, ototoxic drugs, or Hirschsprung disease. The temporal CT scan and head MRI showed no obvious abnormalities (Figure 1d and 1e).
Figure 1.
Pedigree diagrams (a), audiogram information (b), iris evaluation (c), the temporal computed tomography (CT) scan (d), and head magnetic resonance imaging (MRI) results (e). Clinical examinations of the family. (a) Pedigree diagram of the family. Filled symbols represent affected individuals (II: 2, III: 1 and III: 2); empty symbols represent unaffected members; squares and circles represent men and women, respectively, and the arrow denotes the proband (III:2). (b) Audiograms of the affected family members, indicating bilateral severe sensorineural deafness. The horizontal axis shows tone frequency (Hz); the vertical axis shows hearing level (dB). (c) Iris evaluation of the affected siblings. The mother (II-2) and the proband (III-2) presented with bilateral blue irises, and the sister (III-1) had a blue left iris. (d) and (e) Temporal CT scan and head MRI results of the affected family members, showing no obvious abnormalities.
Targeted high-throughput sequencing
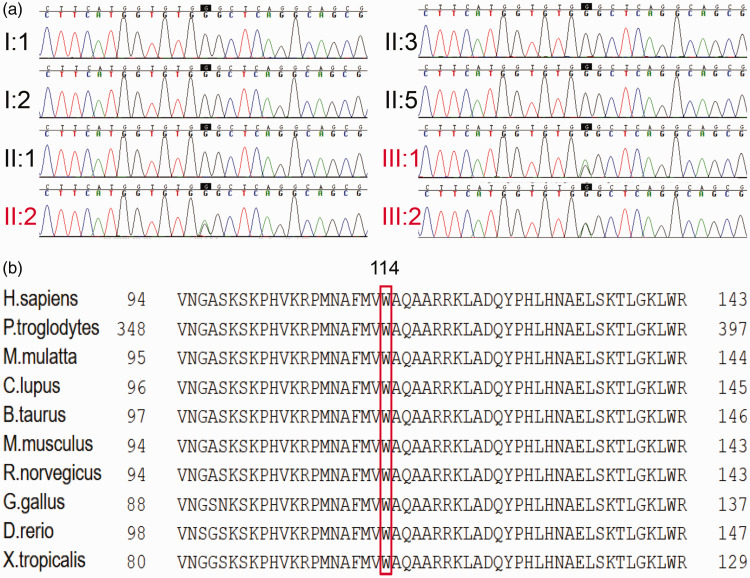
We sequenced all coding exons plus approximately 100 bp of the flanking intronic sequences of 139 deafness genes in the proband (III-2). The average depth of coverage for the targeted regions was 110 to 160×, with >98% of bases having >20× depth of coverage. One novel heterozygous variant NM_006941.4: c.342G>A (p.Trp114Ter) was detected in SOX10. This mutation involves a base replacement at position 342 of the coding region of SOX10, which results in a new termination codon that causes early termination of protein translation. This mutation has not yet appeared in a large population database and has not been reported in the literature (Figure 2a). Furthermore, the NM_006941.4: c.342G>A (p.Trp114Ter) mutation was located in a highly conserved region of SOX10 (Figure 2b).
Figure 2.
Mutation analysis of the family. (a) DNA sequence chromatograms showed a novel nonsense heterozygous mutation (c.342G>A) in the coding region of exon 2 in the SOX10 gene in II-2, III-1, and III-2. (b) Protein alignment analysis showed that the Trp residue (W) at position 114 in SOX10 is conserved across 10 species.
Sanger sequencing
We screened the unaffected family members (I-1, I-2, II-1, II-3, II-4, II-5, and III-3), 500 healthy Chinese individuals, and 122 unrelated Chinese families with apparent hearing loss, but did not detect this variation. Based on current evidence, we confirmed that NM_006941.4: c.342G>A (p.Trp114Ter) was a pathogenic variation.
Discussion
We analyzed the clinical features and molecular etiology of three members of a three-generation Chinese family with WS and other relevant family members. The main symptoms of the affected members of this family (II-2, III-1, III-2) were sensorineural deafness and blue irises. All patients had severe bilateral sensorineural deafness. The proband (III-2) and her sister (III-1) received cochlear implants, and their speech developed well after surgery. The blue irises of the proband (III-2) showed abnormal pigmentation in a fan shape. The mother (II-2) had completely blue irises, whereas the sister (III-1) had a bright blue iris on the left and a normal iris on the right (Figure 1c). None of the three patients had hair or skin pigment changes or gray hair. According to the main diagnostic criteria of WS recommended by the Waardenburg Association, the family conformed to three main diagnostic criteria: congenital sensorineural deafness; complete or partial heterochromia iridis; and affected first-degree relatives. All three patients showed the same base replacement variation [NM_006941.4: c.342G>A (p.Trp114Ter)] at position 342 of the coding region of SOX10, which was verified by Sanger sequencing. The unaffected family members (I-1, I-2, II-1, II-3, II-4, II-5, and III-3) did not carry this variation, and the family was diagnosed as having the WS2E subtype on the basis of genetic typing.
In this study, the inner canthal distances of the proband (III-2), her elder sister (III-1), and their mother (II-2) were 2.03, 2.11, and 1.68 cm, respectively. The current thought, in reference to similar phenotypic cases, is based on classifying WS1 and WS2 according to the angular distance. However, Reynolds et al.17 analyzed clinical phenotypes in 26 WS1 families and 8 WS2 families and found that the W value gradually decreases with age in female patients. They also used statistical analysis to show that because of genetic heterogeneity, WS patients with a W value between 1.51 and 2.27 could have type WS2. In our patient’s family, the proband (III-2) and her sister (III-1) were 5 and 7 years old, respectively, which may explain the high W values.
In previous studies, controversy remains over whether temporal bone CT and head MRI should be routinely conducted in WS patients. According to existing reports, the incidence of temporal bone malformation in WS patients ranges from 0% to 100%, with large variability.18 In 2014, a retrospective study by Kontorinis et al.19 found that inner ear malformation was not a main clinical feature of WS patients. However, studies have found that all patients with WS caused by SOX10 gene variations have inner ear malformations, suggesting that there is a strong correlation between SOX10 mutations and inner ear malformations.18,19 In this family, temporal bone CT and head MRI examination showed no obvious abnormalities in the proband (III-2) (Figure 1d and 1e), and no obvious middle or inner ear malformations were found during cochlear implantation.
Because of the high clinical and genetic heterogeneity of WS, it is difficult to classify and diagnose the disease clinically. The three patients in this study had abnormal W values and SOX10 variations were detected, but there were no obvious inner ear deformities or other clinical symptoms or signs, which reflects the difficulty in classifying this disease.
In our study, targeted next-generation sequencing identified a base substitution at position 342 in SOX10 [NM_006941.4: c.342G>A (p.Trp114Ter)], which, in theory, creates a new stop codon and would be predicted to generate a truncated SOX10 protein. Several nonsense mutations have been reported downstream of this location, suggesting that nonsense mutations are a common pathogenic mechanism.20–22 Moreover, the amino acid residue at the corresponding position of the SRY box protein is highly conserved among different species.
The American College of Medical Genetics and Genomics (ACMG) guidelines are widely used to interpret the significance of genetic variants.23 The variant identified in this family was seen in all affected family members but not in the controls (PS4). The variant is a nonsense variant (PVS1) and co-segregates with the disease in all affected family members (PP1). Bioinformatic analyses predicts it to generate a truncated SOX10 protein (PP3). It located in the highly conserved peptide sequences of SRY box protein, which are highly conserved among different ethnicities (PM4). It has not been reported in the Single Nucleotide Polymorphism Database, The Human Gene Mutation Database, 1000 Genomes Project, ClinVar, or Exome Sequencing Project v. 6500 (PM2). Accordingly, this variant is categorized as pathogenic.
Conclusions
We identified a novel heterozygous nonsense variant, NM_006941.4: c.342G>A (p.Trp114Ter), in a family with autosomal dominant syndromic hearing loss with WS2. Our findings expand the pathogenic spectrum and strengthen the clinical diagnostic role of the SOX10 gene in patients with WS2.
Acknowledgements
We thank the families for their invaluable cooperation and participation.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by grants from the National Natural Science Foundation of China (Nos. 81570926, 81960192), the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (Nos. CY2017-MS18 and CY2017-QN14), the Gansu Provincial Health Industry Research Plan Project (No. GSWSKY2017-11), the Research Fund for Doctoral Tutor of Lanzhou University Second Hospital (No. bdkyjj-02), the Gansu Provincial Youth Science and Technology Fund Projects (No. 1606RJYA227), and Science and Technology Research and Developmental Guidance Plan Project of Lanzhou City (No. 2018-ZD-41).
ORCID iDs
Su-Yang Wang https://orcid.org/0000-0003-1998-2908
Yu-Fen Guo https://orcid.org/0000-0002-9344-8087
References
- 1.Read AP, Newton VE. Waardenburg syndrome. J Med Genet 1997; 34: 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song J, Feng Y, Acke FR, et al. Hearing loss in Waardenburg syndrome: a systematic review. Clin Genet 2016; 89: 416–425. [DOI] [PubMed] [Google Scholar]
- 3.Sankar R. Shah-Waardenburg syndrome. Dermatol Online J 2008; 14: 19. [PubMed] [Google Scholar]
- 4.Liu XZ, Newton VE, Read AP. Waardenburg syndrome type II: phenotypic findings and diagnostic criteria. Am J Med Genet 1995; 55: 95–100. [DOI] [PubMed] [Google Scholar]
- 5.Zaman A, Capper R, Baddoo W. Waardenburg syndrome: more common than you think!. Clin Otolaryngol 2015; 40: 44–48. [DOI] [PubMed] [Google Scholar]
- 6.Silan F, Zafer C, Onder I. Waardenburg syndrome in the Turkish deaf population. Genet Couns 2006; 17: 41–48. [PubMed] [Google Scholar]
- 7.Tamayo ML, Gelvez N, Rodriguez M, et al. Screening program for Waardenburg syndrome in Colombia: clinical definition and phenotypic variability. Am J Med Genet A 2008; 146A: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 8.Tassabehji M, Newton VE, Read AP. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet 1994; 8: 251–255. [DOI] [PubMed] [Google Scholar]
- 9.Lalwani AK, Baldwin CT, Morell R, et al. A locus for Waardenburg syndrome type II maps to chromosome 1p13.3-2.1. Am J Hum Genet 1994; 55: A14. [PMC free article] [PubMed] [Google Scholar]
- 10.Selicorni A, Guerneri S, Ratti A, et al. Cytogenetic mapping of a novel locus for type II Waardenburg syndrome. Hum Genet 2002; 110: 64–67. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Martin M, Rodriguez-Garcia A, Perez-Losada J, et al. SLUG (SNAI2) deletions in patients with Waardenburg disease. Hum Mol Genet 2002; 11: 3231–3236. [DOI] [PubMed] [Google Scholar]
- 12.Hennekam RC, Gorlin RJ. Confirmation of the Yemenite (Warburg) deaf-blind hypopigmentation syndrome. Am J Med Genet 1996; 65: 146–148. [DOI] [PubMed] [Google Scholar]
- 13.Kim NK, Kim AR, Park KT, et al. Whole-exome sequencing reveals diverse modes of inheritance in sporadic mild to moderate sensorineural hearing loss in a pediatric population. Genet Med 2015; 17: 901–911. [DOI] [PubMed] [Google Scholar]
- 14.Farrer LA, Arnos KS, Asher JH, Jr, et al. Locus heterogeneity for Waardenburg syndrome is predictive of clinical subtypes. Am J Hum Genet 1994; 55: 728–737. [PMC free article] [PubMed] [Google Scholar]
- 15.Arias S, Mota M. Apparent non-penetrance for dystopia in Waardenburg syndrome type I, with some hints on the diagnosis of dystopia canthorum. J Genet Hum 1978; 26: 103–131. [PubMed] [Google Scholar]
- 16.Liu XW, Wang JC, Wang SY, et al. The mutation frequencies of GJB2, GJB3, SLC26A4 and MT-RNR1 of patients with severe to profound sensorineural hearing loss in northwest China. Int J Pediatr Otorhinolaryngol 2020; 136: 110143. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds JE, Meyer JM, Landa B, et al. Analysis of variability of clinical manifestations in Waardenburg syndrome. Am J Med Genet 1995; 57: 540–547. [DOI] [PubMed] [Google Scholar]
- 18.Elmaleh-Berges M, Baumann C, Noel-Petroff N, et al. Spectrum of temporal bone abnormalities in patients with Waardenburg syndrome and SOX10 mutations. AJNR Am J Neuroradiol 2013; 34: 1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontorinis G, Goetz F, Lanfermann H, et al. Inner ear anatomy in Waardenburg syndrome: radiological assessment and comparison with normative data. Int J Pediatr Otorhinolaryngol 2014; 78: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 20.Akutsu Y, Shirai K, Takei A, et al. A patient with peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome, and severe hypoganglionosis associated with a novel SOX10 mutation. Am J Med Genet A 2018; 176: 1195–1199. [DOI] [PubMed] [Google Scholar]
- 21.Bocángel MAP, Melo US, Alves LU, et al. Waardenburg syndrome: Novel mutations in a large Brazilian sample. Eur J Med Genet 2018; 61: 348–354. [DOI] [PubMed] [Google Scholar]
- 22.Chen KT, Zong L, Zhan Y, et al. Genetic counseling for a three-generation Chinese family with Waardenburg syndrome type II associated with a rare SOX10 mutation. Int J Pediatr Otorhinolaryngol 2015; 79: 745–748. [DOI] [PubMed] [Google Scholar]
- 23.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]