Highlights
-
•
The first reported case of Trousseau’s syndrome associated with rapidly emerging pancreatic cancer potentially triggered esophagectomy.
-
•
The aggressively emerging pancreatic cancer with mucin production may be a potential mechanism for cancer-related thrombosis.
-
•
When a patient with cancer encountered small, multiple cerebral infarctions postoperatively, the body should be checked for occult malignancy.
Keywords: Trousseau’s syndrome, Surgical stress, Cancer-associated thrombosis, Occult malignant tumor, Case report
Abstract
Introduction
Trousseau’s syndrome is characterized as an unexpected, cancer-associated thrombotic event. We describe the first reported case of Trousseau’s syndrome associated with rapidly emerging pancreatic cancer potentially triggered by esophagectomy.
Presentation of case
A 79-year-old asymptomatic male with clinical stage I esophageal squamous cell carcinoma underwent thoracoscopic subtotal esophagectomy. On postoperative day 46, the patient presented with weakness of his left upper extremity due to multiple cerebral and cerebellar infarctions, with no evidence of atherosclerotic or cardiogenic thrombi. An abdominal computed tomography (CT) showed a pancreatic tumor with multiple liver metastases. Extremely high D-dimer and the CT findings suggested Trousseau’s syndrome associated with a rapidly emerging neoplasm as the etiology of the brain infarction. Although further thrombotic events did not occur, his condition deteriorated rapidly and died on the 31st days of onset. The autopsy revealed multiple small infarctions, with multiple thrombi in the cerebral hemispheres, brain stem, and cerebellum. Histological evaluation revealed pancreatic adenocarcinoma with nodal and liver metastases.
Discussion
A hypercoagulable state associated with the aggressively emerging pancreatic adenocarcinoma, accompanied by cancer cell production of mucin, may be a potential mechanism for cancer-related thrombosis.
Conclusion
In patients who received intensive surgical treatment and encountered unexplained brain infarctions in the multi-arterial territory, Trousseau’s syndrome should be considered, and investigation for occult malignancy is required.
1. Introduction
Trousseau’s syndrome currently refers to thrombotic events, such as cerebral infarction, deep vein thrombosis (DVT) or pulmonary embolism, in patients with malignancy, unexplained by any apparent factors that occur concomitantly with either occult or a recently diagnosed carcinoma [[1], [2], [3], [4]]. This disorder is considered to be a fatal condition with a poor prognosis [5].
Patients with malignant disease have a higher risk of a hypercoagulable condition even before cancer diagnosis, and its risk is more significant in more advanced stages of cancer [1,6,7]. Although the apparent correlation between cancer stage and a hypercoagulable risk suggests a biological gradient between cancer activity and arterial thromboembolism risk [6], little is known of the precise mechanism underlying Trousseau’s syndrome.
It has been known that pancreatic cancers could be associated with a wide spectrum of paraneoplastic syndromes, including Trousseau’s syndrome [8], to our knowledge, there has been no reported case of Trousseau’s syndrome in association with rapidly emerging pancreatic cancer potentially triggered by surgery. Here, we present a rare case of Trousseau’s syndrome associated with rapidly emerging pancreatic adenocarcinoma at six weeks after surgery for esophageal squamous cell carcinoma (SCC). The work has been reported in line with the SCARE 2018 criteria [9].
2. Presentation of case
A 79-year-old asymptomatic male was diagnosed with esophageal SCC in an annual medical checkup and referred to our department for surgical treatment. He consumed 20 drinks of alcohol a week and had a past 40-pack-year smoking history. He was on medication for hypertension and had no other specific systemic disease. Endoscopy and endoscopic ultrasonography of the esophagus showed a 0-IIa type tumor with submucosal involvement in the lower thoracic esophagus (Fig. 1). Laboratory tests on admission showed elevated levels of cytokeratin fragment 21-1 at 3.7 ng/mL and carbohydrate antigen 19-9 (CA 19-9) at 63.8 U/mL but did not show any disorder in other tests including blood coagulation tests and other tumor markers: a prothrombin time-international normalized ratio (PT-INR), 0.94; D-dimer, 0.5 μg/mL; carcinoembryonic antigen, 1.8 ng/mL; and SCC-related antigen, 1.1 ng/mL. Venous ultrasonography of the lower limbs, performed as a part of preoperative workup, did not show any DVT. Doppler ultrasound of the neck showed only thick intima within bilateral carotid arteries. Computed tomography (CT) from the neck to pelvis, which was taken 24 days befor surgery, did not reveal lymph node involvement, distant metastasis or other concomitant tumors (Fig. 2). The patient was diagnosed with stage I disease (cT1b N0 M0) and underwent thoracoscopic subtotal esophagectomy with cervical esophagogastric anastomosis. Anastomotic leakage occurred postoperatively and was managed by percutaneous drainage and enteral nutrition (Clavien-Dindo grade 3a).
Fig. 1.
(A) Endoscopic image showing a slightly elevated lesion (0-IIa type), occupying one-fourth of the circumference of the lower thoracic esophagus. (B) Endoscopic ultrasonography showing hypoechogenic destruction of the interface between the second and third sonographic layers.
Fig. 2.
Preoperative abdominal CT showing (A) no findings of liver metastasis and (B) no evidence of pancreatic head tumor and lymph nodes swelling.
On postoperative day 46, the patient complained of weakness of his left upper extremity. Although magnetic resonance angiography showed no overt occlusion of head and neck arteries, magnetic resonance imaging with diffusion-weighted image revealed multiple high-intensity areas in both cerebral hemispheres and the cerebellum (Fig. 3). Electrocardiogram, Holter monitoring and echocardiography showed no arrhythmia and no evidence of atherosclerotic or cardiac thrombus. Laboratory tests at the onset revealed a platelet count of 17.3 104/μL, a PT-INR of 1.12 and fibrinogen of 562 mg/dL, and markedly high levels of D-dimer at 28.2 μg/mL, CA 19-9 at 5594.0 U/mL, and carbohydrate antigen 125 (CA 125) at 277.3 U/mL. The patient’s condition did not fulfill the diagnostic criteria for disseminated intravascular coagulation (DIC). Extremely high levels of CA 19-9 and CA125 reminded us to investigate concealed cancer. The contrast-enhanced CT showed a pancreatic head tumor with multiple liver metastases (Fig. 4). Although further embolic strokes did not occur after commencing injection of intravenous heparin (10,000 units/day), the patient’s condition deteriorated gradually, and he died on the 31 st days of onset.
Fig. 3.
Diffusion weighted magnetic resonance imaging of the brain showing multiple high-intensity areas in both (A) cerebral hemispheres and (B) the cerebellum.
Fig. 4.
On postoperative day 46, abdominal CT showing findings of (A) multiple liver metastases and (B) pancreatic head tumor with regional lymph nodes swelling.
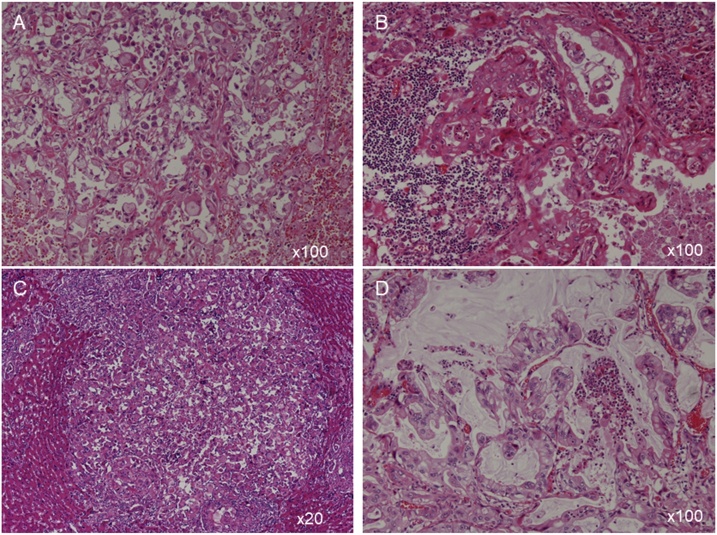
The autopsy was performed 5 h after his death. An 8-cm tumor was present in the head of the pancreas. The pancreatic adenocarcinoma, histologically, has various morphological features, including glandular, papillary, solid structures and signet ring cells with abundant mucin (Fig. 5A). Multiple cancerous lesions in para-pancreatic lymph nodes, liver, both lungs, peritoneum and a right adrenal gland had similar pathological features (Fig. 5B–D), indicating they were metastases from pancreatic cancer. In the right lung, red thrombus was detected in the pulmonary artery. In the brain, multiple small infarctions were found in the cerebrum and pons, and carcinoma cells were absent. Hence, the patient contracted Trousseau’s syndrome associated with rapidly emerging pancreatic adenocarcinoma at 46 days after the surgical intervention for esophageal SCC.
Fig. 5.
Histological examination of (A) the pancreas (×100), (B) para-pancreatic lymph node (×100), (C) liver (×20) and (D), right lung (×100), showing various morphological features, including glandular, papillary, solid structures and signet ring cells with abundant mucin.
3. Discussion
Trousseau’s syndrome, also referred to as Trousseau’s sign of malignancy, was first described by a French physician Armand Trousseau (1801–1867), among his many contributions to modern medicine including Trousseau’s sign of latent tetany and Trousseau-Lallemand bodies (now referred to as Bence-Jones proteins), and is a type of paraneoplastic syndrome caused by predominantly adenocarcinomas, presenting as migrating and recurrent episodes of thrombophlebitis [2,3,[10], [11], [12], [13]]. Trousseau’s syndrome, which he called “phlegmasia alba dolens” referring to the DVT of extremities in 1865, has become a popular synonym of cancer-related hypercoagulability. Trousseau’s syndrome involves a much wider spectrum of clinical manifestations than the DVT and includes pulmonary thromboembolism, thrombophlebitis, nonbacterial verrucous endocarditis, arterial thromboembolization to the chronic DIC [3]. Ironically, he diagnosed himself with a DVT of the left upper limb, which led to him being diagnosed with a malignant disease and subsequently dying of gastric adenocarcinoma in that year [12,13].
Potential causes of brain infarction that follow subtotal esophagectomy for cancer include atherosclerotic thrombus, cardiogenic thrombus, thrombus from the stump of the arch of the azygos vein, tumor embolization, and Trousseau’s syndrome. Trousseau’s syndrome commonly occurs in lung (17%), pancreas (10%), colon and rectum (8%), kidney (8%), and prostate (7%) cancers [14]. Frequency of thrombotic events in pancreatic carcinomas highly varies in different studies (17–57%) [15], but it is evident that the relative risk for venous thromboembolic events is significantly increased. Meta-analysis data from 40 reports indicated a 6.1-fold elevation, that figure is among the tumor with the highest risk [16]. Compared to the patients with squamous cell carcinoma, those with adenocarcinoma have a 1.65-fold higher probability of developing an embolism (98% confidence interval: 1.20–2.29) [17].
In a series of 19 cancer cases presenting with cerebral infarction Chen et al. reported that 84% of patients had infarctions in the multi-arterial territory and 79% had significantly elevated D-dimer [18], which was consistent with other case series in the literature [19,20]. When extensive stroke evaluation failed to identify a primary cerebrovascular explanation in patients whose imaging showed multiple infarct lesions that exceed the territory of a single vessel, it was a key clue for Trousseau’s syndrome. High level of plasma D-dimer in patients with Trousseau’s syndrome suggested hypercoagulability, even though they did not reach international DIC criteria [[21], [22], [23]]. In our case, multiple cerebral and cerebellar infarctions occurred, with no evidence of atherosclerotic or cardiogenic thrombi, and extremely high value of D-dimer observed at the onset.
Previous studies revealed that venous thromboembolism was strongly linked to a malignant tumor, and the highest risk was seen in mucin-producing adenocarcinomas and metastatic tumors [24,25]. Although the pathophysiology of the phenomena is not well established, the mucin compounds produced by adenocarcinomas have been found to interact with the selectin family of adhesion molecules, leading to platelet activation and aggregation without the help of thrombin [2,22,[26], [27], [28], [29]]. In our case, the elevated CA125 level, a potential biomarker for a mucin-producing tumor [28], was consistent with the presence of mucin in the tumor. Tissue factor is produced in various types of tumor cells, and a primary cellular initiator of fluid-phase blood coagulation that changes factor VII (FVII) to its activated form (FVIIa) and initiates the extrinsic coagulation pathway [2]. An increase in tissue factor production might explain the higher incidence of Trousseau's syndrome in patients with aggressive tumor features.
Recovery is impaired in patients with Trousseau’s syndrome [5,18,30] and there is no established evidence supporting anticoagulant treatment for this condition [1,4]. Systemic heparinization is considered an effective treatment strategy to ameliorate symptoms and prevent further thromboembolic events to a certain extent [4,22,31,32]. Previous studies suggest that warfarin is less effective than heparin in reducing the rate of recurrent embolization [21,33]. Combination therapies comprising anti-coagulation treatment and chemotherapy or targeted molecular treatment has recently succeeded in inhibiting reported thrombosis and controlling the tumor [34,35].
Surgery is considered to be a common and effective treatment for patients with solid malignancies. However, evidence from animal and clinical trials demonstrated that surgical stress is potentially a powerful factor promoting malignant growth and enhancing metastatic seeding of tumor cells [[36], [37], [38], [39]]. Various factors, such as inflammatory response to surgery, ischemia/reperfusion damage, infection, stress on the immune system, and interruption of tumor dormancy in the systemic response to surgery trigger, may promote cancer metastasis [40,41]. In our patient, the initial enhanced CT scan, which was taken 24 days before surgery, did not show any evidence of liver metastases. If a 3-phase liver scan were performed in the initial enhanced CT examination, there have been a possibility that could detect liver metastases before the esophagectomy. However, in fact, the initial CT scan did not detect any enlarged lymph nodes before the surgery. Therefore, we speculated that residual occult pancreatic adenocarcinoma rapidly grew and spread throughout the body shortly after thoracoscopic esophagectomy, which could result in surgical stress-induced worsening of the patient’s prognosis.
In our case, multiple cerebral and cerebellar infarctions occurred, with no evidence of atherosclerotic or cardiogenic thrombi. Moreover, extremely elevated D-dimer level indicated a hypercoagulable state at the onset, while elevated CA125 and the presence of mucin-producing adenocarcinoma as a component of the pancreatic carcinoma indicated the possibility of a cancer-related thrombus as an etiology of the brain infarction. These findings suggested that Trousseau’s syndrome, linked to a rapidly emerging pancreatic adenocarcinoma potentially triggered by surgical stress of esophagectomy, was the most probable etiology.
4. Conclusion
We report the first case of Trousseau’s syndrome associated with rapidly emerging pancreatic adenocarcinoma triggered by surgical stress of esophagectomy. When a patient with cancer encountered small, multiple cerebral infarctions postoperatively, the whole body should be carefully checked for any evidence of concomitant occult malignancy. Furthermore, monitoring of D-dimer level during postoperative care, regardless of the primary tumor site and histological type, could help early detection and treatment of a life-threatening cancer-related thrombosis.
Declaration of Competing Interest
Neither the authors nor Osaka Medical College Hospital has gained financially or otherwise by advancing this research. No conflicts of interest are declared.
Funding
All authors have no source of funding to disclose.
Ethical approval
Ethical approval for this report has been exempted by our institution.
Consent
Written informed consent was obtained from the patient’s kin for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors contribution
SWL wrote the manuscript and prepared the manuscript under the supervision of YH and KU. SWL, RT and YI performed the surgery including the patient’s care. HK and YH performed pathological investigation. The authors read and approved the final manuscript.
Registration of research studies
Our paper is a case report, no registration was done for it.
Guarantor
Dr Sang-Woong Lee, corresponding author of this article.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
The authors are grateful for the cooperation of all staff engaged in the patient’s treatment.
References
- 1.Evans T.R., Mansi J.L., Bevan D.H. Trousseau’s syndrome in association with ovarian carcinoma. Cancer. 1996;77(12):2544–2549. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2544::AID-CNCR18>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrier M., Le Gal G., Wells P.S., Fergusson D., Ramsay T., Rodger M.A. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann. Intern. Med. 2008;149(5):323–333. doi: 10.7326/0003-4819-149-5-200809020-00007. [DOI] [PubMed] [Google Scholar]
- 4.Ikushima S., Ono R., Fukuda K., Sakayori M., Awano N., Kondo K. Trousseau’s syndrome: cancer-associated thrombosis. Jpn. J. Clin. Oncol. 2016;46(3):204–208. doi: 10.1093/jjco/hyv165. [DOI] [PubMed] [Google Scholar]
- 5.Cestari D.M., Weine D.M., Panageas K.S., Segal A.Z., DeAngelis L.M. Stroke in patients with cancer: incidence and etiology. Neurology. 2004;62(11):2025–2030. doi: 10.1212/01.wnl.0000129912.56486.2b. [DOI] [PubMed] [Google Scholar]
- 6.Navi B.B., Reiner A.S., Kamel H., Iadecola C., Okin P.M., Elkind M.S.V., Panageas K.S., DeAngelis L.M. Risk of arterial thromboembolism in patients with cancer. J. Am. Coll. Cardiol. 2017;70(8):926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navi B.B., Reiner A.S., Kamel H., Iadecola C., Okin P.M., Tagawa S.T., Panageas K.S., DeAngelis L.M. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2019;133(8):781–789. doi: 10.1182/blood-2018-06-860874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zalatnai A., Perjesi E., Galambos E. Much more than trousseau syndrome. The broad spectrum of the pancreatic paraneoplastic syndromes. Pathol. Oncol. Res. 2018;24(1):1–10. doi: 10.1007/s12253-017-0206-6. [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A.J., Orgill D.P., Group S The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Trousseau A. vol. 5. Edited by Society TNS; London: 1865. Phlegmasia alba dolens; pp. 281–332. (Lectures on Clinical Medicine). [Google Scholar]
- 11.Callander N., Rapaport S.I. Trousseau’s syndrome. West. J. Med. 1993;158(4):364–371. [PMC free article] [PubMed] [Google Scholar]
- 12.Khorana A.A. Malignancy, thrombosis and Trousseau: the case for an eponym. J. Thromb. Haemost. 2003;1(12):2463–2465. doi: 10.1111/j.1538-7836.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 13.Young P., Bravo M.A., Gonzalez M.G., Finn B.C., Quezel M.A., Bruetman J.E. Armand Trousseau (1801-1867), his history and the signs of hypocalcemia. Rev. Med. Chil. 2014;142(10):1341–1347. doi: 10.4067/S0034-98872014001000017. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen H.T., Mellemkjaer L., Olsen J.H., Baron J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 15.Khorana A.A., Fine R.L. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5(11):655–663. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
- 16.Iodice S., Gandini S., Lohr M., Lowenfels A.B., Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J. Thromb. Haemost. 2008;6(5):781–788. doi: 10.1111/j.1538-7836.2008.02928.x. [DOI] [PubMed] [Google Scholar]
- 17.Bick R.L. Coagulation abnormalities in malignancy: a review. Semin. Thromb. Hemost. 1992;18(4):353–372. doi: 10.1055/s-2007-1002575. [DOI] [PubMed] [Google Scholar]
- 18.Chen W., He Y., Su Y. Multifocal cerebral infarction as the first manifestation of occult malignancy: case series of trousseau’s syndrome and literature review. Brain Circ. 2018;4(2):65–72. doi: 10.4103/bc.bc_1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon H.M., Kang B.S., Yoon B.W. Stroke as the first manifestation of concealed cancer. J. Neurol. Sci. 2007;258(1–2):80–83. doi: 10.1016/j.jns.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Mai H., Xia J., Wu Y., Ke J., Li J., Pan J., Chen W., Shao Y., Yang Z., Luo S. Clinical presentation and imaging characteristics of occult lung cancer associated ischemic stroke. J. Clin. Neurosci. 2015;22(2):296–302. doi: 10.1016/j.jocn.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 21.el-Shami K., Griffiths E., Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12(5):518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 22.Ladizinski B., Federman D.G. Trousseau syndrome. CMAJ. 2013;185(12):1063. doi: 10.1503/cmaj.121344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferroni P., Martini F., Portarena I., Massimiani G., Riondino S., La Farina F., Mariotti S., Guadagni F., Roselli M. Novel high-sensitive D-dimer determination predicts chemotherapy-associated venous thromboembolism in intermediate risk lung cancer patients. Clin. Lung Cancer. 2012;13(6):482–487. doi: 10.1016/j.cllc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Hillen H.F. Thrombosis in cancer patients. Ann. Oncol. 2000;11(Suppl. 3):273–276. doi: 10.1093/annonc/11.suppl_3.273. [DOI] [PubMed] [Google Scholar]
- 25.Hisada Y., Geddings J.E., Ay C., Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J. Thromb. Haemost. 2015;13(8):1372–1382. doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahrenbrock M., Borsig L., Le D., Varki N., Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J. Clin. Invest. 2003;112(6):853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasthuri R.S., Taubman M.B., Mackman N. Role of tissue factor in cancer. J. Clin. Oncol. 2009;27(29):4834–4838. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jovin T.G., Boosupalli V., Zivkovic S.A., Wechsler L.R., Gebel J.M. High titers of CA-125 may be associated with recurrent ischemic strokes in patients with cancer. Neurology. 2005;64(11):1944–1945. doi: 10.1212/01.WNL.0000163850.07976.63. [DOI] [PubMed] [Google Scholar]
- 29.Tachihara M., Nikaido T., Wang X., Sato Y., Ishii T., Saito K., Sekine S., Tanino Y., Ishida T., Munakata M. Four cases of Trousseau’s syndrome associated with lung adenocarcinoma. Intern. Med. 2012;51(9):1099–1102. doi: 10.2169/internalmedicine.51.6453. [DOI] [PubMed] [Google Scholar]
- 30.Hong C.T., Tsai L.K., Jeng J.S. Patterns of acute cerebral infarcts in patients with active malignancy using diffusion-weighted imaging. Cerebrovasc. Dis. 2009;28(4):411–416. doi: 10.1159/000235629. [DOI] [PubMed] [Google Scholar]
- 31.Taccone F.S., Jeangette S.M., Blecic S.A. First-ever stroke as initial presentation of systemic cancer. J. Stroke Cerebrovasc. Dis. 2008;17(4):169–174. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Finelli P.F., Nouh A. Three-territory DWI acute infarcts: diagnostic value in cancer-associated hypercoagulation stroke (Trousseau syndrome) AJNR Am. J. Neuroradiol. 2016;37(11):2033–2036. doi: 10.3174/ajnr.A4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers L.R., Cho E.S., Kempin S., Posner J.B. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am. J. Med. 1987;83(4):746–756. doi: 10.1016/0002-9343(87)90908-9. [DOI] [PubMed] [Google Scholar]
- 34.Masubuchi H., Maeno T., Uchida M., Kono S., Suzuki M., Takemura M., Yamaguchi A., Yamaguchi K., Kanbe M., Kitahara S. A case of Trousseau syndrome caused by pulmonary adenocarcinoma that was controlled for one year and 10 months with thrombosis treatment using an EGFR tyrosine kinase inhibitor and chemotherapy. Respir. Med. Case Rep. 2015;15:101–105. doi: 10.1016/j.rmcr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nonagase Y., Takeda M., Tanaka K., Hayashi H., Iwasa T., Nakagawa K. Treatment of EGFR mutation-positive non-small cell lung cancer complicated by Trousseau syndrome with gefitinib followed by osimertinib: a case report. Oncotarget. 2018;9(50):29532–29535. doi: 10.18632/oncotarget.25687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krall J.A., Reinhardt F., Mercury O.A., Pattabiraman D.R., Brooks M.W., Dougan M., Lambert A.W., Bierie B., Ploegh H.L., Dougan S.K. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018;10(436) doi: 10.1126/scitranslmed.aan3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coffey J.C., Wang J.H., Smith M.J., Bouchier-Hayes D., Cotter T.G., Redmond H.P. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4(12):760–768. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya Y., Sawada S., Yoshioka I., Ohashi Y., Matsuo M., Harimaya Y., Tsukada K., Saiki I. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133(5):547–555. doi: 10.1067/msy.2003.141. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Eliyahu S., Page G.G., Yirmiya R., Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer. 1999;80(6):880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Zhang P., Xu Y., Yan J., Liu Z., Lau W.B., Lau W.B., Li Y., Zhao X., Wei Y. Surgical stress and cancer progression: the twisted tango. Mol. Cancer. 2019;18(1):132. doi: 10.1186/s12943-019-1058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav. Immun. 2003;17(Suppl. 1):S27–36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]