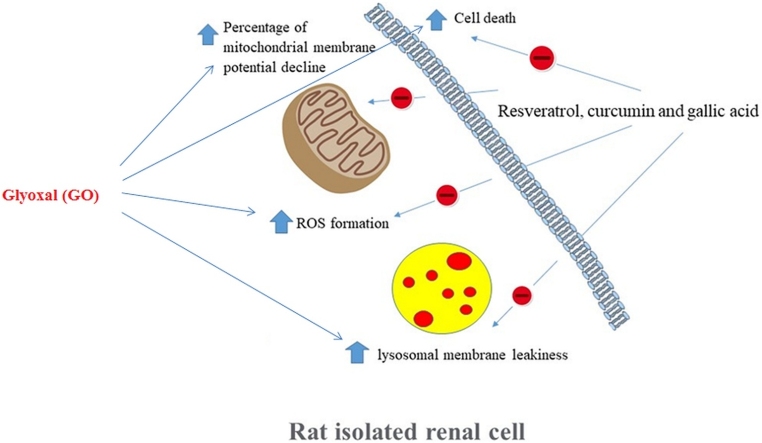
Graphical abstract
Keywords: Diabetes, Curcumin, Gallic acid, Glyoxal, Kidney, Animal, Polyphenols, Natural products
Highlights
-
•
RES, CUR and GA protected renal cells from GO–induced cells death.
-
•
RES, CUR and GA reduced formation of ROS.
-
•
RES, CUR and GA diminished lipid peroxidation products.
-
•
RES, CUR and GA repressed GO-induced mitochondrial membrane potential collapse.
-
•
RES, CUR and GA decreased lysosomal membrane leakage in GO-treated cells.
Abstract
Glyoxal (GO), a by-product of glucose auto-oxidation, is involved in the glycation of proteins/ lipids and formation of advanced glycation (AGE) and lipoxidation (ALE) end products. AGE/ALE were shown to contribute to diabetic complications development/progression such as nephropathy. Diabetic nephropathy progression has an oxidative nature. Given the antioxidant effects of polyphenols, potential protective effects of resveratrol, curcumin and gallic acid, in rat renal cells treated with GO, were evaluated in the present work. According to our results, incubation of GO with the cells reduced their viability and led to membrane lysis, reactive oxygen species (ROS) formation, lipid peroxidation, mitochondrial membrane potential collapse, and lysosomal membrane leakage. These findings were prevented by pre-treatment with resveratrol, curcumin and gallic acid. Mitochondrial and lysosomal toxic interactions appear to worsen oxidative stress/cytotoxicity produced by GO. Resveratrol, curcumin and gallic acid inhibited ROS formation and attenuated GO-induced renal cell death.
1. Introduction
Advanced glycation (AGE) and lipoxidation (ALE) end products are modified proteins and lipids that are generated after proteins and lipids come into contact with aldose sugars, via non-enzymatic glycation and oxidation reactions. Formation of these products can lead, among others, to Alzheimer’s disease, cancer, aging and complications of chronic increment of blood sugar such as diabetic nephropathy [[1], [2], [3]]. Oxidative stress contributes to diabetic nephropathy pathogenesis. Binding of AGE to receptors (RAGE) in diabetics, leads to a rise in reactive oxygen species (ROS) and reactive carbonyl species level, but a decline in the capacity of the body to combat oxidative stress [4]. ROS are highly reactive/unstable molecules and in case an appropriate adaptation by endogenous antioxidant arsenal is lacking [5,6], their accumulation triggers stress-sensitive intracellular signaling pathways activation, promoting cellular damage and development/ progression of diabetic complications [7].
The use of phytochemical components has been extensively proposed for amelioration of such oxidative modifications. Demands for herbal medicines in the treatment of various pathological conditions are increasing globally, thus, selection of appropriate phytochemical compounds is of crucial importance in clinical applications [[8], [9], [10]]. Generally, herbal preparations contain numerous secondary metabolites, and determination of bioactive compounds responsible for the pharmacological activities is critically important to produce proper and acceptable medicines in a greater extent [11]. Resveratrol, a phenolic compound, is found in wines and different parts of grape [12,13]. Resveratrol exerts several biological effects including prevention of lipid peroxidation, stimulation of nitric oxide production, scavenging of free radicals, and anti-inflammatory properties [[14], [15], [16], [17], [18]]. Considering in vitro and in vivo findings, resveratrol reduces oxidative stress by scavenging ROS, diminishes apoptosis in renal cells and prevents renal ischemia [[19], [20], [21], [22], [23]]. Curcumin as a polyphenol has shown anti-inflammatory effects, and it has beneficial effects against diabetes, psoriasis, Alzheimer's disease and cancer [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. Curcumin is an antioxidant agent that reduces the damage caused by ROS and it was shown to prevent the occurrence of these diseases [[35], [36], [37]]. In Drosophila melanogaster, copper-induced oxidative stress was alleviated by curcumin [38]. Also, in rats treated with sodium nitrite, curcumin produced hepatoprotective effects [39]. Gallic acid derivatives exert anti-free radical, anti-microbial and anti-allergic properties, and could disrupt ROS signaling pathways [[40], [41], [42], [43], [44], [45]].
Glyoxal (GO) is a natural metabolite of glucose. GO reactive carbonyl group can form AGEs via reacting with proteins’ amino groups. GO level increases in diabetic patients due to impaired glucose metabolism and it is a causative factor in renal dysfunction. In an in vivo study, gallic acid could exert beneficial effects against GO-induced renal fibrosis [46]. Since GO-induced AGE/ALE formation has an oxidative pathophysiology, and given the antioxidant effects of polyphenols, we evaluated the effects of resveratrol, curcumin and gallic acid, in GO-induced oxidative modifications in isolated rat renal cells.
2. Materials and methods
2.1. Reagents
Resveratrol, curcumin, gallic acid, chloroquine, dimethyl sulfoxide (DMSO), Sigma-Aldrich), 2′,7′-Dichlorofluorescein diacetate (DCFDA), trypan blue, thiobarbituric acid (TBA), rhodamine 123 (Rh123), acridine orange and GO (Sigma-Aldrich, USA) were used in the present work.
2.2. Animals
Male three-month-old Wistar rats (220−250 g, from Zabol University of Medical Sciences (Zabol, Iran)) had free access to food and water; they were used for rat kidney proximal tubular cells preparation. During one week before initiation of the experiments, animals’ acclimatization to the laboratory conditions (21 ± 2 ͦ C, with 12 h/12 h dark/light cycles) was done. Protocols for animal experiments from EU Commission Directive 2010/63/EU and those of the Committee of Animal Experimentation of Zabol University of Medical Sciences, were observed. The Ethics Committee of Zabol University of Medical Sciences approved the animals experiments conducted in the current work.
2.3. Isolation and incubation
Using collagenase perfusion method, isolated rat kidney proximal tubular cells were prepared [47]. By employing a hemacytometer, cell concentration was determined using trypan blue (0.2 % w/v). After isolation, cell viability was always >85 %. Cells (106 cells/mL) were incubated (37 °C) in Krebs-Henseleit buffer (pH 7.4) supplemented with 25 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 2.5 mM CaCl2, 25 mM NaHCO3, and 2% (w/v) bovine serum albumin, in constantly rotating 50-ml round-bottom flasks, under 95 % O2 and 5% CO2, in a water bath at 37 °C [48]. To prevent non-toxic or severely toxic conditions, we used GO at EC50. In addition to polyphenols (resveratrol, curcumin and gallic acid), free radical scavengers (mannitol and DMSO), mitochondrial permeability transition (MPT) pore sealing agents (carnitine and l-glutamine) and a lysosomotropic agent (chloroquine) as protective agents were considered at sub-toxic concentrations [49]. Fresh stock solutions of all chemicals (×100 concentrated) were prepared in Krebs-Henseleit buffer [48]. All polyphenols and protective agents were added 15 min before GO, to renal cells. To incubate GO and all other treatments at desired concentrations, 100 μL of concentrated stock solution (×100 concentrated) was added to 10 mL of renal cells suspension in the rotating flask. Because curcumin and resveratrol solubility is low in water, to prepare their concentrated stock solution (×100 concentrated), 200 μL of DMSO per 1 mL buffer was used. At this concentration, DMSO had no effect on the measured factors. The concentrations of the preventing agents mentioned above, were chosen based on a previous report [50].
2.4. Cytotoxicity assessment
Using the trypan blue (0.2 % w/v) exclusion assay, GO-induced cytotoxicity in isolated renal cells was determined. Throughout the 3 -h incubation period, aliquots of the renal cells incubate were collected at various time points and cytotoxicity was assessed. All experiments were done in triplicate [51].
2.5. Determination of ROS
To determine ROS levels, dichlorofluorescin diacetate (DCFDA) was added to the renal cells; within cells, DCF-DA deacetylated by esterases is changed to fluorescent DCF by ROS. Fluorescence intensity was determined using a Shimadzu RF5000U fluorescence spectrophotometer (excitation 500 nm and emission 520 nm) and DCF formation is presented as fluorescence intensity units [52,53]. All experiments were done in triplicate.
2.6. Lipid peroxidation assay
Renal cells lipid peroxidation was determined in terms of the concentration of thiobarbituric acid (TBA) reactive substances (TBARS) produced. Absorbance (at 532 nm) was determined in a Beckman DU-7 spectrophotometer; all experiments were done in triplicate [54].
2.7. Mitochondrial membrane potential assay
The cationic fluorescent dye rhodamine 123 (Rh123) for determination of mitochondrial membrane potential [55] was used as Rh123, via facilitated diffusion, selectively accumulates in the mitochondria [56]. However, there is an indirect relationship between mitochondrial potential and the amount of Rh123 that enters the mitochondria. Aliquots (500 μl) from the cell suspension were incubated (37 °C), at various time points, and centrifuged (at 50 g for 1 min). Next, the cell pellet was re-suspended in 2 mL of fresh incubation medium containing 1.5 μM Rh123, and subsequently, incubated (37 °C) in a thermostatic bath for 10 min while being gently shaken. Renal cells were separated by centrifugation and Rh123 level in the incubation medium, was determined fluorometrically by Hitachi F-2500 fluorescence spectrophotometer (excitation 490 nm and emission 520 nm). The capacity of mitochondria to take up Rh123 was calculated as the difference in fluorescence intensity between control and treated cells and all experiments were done in triplicate [57].
2.8. Lysosomal membrane integrity assay
Using the fluorescent dye acridine orange, lysosomal membrane stability was assessed. The cells were pelleted by 1-minute centrifugation (50 g at 4 °C) and re-suspended in 2 mL of fresh incubation medium. To remove the fluorescent dye from the media, the washing step was carried out two times. Then, acridine orange level was determined using Hitachi F-2500 fluorescence spectrophotometer (excitation 495 nm and emission 530 nm); all experiments were done in triplicate [58].
2.9. Statistical analysis
All continuous parameters are expressed as mean ± standard deviation (SD) of triplicate samples. Comparison between groups was made by one-way analysis of variance (ANOVA) after assessing for normality and homogeneity of variances using the Shapiro-Wilk and the Levene’s test, respectively. Post-hoc comparisons were made by Tukey’s honestly significant difference (HSD) test for multiple comparisons. Statistical significance was set at P <0.05.
3. Results
The EC50, 2h (the concentration at which renal cells viability was decreased by 50 % after 2 h exposure) for GO was 5 mM. GO markedly induced renal cytotoxicity compared to control renal cells (Table 1). GO-induced cytotoxicity was significantly prevented by natural antioxidants (resveratrol, curcumin and gallic acid), and ROS neutralizing (mannitol and DMSO), mitochondrial protective (carnitine and l-glutamine), and lysosome protective (chloroquine) agents (Table 1). Polyphenols as well as mannitol, DMSO, carnitine, l-glutamine and chloroquine did not produce cytotoxicity in intact rat renal cells and cell viability was not reduced over the 3 -h incubation time (data not shown).
Table 1.
Protective effects of various treatments against GO-induced renal cytotoxicity.
| Treatment | Cytotoxicity (%) following 3 h incubation |
|---|---|
| Control isolated renal cells | 15 ± 3 |
| +GO (5 mM) | 80 ± 5a |
| +Resveratrol (50 μM) | 37 ± 4b |
| +Curcumin (5 μM) | 27 ± 2 b |
| +Gallic acid (10 μM) | 30 ± 4b |
| + DMSO (150 mM) | 50 ± 5b |
| +Mannitol (50 mM) | 66 ± 4b |
| +Carnitine (2 mM) | 34 ± 4b |
| +L-Glutamine (1 mM) | 56 ± 2b |
| +Chloroquine (100 mM) | 47 ± 5b |
Data are expressed as mean ± SD of three separate experiments (n = 3).
Significantly different from the control renal cells (P < 0.05).
Significantly different from the GO-treated renal cells (P < 0.05). GO: glyoxal; DMSO: dimethyl sulfoxide and SD: standard deviation.
The ROS formation was significantly augmented when renal cells were incubated with GO (5 mM) (Table 2). ROS formation was significantly prevented by antioxidants (resveratrol, curcumin and gallic acid), and ROS neutralizing (mannitol and DMSO), mitochondrial protective (carnitine and l-glutamine) and lysosome protective (chloroquine) agents (Table 2).
Table 2.
Protective effects of various treatments against GO-induced ROS formation in rat renal cells.
| Treatment | ROS formation (fluorescence intensity) |
||
|---|---|---|---|
| 15 min | 30 min | 60 min | |
| Control isolated renal cells | 47 ± 3 | 55 ± 2 | 62 ± 4 |
| +GO (5 mM) | 62 ± 5a | 75 ± 3a | 95 ± 4a |
| +Resveratrol (50 μM) | 57 ± 3 | 62 ± 4b | 71 ± 3b |
| +Curcumin (5 μM) | 54 ± 5 | 56 ± 3b | 67 ± 5b |
| +Gallic acid (10 μM) | 51 ± 3b | 56 ± 5b | 73 ± 3b |
| +DMSO (150 mM) | 50 ± 5b | 61 ± 3b | 75 ± 6b |
| +Mannitol (50 mM) | 48 ± 4b | 63 ± 4b | 76 ± 4b |
| +Carnitine (2 mM) | 42 ± 3b | 59 ± 2b | 72 ± 5b |
| +L-Glutamine (1 mM) | 49 ± 3b | 67 ± 3b | 78 ± 5b |
| +Chloroquine (100 mM) | 54 ± 4 | 64 ± 5b | 81 ± 3b |
Data are expressed as mean ± SD of three separate experiments (n = 3).
Significantly different from the control renal cells (P < 0.05).
Significantly different from the GO-treated renal cells (P < 0.05). GO: glyoxal; ROS: reactive oxygen species; DMSO: dimethyl sulfoxide and SD: standard deviation.
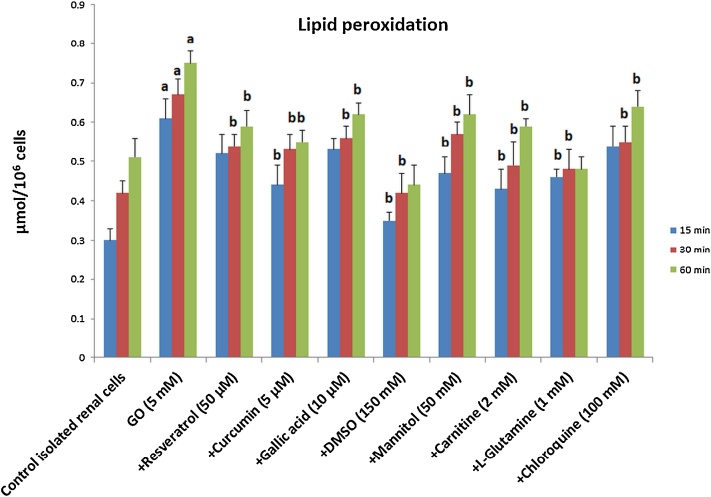
When renal cells were incubated with GO 5 mM (as the EC50,2h), lipid peroxidation significantly increased (Fig.1). GO-induced lipid peroxidation was significantly prevented by antioxidants (resveratrol, curcumin and gallic acid) and ROS neutralizing (mannitol and DMSO), mitochondrial protective (carnitine and l-glutamine) and lysosome protective (chloroquine) agents.
Fig. 1.
Protective effects of various treatments against lipid peroxidation induced by GO in rat renal cells.
Data are expressed as mean ± SD of three separate experiments (n = 3).
aSignificantly different from the control renal cells (P < 0.05). b Significantly different from the GO-treated renal cells (P < 0.05). GO: glyoxal; DMSO: dimethyl sulfoxide and SD: standard deviation.
Via ROS formation, GO produced a rapid decrement of mitochondrial membrane potential, as an indicator of mitochondrial dysfunction. Mitochondrial membrane potential decline was avoided by antioxidants (resveratrol, curcumin and gallic acid) and ROS neutralizing (mannitol and DMSO), mitochondrial protective (carnitine and l-glutamine) and lysosome protective (chloroquine) compounds (Table 3).
Table 3.
Protective effects of various treatments against GO-induced mitochondrial membrane potential decline in rat renal cells.
| Treatment | Percentage of mitochondrial membrane potential decline |
||
|---|---|---|---|
| 15 min | 30 min | 60 min | |
| Control isolated renal cells | 8 ± 2 | 12 ± 1 | 17 ± 2 |
| +GO (5 mM) | 26 ± 3a | 37 ± 4a | 44 ± 4a |
| +Resveratrol (50 μM) | 12 ± 2b | 26 ± 2b | 34 ± 3b |
| +Curcumin (5 μM) | 14 ± 2b | 28 ± 3b | 33 ± 2b |
| +Gallic acid (10 μM) | 21 ± 3 | 26 ± 2b | 35 ± 4b |
| +DMSO (150 mM) | 15 ± 3b | 22 ± 3b | 36 ± 3b |
| +Mannitol (50 mM) | 16 ± 2b | 22 ± 1b | 29 ± 2b |
| +Carnitine (2 mM) | 11 ± 3b | 27 ± 3b | 33 ± 4b |
| +L-Glutamine (1 mM) | 18 ± 1b | 25 ± 4b | 32 ± 2b |
| +Chloroquine (100 mM) | 23 ± 3 | 28 ± 2b | 31 ± 3b |
Data are expressed as mean ± SD of three separate experiments (n = 3).
Significantly different from the control renal cells (P < 0.05).
Significantly different from the GO-treated renal cells (P < 0.05). GO: glyoxal; DMSO: dimethyl sulfoxide and SD: standard deviation.
When renal cells lysosomes were loaded with acridine orange (as a lysosomotropic agent), a marked redistribution of acridine orange into the cytosolic fraction followed within 60 min of GO incubation, showing severe oxidative damage to lysosomal membrane (Table 4). GO-induced acridine orange release was avoided by antioxidants (resveratrol, curcumin and gallic acid) and ROS neutralizing (mannitol and DMSO), mitochondrial protective (carnitine and l-glutamine) and lysosome protective (chloroquine) agents (Table 4).
Table 4.
Protective effects of various treatments against GO- induced lysosomal membrane leakiness in rat renal cells.
| Treatment | Percentage of acridine orange redistribution |
||
|---|---|---|---|
| 15 min | 30 min | 60 min | |
| Control isolated renal cells | 5 ± 1 | 7 ± 1 | 12 ± 2 |
| +GO (5 mM) | 19 ± 2a | 25 ± 3a | 39 ± 2a |
| +Resveratrol (50 μM) | 9 ± 1b | 12 ± 2b | 18 ± 2b |
| +Curcumin (5 μM) | 11 ± 2b | 14 ± 2b | 24 ± 4b |
| + Gallic acid (10 μM) | 11 ± 1b | 13 ± 2b | 19 ± 3b |
| +Dimethyl sulfoxide (150 mM) | 13 ± 2b | 16 ± 1b | 23 ± 3b |
| +Mannitol (50 mM) | 12 ± 1b | 15 ± 2b | 21 ± 2b |
| +Carnitine (2 mM) | 3 ± 1b | 9 ± 2b | 16 ± 2b |
| +L-Glutamine (1 mM) | 11 ± 2b | 17 ± 2b | 21 ± 1b |
| +Chloroquine (100 mM) | 7 ± 1b | 11 ± 3b | 18 ± 3b |
Values are expressed as mean ± SD of three separate experiments (n = 3).
Significantly different from the control renal cells (P < 0.05).
Significantly different from the GO-treated renal cells (P < 0.05). GO: glyoxal; DMSO: dimethyl sulfoxide and SD: standard deviation.
Antioxidants (resveratrol, curcumin and gallic acid) and ROS neutralizing (mannitol and DMSO), mitochondrial protective (carnitine and l-glutamine) and lysosome protective (chloroquine) agents did not induce any damage to rat renal cells over the 3 -h time period (data not shown).
4. Discussion
In the current study, resveratrol, curcumin and gallic acid protective effects against detrimental effects of glyoxal (GO) in renal rat cells, were demonstrated. Considering the increasing incidence rate of redox-state related diseases, there is an urgent need to enhance the organisms defense system with minimal side effects. Therefore, naturally occurring compounds with potent antioxidant properties have been extensively used for alleviating the detrimental impacts of such diseases [59,60]. Previous in vitro studies indicated that hyperglycemia is the direct cause of the glycation of cellular matrix proteins of mesonephric kidney cells and renal mesangial cell death [61,62]. Moreover, GO as one of the by-products of the auto-oxidation of glucose, was shown to be involved in the glycation of proteins and lipids [4].4 GO was used in the current study to create a cellular model of renal damage due to increased blood sugar.
Synergistic effects between different compounds might lead to enhanced pharmacological effects. Specifically, curcumin and resveratrol co-administration in cell lines potentiated the effects of each single compound [63]. Similar results were observed for gallic acid and curcumin combination. Specifically, in this study, the capacity of curcumin in preventing gallic acid-induced depletion of the antioxidant system was shown [64] which was in line with studies that validated the antioxidant potential of curcumin [65,66]. Moreover, the specific effects of phytochemical compounds may be dependent on their molecular polarities and on the dosage of each treatment, a fact that could explain the controversial functions of antioxidants in combination or single administration [67].
Our results indicated that GO is able to cause death in rat renal cells. In general, studies conducted on humans and animals demonstrated that several antioxidants (e.g. vitamins C and E) can block and prevent complications stemming from the rise of sugar level in the blood [4]. Several studies revealed that phytochemical compounds such as resveratrol might hinder and decelerate the progression of cancer, ischemic injuries and cardio-vascular diseases and many other human related diseases, but enhance stress resistance and prolong organisms life-span [[68], [69], [70]]. Our study also showed that natural antioxidant compounds (polyphenols) as well as the ROS neutralizing ones (mannitol and DMSO) significantly protected the rat renal cells against the destructive effects of GO-induced oxidative stress. Furthermore, the antioxidant (polyphenols) and ROS neutralizing (mannitol and DMSO) compounds not only reduced ROS levels and GO-induced oxidative stress (Table 2), but also reduced cell death in rat renal cells (Table 1), which clearly illustrates that ROS formation has a major role in GO-triggered renal cells death. Other studies also showed that ROS production can be strongly inhibited by resveratrol [71]. In rats treated with methylglyoxal, resveratrol protected the animals from glycoxidative stress [72]. Resveratrol also showed the potential of diminishing glycation in foods and retarding carbohydrate-hydrolyzing enzyme function [73]. Moreover, use of resveratrol in stroke-prone spontaneously hypersensitive rats was proposed to minimize several urinary markers of oxidative stress [74]. A proposed mechanism for these protective effects is that it might target blood cells and lipoproteins. Specifically, being lipophilic, resveratrol has the opportunity to bind to lipoprotein particles which may enhance antioxidant capacity of this molecule [75]. Other proposed mechanisms of action for resveratrol include activation of sirtuins, modulation of regulatory genes related with cell cycle and suppression of protein kinases [76]. Similar results have also been proposed for gallic acid and curcumin, in vivo [77,78].
Biochemical pathways including protein kinase C (PKC), AGE, polyol and hexose amines pathways, which are activated by hyperglycemia, cause ROS formation. Glucose under hyperglycemic conditions in the polyol path, first transforms to poly-alcohol sorbitol and later to fructose, resulting in the excessive production of superoxide anion radical, inhibition of glucose-6-phosphate dehydrogenase, and subsequent decrement of the concentration of intracellular factors involved in antioxidant defense (NADPH and GSH) and increment of cellular susceptibility to ROS [79].
Also, in the AGE pathway, the rise in glucose concentration causes the auto-oxidation of glucose to GO, and conversion of Amadori products (derivative of 1-amino-1-deoxy ribose glucose, fructoselysine) to 3-deoxyribose glucosone and conversion of glyceraldehyde 3-phosphate and dihydroxyacetone phosphate to methyl glyoxylate which reacts with proteins free amino acid groups to produce AGE. Moreover, sorbitol is converted to metabolized fructose by sorbitol dehydrogenase leading to increased level of NADH/NAD + which in turn, inhibits glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and increases the triose phosphate concentration. This increase in concentration induces the formation of methyl glyoxylate and diacylglycerol (DAG), whereby the produced methyl glyoxylate intensifies the AEG production [4]. Eventually, ROS production augments due to hyperglycemia induced by AGE production [46].
In the PKC pathway, increased DAG synthesis preceded by hyperglycemia, which is known as a PKC endogenous activator, increases PKC activity. Some studies carried out in animals indicated that increased PKC activity is followed by cardiomyopathy, retinopathy and diabetic nephropathy [80]. Meanwhile, ROS assist the activation of PKC in an individual suffering from diabetes; thereby, this cycle intensifies cell damage triggered by chronic hyperglycemia [81].
Additionally, hyperglycemia causes increased ROS production through activation of the hexosamine pathway [4,82]. It should be noted that oxidative stress occurs under conditions where the ROS concentration is beyond the defensive capacity of cellular antioxidants. Moreover, oxidative stress activates intracellular signaling pathways that are sensitive to stress thus increasing cell susceptibility and progression of complications due to increased blood sugar levels [7].
The present study showed that ROS increases in rat renal cells following treatment with GO, were inhibited by mitochondrial protective agents including MPT pore blocking factors (carnitine), ATP producing compound (L-glutamine), and lysosomes protective agent (chloroquine) (Table 2). Thus, it may be concluded that mitochondria and lysosome might be the main sources of ROS production in rat renal cells following exposure to GO. It is noteworthy that the ROS produced within the cell, cause cellular damage only if the cellular antioxidant arsenal fails to eliminate them [4]. Furthermore, the low antioxidant capacity of the cell intensifies the damage caused by radicals leading to the eventual cell death.
Also, glucose is changed to sorbitol when subjected to hyperglycemic conditions, leading to the overproduction of superoxide anion radicals and reduction of intracellular concentrations of agents that are involved in the cell antioxidant defense (e.g. NADPH and GSH), as well as increment of the risk of oxidative stress occurrence [47,83]. It should be mentioned that biological membranes are highly susceptible to damage caused by ROS and such damage increases lipid peroxidation if not inhibited by the cellular antioxidant defense mechanism(s) [83]. Since reduction of the cellular antioxidant defense causes oxidative stress, polyphenols possessing antioxidant properties can reduce the destructive effects of the above-noted stress. Furthermore, our study showed that the lipid peroxidation level in renal cells increases once exposed to GO. Also, lipid peroxidation due to GO in rat renal cells was inhibited by antioxidant (polyphenols) and ROS neutralizing compounds (mannitol and DMSO) (Fig.1). Decreased lipid peroxidation was also proposed by other studies after consumption of resveratrol that might be attributed to its ability in preventing oxidation of LDL by chelating copper and by scavenging ROS [84,85]. Considering the association between oxidation of LDL particles and risk of heart diseases and that resveratrol can be detected in human LDL particles after wine consumption [69], we can assume that resveratrol might prevent diseases which is consistent with its ability to reduce lipid peroxidation. Consistently, gallic acid and curcumin produced similar effects, in vivo [77,86]. Mechanisms involved in gallic acid pharmacological effects were recently reviewed [87].
Our data demonstrated that lipid peroxidation caused by GO is the result of ROS overproduction induced by GO in rat renal cells. Mitochondrial protective agents, carnitine and l-glutamine, as well as the lysosome protective agent chloroquine reduced GO-induced lipid peroxidation level (Fig.1). These findings clearly demonstrated that decline of ROS through mitochondrial and lysosome pathways, reduced membrane lipid peroxidation and lowered GO-induced mitochondrial and lysosome toxicity. Hyperglycemia causes mitochondrial dysfunction, whereby in hyperglycemic conditions, increased levels of pyruvate derived from glucose lead to increases in NADH and FADH2 flow into the electron transfer chain, and consequently, create the voltage gradient between the mitochondrial intermembrane space and mitochondrial matrix. The accumulation of electrons in the electron transport chain increases the likelihood of electrons colliding with oxygen and producing oxygen radicals. Moreover, excessive ROS levels beyond the cellular antioxidant capacity, can oxidize thiol groups around the MPT pores on the membrane, and trigger conformational changes over the mitochondrial membrane resulting in MPT pore opening [88]. The MPT pores are rather large openings which link the extra- and intra-mitochondrial membranes [89]. The potential of mitochondrial membrane (Ψm) is established via constant pumping of proton from the matrix towards the gaps within the mitochondrial membranes. This difference in potential is necessary for ATP synthesis inside the cell. The opening of the MPT pore initiates proton imbalance and a drop in mitochondrial membrane potential [88]. Our data showed that GO is the cause of mitochondrial membrane potential drop in rat renal cells which was inhibited by antioxidant agents (polyphenols), and ROS neutralizing (mannitol and DMSO), mitochondrial protective (carnitine and l- glutamine) and lysosome protective (chloroquine) agents (Table 3). Our data also indicated that the drop in mitochondrial membrane potential in rat renal cells following exposure to GO is because of ROS overproduction. Biogenesis of such dynamic organelles like mitochondria and lysosomes has been extensively documented and correlated with regulation of cellular metabolism and redox state. Damage to mitochondrial biogenesis was indicated in pathological conditions and diseases like aging, diabetes and metabolic syndrome. In most of these conditions, the damage contributes to oxidative stress and organ dysfunction. Several studies indicated physiological improvements accompanied by increases in mitochondrial content of several organs, attributed to resveratrol consumption in aging and metabolic syndrome models in rodents [4,19]. Augmented mitochondria ROS levels were suggested to inactivate major enzymes which are related with mitochondrial metabolism such as α-ketoglutarate dehydrogenase and aconitase, which in the next step, may diminish ATP production [90]. Resveratrol treatment also produced significant decreases in NF-κB -dependent gene expression in aged mice and mice with type 2 diabetes [91]. Similar results were observed for the other two substances as well. For instance, gallic acid protected rat liver mitochondria ex vivo from bisphenol A-induced oxidative stress injuries [92]. Moreover, mitochondrially targeted gallic acid can be used for preventing mitochondrial damage induced by oxidative stress in isolated mitochondria [93]. Curcumin also ameliorates the detrimental impact of oxidative stress through several mechanisms in isolated mitochondria [94,95]. In endothelial injury, curcumin exerted protective properties against carbonyl stress and pro-inflammatory signals, through methylglyoxal trapping [96].
Our results further illustrated that GO exposure leads to increment of lysosome membrane damage in rat renal cells. Nevertheless, GO-induced damage to lysosome membrane in rat renal cells was inhibited by antioxidant (polyphenols), ROS neutralizing (mannitol and DMSO), and mitochondrial protective (carnitine and l- glutamine) agents (Table 4).
The lysosomal protective agents prevented mitochondrial membrane damage in rat renal cells. In addition, they prevented damage to the lysosome membrane. Therefore, a cross-link between mitochondrial and lysosomal damages was shown to strengthen GO toxicity leading to eventual emergence of damages in rat renal cells.
In summary, GO led to increased ROS production in renal cells. Moreover, excessive GO produced ROS, inhibited cellular antioxidant defense and blocked the elimination of the harmful ROS. The ROS link sulfhydryl groups to form disulfide bonds and intensify lipid peroxidation to open MPT pores, reducing mitochondrial membrane potential. Mitochondrial damage can lead to increased ROS production, whereby the H2O2 type ROS exit the mitochondria and enter the cytosol and then lysosomes, to produce dangerous hydroxyl radicals that damage the lysosome membrane forcing the release of lysosomal enzymes into the cytosol. These detrimental effects seem to be reversed by administration of naturally occurring molecules such as resveratrol, curcumin and gallic acid. Additional studies searching for the synergistic effects of these compounds in these pathophysiological conditions would provide important information.
5. Conclusions
Incubation of GO with rat renal cells led to lysis of their membrane, formation of ROS, lipid peroxidation, collapse of mitochondrial membrane potential, and induction of lysosomal membrane leakage and cell death. These effects were inhibited by pre-treatment with resveratrol, curcumin and gallic acid. Mitochondrial and lysosomal toxic interactions appear to be responsible for potentiating oxidative stress/cytotoxicity. Resveratrol, curcumin and gallic acid inhibited ROS formation and lipid peroxidation and attenuated GO-induced renal cytotoxicity.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The present work was submitted as theses of Zeinab Alizadeh and Sedigheh Pasandideh, supervised by Dr Jafar Shahraki and funded by Zabol University of Medical Sciences.
Contributor Information
Ramin Rezaee, Email: rezaeera@mums.ac.ir.
Jafar Shahraki, Email: jafar.shahraki@gmail.com.
References
- 1.Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann. Intern. Med. 1984;101(4):527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- 2.Thornalley P.J. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell. Mol. Biol. (Noisy-le-grand) 1998;44(7):1013–1023. [PubMed] [Google Scholar]
- 3.Vitek M.P. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. 1994;91(11):4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanessa Fiorentino T. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013;19(32):5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 5.Bech P., Moses R., Gomis R. The effect of prandial glucose regulation with repaglinide on treatment satisfaction, wellbeing and health status in patients with pharmacotherapy-naïve Type 2 diabetes: a placebo-controlled, multicentre study. Qual. Life Res. 2003;12(4):413–425. doi: 10.1023/a:1023495106160. [DOI] [PubMed] [Google Scholar]
- 6.Ulusu N.N. Pentose phosphate pathway, glutathione-dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: effects of stobadine and vitamin E. Neurochem. Res. 2003;28(6):815–823. doi: 10.1023/a:1023202805255. [DOI] [PubMed] [Google Scholar]
- 7.Folli F. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro-and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011;7(5):313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 8.Rezaee R. Antigenotoxic activities of the natural dietary coumarins umbelliferone, herniarin and 7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative stress. Drug Chem. Toxicol. 2014;37(2):144–148. doi: 10.3109/01480545.2013.834352. [DOI] [PubMed] [Google Scholar]
- 9.Hashemzaei M. Crocin: a fighter against inflammation and pain. Food Chem. Toxicol. 2020:111521. doi: 10.1016/j.fct.2020.111521. [DOI] [PubMed] [Google Scholar]
- 10.Panahi Y. Comparative trial of Aloe vera/olive oil combination cream versus phenytoin cream in the treatment of chronic wounds. J. Wound Care. 2015;24(10):459–465. doi: 10.12968/jowc.2015.24.10.459. [DOI] [PubMed] [Google Scholar]
- 11.Shi Z.-Q. Identification of effective combinatorial markers for quality standardization of herbal medicines. J. Chromatogr. A. 2014;1345:78–85. doi: 10.1016/j.chroma.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Kitada M., Koya D. Renal protective effects of resveratrol. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soleas G.J., Diamandis E.P., Goldberg D.M. Resveratrol: a molecule whose time has come? And gone? Clin. Biochem. 1997;30(2):91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 14.Hashemzaei M. Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2016;46:110–115. doi: 10.1016/j.etap.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Hashemzaei M. Anticancer effects of co-administration of daunorubicin and resveratrol in MOLT-4, U266 B1 and RAJI cell lines. Farmacia. 2016;64(1):36–42. [Google Scholar]
- 16.Tabrizian K. Behavioral and molecular effects of intrahippocampal infusion of auraptene, resveratrol, and curcumin on H-89-induced deficits on spatial memory acquisition and retention in Morris water maze. Hum. Exp. Toxicol. 2019;38(7):775–784. doi: 10.1177/0960327119839160. [DOI] [PubMed] [Google Scholar]
- 17.Ovesná Z. Antioxidant activity of resveratrol, piceatannol and 3, 3’, 4, 4’, 5, 5’-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncol. Rep. 2006;16(3):617–624. [PubMed] [Google Scholar]
- 18.Hamza R.Z., El-Shenawy N.S. Anti-inflammatory and antioxidant role of resveratrol on nicotine-induced lung changes in male rats. Toxicol. Rep. 2017;4:399–407. doi: 10.1016/j.toxrep.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y.T. Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro. Int. J. Mol. Med. 2016;37(1):83–91. doi: 10.3892/ijmm.2015.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezaee R. Effects of resveratrol on the acquisition and reinstatement of morphine‑induced conditioned place preference in mice. World Acad. Sci. J. 2020;2(2):77–82. [Google Scholar]
- 22.Şener G. Resveratrol improves ischemia/reperfusion-induced oxidative renal injury in rats. Arch. Med. Res. 2006;37(7):822–829. doi: 10.1016/j.arcmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Tabrizian K. Neuro‐protective effects of resveratrol on carbon monoxide‐induced toxicity in male rats. Phytother. Res. 2017;31(9):1310–1315. doi: 10.1002/ptr.5855. [DOI] [PubMed] [Google Scholar]
- 24.Hashemzaei M. Anticancer and apoptosis‑inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017;38(2):819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suskind D.L. Tolerability of Curcumin in Pediatric Inflammatory Bowel Disease: a forced dose titration study. J. Pediatr. Gastroenterol. Nutr. 2013;56(3):277. doi: 10.1097/MPG.0b013e318276977d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabeshpour J. Effects of curcumin on ion channels and pumps: a review. IUBMB Life. 2019;71(7):812–820. doi: 10.1002/iub.2054. [DOI] [PubMed] [Google Scholar]
- 27.Tabeshpour J., Hashemzaei M., Sahebkar A. The regulatory role of curcumin on platelet functions. J. Cell. Biochem. 2018;119(11):8713–8722. doi: 10.1002/jcb.27192. [DOI] [PubMed] [Google Scholar]
- 28.Tabrizian K. Auraptene consolidates memory, reverses scopolamine-disrupted memory in passive avoidance task, and ameliorates retention deficits in mice. Iran. J. Basic Med. Sci. 2015;18(10):1014. [PMC free article] [PubMed] [Google Scholar]
- 29.Shahraki A. Effects of resveratrol nanocapsules on the quantitative insulin sensitivity check index in insulin resistance: a study on metabolic syndrome induce mice. SN Applied Sciences. 2020;2(5):1–7. [Google Scholar]
- 30.Hassani S. Protective effects of curcumin and vitamin E against chlorpyrifos-induced lung oxidative damage. Hum. Exp. Toxicol. 2015;34(6):668–676. doi: 10.1177/0960327114550888. [DOI] [PubMed] [Google Scholar]
- 31.Rezaee R. Curcumin: a potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol. Res. 2017;117:218–227. doi: 10.1016/j.phrs.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Mirzaei H. Curcumin: a new candidate for melanoma therapy? Int. J. Cancer. 2016;139(8):1683–1695. doi: 10.1002/ijc.30224. [DOI] [PubMed] [Google Scholar]
- 33.Salehi B. Curcumin’s nanomedicine formulations for therapeutic application in neurological diseases. J. Clin. Med. 2020;9(2):430. doi: 10.3390/jcm9020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharifi-Rad M. Impact of natural compounds on neurodegenerative disorders: from preclinical to Pharmacotherapeutics. J. Clin. Med. 2020;9(4):1061. doi: 10.3390/jcm9041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pari L., Tewas D., Eckel J. Role of curcumin in health and disease. Arch. Physiol. Biochem. 2008;114(2):127–149. doi: 10.1080/13813450802033958. [DOI] [PubMed] [Google Scholar]
- 36.Sharma R., Gescher A., Steward W. Curcumin: the story so far. Eur. J. Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Hongsibsong S. Dietary exposure to continuous small doses of α-cypermethrin in the presence or absence of dietary curcumin does not induce oxidative stress in male Wistar rats. Toxicol. Rep. 2014;1:1106–1114. doi: 10.1016/j.toxrep.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abolaji A.O. Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Toxicol. Rep. 2020;7:261–268. doi: 10.1016/j.toxrep.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adewale O.O. Curcumin protects sodium nitrite-induced hepatotoxicity in Wistar rats. Toxicol. Rep. 2019;6:1006–1011. doi: 10.1016/j.toxrep.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haute G.V. Gallic acid reduces the effect of LPS on apoptosis and inhibits the formation of neutrophil extracellular traps. Toxicol. Vitr. 2015;30(1):309–317. doi: 10.1016/j.tiv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Huang D.-W. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016;36(2):150–160. doi: 10.1016/j.nutres.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Lu Z. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006;48(4):263–274. doi: 10.1016/j.neuint.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Pal C. Gallic acid prevents nonsteroidal anti-inflammatory drug-induced gastropathy in rat by blocking oxidative stress and apoptosis. Free Radic. Biol. Med. 2010;49(2):258–267. doi: 10.1016/j.freeradbiomed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Abarikwu S.O. Evaluation of the protective effects of quercetin and gallic acid against oxidative toxicity in rat’s kidney and HEK-293 cells. Toxicol. Rep. 2020;7:955–962. doi: 10.1016/j.toxrep.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur S., Muthuraman A. Ameliorative effect of gallic acid in paclitaxel-induced neuropathic pain in mice. Toxicol. Rep. 2019;6:505–513. doi: 10.1016/j.toxrep.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yousuf M.J., Vellaichamy E. Protective activity of gallic acid against glyoxal-induced renal fibrosis in experimental rats. Toxicol. Rep. 2015;2:1246–1254. doi: 10.1016/j.toxrep.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones D.P. Use of isolated kidney cells for study of drug metabolism. Biochem. Pharmacol. 1979;28(6):929–935. doi: 10.1016/0006-2952(79)90378-2. [DOI] [PubMed] [Google Scholar]
- 48.Shahraki J. Cytoprotective effects of hydrophilic and lipophilic extracts of Pistacia vera against oxidative versus carbonyl stress in rat hepatocytes. Iranian journal of pharmaceutical research: IJPR. 2014;13(4):1263. [PMC free article] [PubMed] [Google Scholar]
- 49.Eskandari M.R. A comparison of cardiomyocyte cytotoxic mechanisms for 5-fluorouracil and its pro-drug capecitabine. Xenobiotica. 2015;45(1):79–87. doi: 10.3109/00498254.2014.942809. [DOI] [PubMed] [Google Scholar]
- 50.Shahraki J. Ichthyotoxic cochlodinium polykrikoides induces mitochondrial mediated oxidative stress and apoptosis in rat liver hepatocytes. Iranian journal of pharmaceutical research: IJPR. 2013;12(4):829. [PMC free article] [PubMed] [Google Scholar]
- 51.Pourahmad J., O’Brien P.J. A comparison of hepatocyte cytotoxic mechanisms for Cu2+ and Cd2+ Toxicology. 2000;143(3):263–273. doi: 10.1016/s0300-483x(99)00178-x. [DOI] [PubMed] [Google Scholar]
- 52.Shen H.-M. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic. Biol. Med. 1996;21(2):139–146. doi: 10.1016/0891-5849(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 53.Pourahmad J. A new approach on valproic acid induced hepatotoxicity: involvement of lysosomal membrane leakiness and cellular proteolysis. Toxicol. Vitr. 2012;26(4):545–551. doi: 10.1016/j.tiv.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Smith M.T. The measurement of lipid peroxidation in isolated hepatocytes. Biochem. Pharmacol. 1982;31(1):19–26. doi: 10.1016/0006-2952(82)90230-1. [DOI] [PubMed] [Google Scholar]
- 55.Verma A. Phytochemical analysis and in vitro cytostatic potential of ethnopharmacological important medicinal plants. Toxicol. Rep. 2020 doi: 10.1016/j.toxrep.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersson B., Aw T.Y., Jones D.P. Mitochondrial transmembrane potential and pH gradient during anoxia. Am. J. Cell Physiol. 1987;252(4):C349–C355. doi: 10.1152/ajpcell.1987.252.4.C349. [DOI] [PubMed] [Google Scholar]
- 57.Hashemzaei M. Antimony induces oxidative stress and cell death in normal hepatocytes. Toxicol. Environ. Chem. 2015;97(2):256–265. [Google Scholar]
- 58.Pourahmad J., Ross S., O’Brien P.J. Lysosomal involvement in hepatocyte cytotoxicity induced by Cu2+ but not Cd2+ Free Radic. Biol. Med. 2001;30(1):89–97. doi: 10.1016/s0891-5849(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 59.Margaritis I. Effect of crocin on antioxidant gene expression, fibrinolytic parameters, redox status and blood biochemistry in nicotinamide-streptozotocin-induced diabetic rats. J. Biol. Res. 2020;27(1):1–15. doi: 10.1186/s40709-020-00114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsamouri M.-M. Histopathological evaluation and redox assessment in blood and kidney tissues in a rabbit contrast-induced nephrotoxicity model. Food Chem. Toxicol. 2017;108:186–193. doi: 10.1016/j.fct.2017.07.058. [DOI] [PubMed] [Google Scholar]
- 61.Harris R.D. Global glomerular sclerosis and glomerular arteriolar hyalinosis in insulin dependent diabetes. Kidney Int. 1991;40(1):107–114. doi: 10.1038/ki.1991.187. [DOI] [PubMed] [Google Scholar]
- 62.Heilig C.W. Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J. Clin. Invest. 1995;96(4):1802–1814. doi: 10.1172/JCI118226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaky A. A combination of resveratrol and curcumin is effective against aluminum chloride-induced neuroinflammation in rats. J. Alzheimer Dis. 2017;60(s1):S221–S235. doi: 10.3233/JAD-161115. [DOI] [PubMed] [Google Scholar]
- 64.Abarikwu S.O. Combined administration of curcumin and gallic acid inhibits gallic acid-induced suppression of steroidogenesis, sperm output, antioxidant defenses and inflammatory responsive genes. J. Steroid Biochem. Mol. Biol. 2014;143:49–60. doi: 10.1016/j.jsbmb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Chandra A.K. Effect of curcumin on chromium-induced oxidative damage in male reproductive system. Environ. Toxicol. Pharmacol. 2007;24(2):160–166. doi: 10.1016/j.etap.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Sahoo D.K., Roy A., Chainy G.B. Protective effects of vitamin E and curcumin on L-thyroxine-induced rat testicular oxidative stress. Chem. Biol. Interact. 2008;176(2-3):121–128. doi: 10.1016/j.cbi.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Naksuriya O., Okonogi S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discov. Ther. 2015;9(2):136–141. doi: 10.5582/ddt.2015.01013. [DOI] [PubMed] [Google Scholar]
- 68.Jang M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 69.Markus M.A., Morris B.J. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin. Interv. Aging. 2008;3(2):331. [PMC free article] [PubMed] [Google Scholar]
- 70.Pandey K.B., Rizvi S.I. Pleiotropic biological effects of resveratrol: implications for human health. Natl. Acad. Sci. Lett. 2009;32(11/12):321–326. [Google Scholar]
- 71.Rotondo S. Effect of trans‐resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br. J. Pharmacol. 1998;123(8):1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yılmaz Z. The effect of resveratrol on glycation and oxidation products in plasma and liver of chronic methylglyoxal-treated rats. Pharmacol. Rep. 2018;70(3):584–590. doi: 10.1016/j.pharep.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Shen Y., Xu Z., Sheng Z. Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity, and to conjugate methylglyoxal. Food Chem. 2017;216:153–160. doi: 10.1016/j.foodchem.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 74.Mizutani K. Protective effect of resveratrol on oxidative damage in male and female stroke‐prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2001;28(1-2):55–59. doi: 10.1046/j.1440-1681.2001.03415.x. [DOI] [PubMed] [Google Scholar]
- 75.Belguendouz L., Frémont L., Gozzelino M.-T. Interaction of transresveratrol with plasma lipoproteins. Biochem. Pharmacol. 1998;55(6):811–816. doi: 10.1016/s0006-2952(97)00544-3. [DOI] [PubMed] [Google Scholar]
- 76.Marques F.Z., Markus M.A., Morris B.J. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 2009;41(11):2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Ghosh S. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep. 2015;2:365–376. doi: 10.1016/j.toxrep.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramkumar K. Protective effect of gallic acid on alloxan-induced oxidative stress and osmotic fragility in rats. Hum. Exp. Toxicol. 2014;33(6):638–649. doi: 10.1177/0960327113504792. [DOI] [PubMed] [Google Scholar]
- 79.Yagihashi S. International Review of Neurobiology. Elsevier; 2016. Glucotoxic mechanisms and related therapeutic approaches; pp. 121–149. [DOI] [PubMed] [Google Scholar]
- 80.Dahl-Jørgensen K. Long-term glycemie control and kidney function in insulin-dependent diabetes mellitus. Kidney Int. 1992;41(4):920–923. doi: 10.1038/ki.1992.140. [DOI] [PubMed] [Google Scholar]
- 81.Wang P.H., Lau J., Chalmers T.C. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341(8856):1306–1309. doi: 10.1016/0140-6736(93)90816-y. [DOI] [PubMed] [Google Scholar]
- 82.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 83.Teng S. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem. Biol. Interact. 2001;130:285–296. doi: 10.1016/s0009-2797(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 84.Kasdallah-Grissa A. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006;41(3):236–239. doi: 10.1093/alcalc/agh256. [DOI] [PubMed] [Google Scholar]
- 85.Franco J.G. Resveratrol reduces lipid peroxidation and increases sirtuin 1 expression in adult animals programmed by neonatal protein restriction. J. Endocrinol. 2010;207(3):319–328. doi: 10.1677/JOE-10-0124. [DOI] [PubMed] [Google Scholar]
- 86.Patel S.S., Goyal R.K. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacognosy Res. 2011;3(4):239. doi: 10.4103/0974-8490.89743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kahkeshani N. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran. J. Basic Med. Sci. 2019;22(3):225. doi: 10.22038/ijbms.2019.32806.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kowaltowski A.J., Castilho R.F., Vercesi A.E. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495(1-2):12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- 89.Barrientos A., Moraes C.T. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J. Biol. Chem. 1999;274(23):16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 90.Ungvari Z. Mitochondrial protection by resveratrol. Exerc. Sport Sci. Rev. 2011;39(3):128. doi: 10.1097/JES.0b013e3182141f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pearson K.J. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dutta M., Paul G. Gallic acid protects rat liver mitochondria ex vivo from bisphenol A induced oxidative stress mediated damages. Toxicol. Rep. 2019;6:578–589. doi: 10.1016/j.toxrep.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parihar P. Efficiency of mitochondrially targeted gallic acid in reducing brain mitochondrial oxidative damage. Cell. Mol. Biol. 2014;60(2):35–41. [PubMed] [Google Scholar]
- 94.Jat D. Curcumin reduces oxidative damage by increasing reduced glutathione and preventing membrane permeability transition in isolated brain mitochondria. Cell. Mol. Biol. (Noisy-le-grand) 2013;59(Suppl):OL1899–OL1905. [PubMed] [Google Scholar]
- 95.Martínez-Morúa A. Curcumin decreases oxidative stress in mitochondria isolated from liver and kidneys of high-fat diet-induced obese mice. J. Asian Nat. Prod. Res. 2013;15(8):905–915. doi: 10.1080/10286020.2013.802687. [DOI] [PubMed] [Google Scholar]
- 96.Sun Y.P. Curcumin inhibits advanced glycation end product‑induced oxidative stress and inflammatory responses in endothelial cell damage via trapping methylglyoxal. Mol. Med. Rep. 2016;13(2):1475–1486. doi: 10.3892/mmr.2015.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]