Abstract
In rare instances, endometrial glandular tissue can implant in the thorax of women suffering from endometriosis. The presentation is variable depending on site of implant and can be a rare cause of hemothorax in women. A 28-year-old woman presented with shortness of breath and was found to have a significant right sided hemothorax. The hemothorax was drained but subsequently recurred, with shortness of breath increasing around the time of her menses. Considerable workup was performed and ultimately surgery was required to diagnose her with thoracic endometriosis. This case describes how thoracic endometriosis is a challenging diagnosis and may be under reported in the literature. However, there are key elements of the disease that can prevent delay in diagnosis to reduce pain and suffering.
Keywords: Thoracic endometriosis, Hemothorax, Pleural effusion, Pneumothorax, Fibroid, Catamenial
Introduction
Endometriosis is a benign condition where endometrial glands implant outside the uterine cavity. The disease most commonly affects the pelvic organs, however, the thorax is the most common extra-pelvic location affected [2]. The most common presentation of thoracic endometriosis is pneumothorax associated with menses [6]. Symptoms typically begin with the onset of menses and most commonly include chest or scapular pain (90%), and dyspnea (33%). Symptoms arise intermittently, not usually with each menses [6].
Additionally, thoracic endometriosis is associated with hemorrhage into the pleural space in 12%-14% of cases and is typically associated with large burden of endometrial implants [4].
Diagnosis of thoracic endometriosis is difficult. Imaging of the chest with x-ray, computed tomography (CT), or magnetic resonance imaging (MRI) can rarely identify endometriosis as the cause of pneumothorax or hemothorax. Occasionally cytology can identify endometrial epithelial cells on thoracentesis or chest tube drainage of a hemothorax, but the sensitivity is low [5]. Typically, diagnosis is made through thoracoscopic evaluation and identification of endometrial tissue after biopsy of suspicious lesions. Thoracoscopy is recommended during or within 48 hours of menstruation to improve histological yield [3].
Initial management of thoracic endometriosis involves chest tube drainage of pleural air or blood product. Later, video assisted thoracoscopic surgery is recommended with resection of visible lesions, without or without pleurodesis [7]. Following resection hormonal suppression is recommended for 6-12 months since the endometrial tissue is estrogen dependent [5]
Case report
A 28-year-old African American female with a known multi-year history of severe endometriosis and leiomyomata uteri presented with new onset shortness of breath (SOB). She was found to have a right sided pleural effusion and small apical pneumothorax on x-ray (Figs. 1A and B). Thoracentesis demonstrated hemothorax with drainage of 1500 mL red fluid. Cytology of the bloody fluid showed atypical cells, but no definitive malignancy. Upon further workup her CA-125 was found to be 102 U/mL (reference range 0-35 U/mL). At this time pulmonology suspected malignancy given hemothorax of unknown etiology and elevated CA-125 so gynecology-oncology was consulted. Concurrently she was being followed for many years by gynecology for endometriosis and fibroids, which were diagnosed with pelvic ultrasound and assumed to be localized to the pelvis.
Fig. 1.
(A) Initial posteroanterior chest radiograph showing moderate to large right sided pleural effusion (orange arrow) and a small apical right sided pneumothorax (blue arrows). (B) A lateral chest radiograph again demonstrating moderate to large right sided pleural effusion (orange arrow).
Two months after initial presentation MRI of the pelvis with and without contrast was performed to characterize her fibroids and evaluate for possible malignancy. Multiple fibroids were visualized, but no evidence of malignancy or endometrial implants were identified on the pelvic MRI (Figs. 2A and B). Suspicion for malignancy was high despite pelvic MRI so positron emission tomography CT was performed. Again, no malignancy was noted, but an ametabolic right sided pleural effusion was seen (Fig. 3).
Fig. 2.
Coronal (A) and sagittal (B) T2 weighted MRI of pelvis showing an enlarged fibroid uterus with a dominant 6.0 cm sub serosal fibroid at the fundus of the uterus (white arrow). Additionally, a 3.0 cm intracavitary fibroid is seen (red arrow).
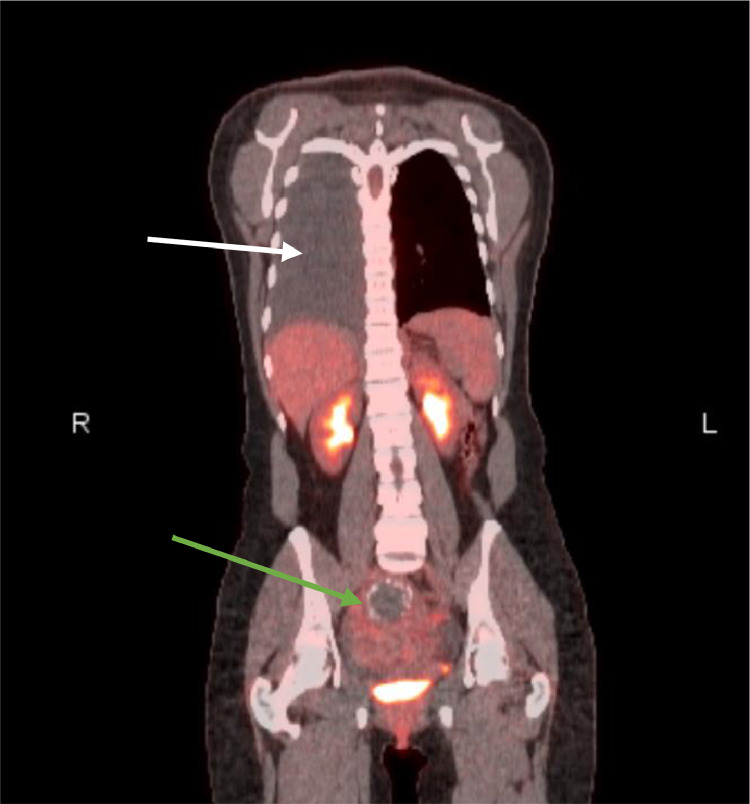
Fig. 3.
Coronal slice of a PET CT showing ametabolic moderately large right sided pleural effusion (white arrow). Additionally, an enlarged heterogenous uterus with calcified fibroids is demonstrated (green arrow).
Three months after initial presentation she had worsening SOB, with thoracentesis showing recurrent bloody pleural effusion. At that time, she was referred to cardiothoracic surgery for surgical exploration. Upon further inquiry she admitted increasing SOB a week prior to her menstrual cycle, with improvement in symptoms at the end of her period.
Four months after initial presentation she underwent robotic assisted thoracoscopy. Intraoperatively numerous brownish deposits were noted on the parietal and visceral pleural surfaces, as well as involvement of the diaphragm. Due to these findings total parietal pleurectomy and spot ablation were performed. Surgical pathology identified the pleural specimens to be consistent with endometriosis. At this time, she was diagnosed with thoracic endometriosis.
She was discharged on post-op day #5 without complication. A gonadotropin-releasing hormone antagonist was initiated at follow up for hormonal suppression. In the year since her surgery she has had no recurrence of pleural effusions.
Discussion
Thoracic endometriosis is poorly understood, under recognized, and often only diagnosed after a delay from initial presentation. However, this case exemplifies characteristics of the disease that can dramatically increase the suspicion for thoracic endometriosis and lead to an earlier diagnosis. The first clue to the diagnosis is demographic information. The disease occurs in women and is much more commonly seen in the young adult population (20s-30s). The woman in this case was 28 years old. Next, this disease process typically exhibits cyclical increase in symptoms around the time of menses when the glandular tissues are activated by spikes in hormone levels. Our patient admitted increase in SOB prior to menses, with improvement in symptoms as her period resolved. Interestingly, the right side of the thorax is affected in most cases (around 85% [4]), which is consistent with findings in our patient. There are many theories to explain this right sided predominance, but the pathogenesis has yet to be fully explained. Finally, the recurrent nature of this disease without other obvious explanation can help hint at the diagnosis. Patients will almost certainly develop recurrence of symptoms (pneumothorax, hemothorax, etc.) without medical and/or surgical management to treat the ectopic glandular tissue. This concept is seen in our case where the patient experienced recurrence of SOB and hemothorax, which finally prompted video assisted thoracoscopic surgery leading to the final diagnosis.
It has been postulated in the literature that thoracic endometriosis is underreported, especially when presenting as pneumothorax. Cases are often misclassified as spontaneous pneumothorax, likely due to the under recognized nature of the disease. Only after multiple recurrences or development of additional symptoms are patients finally given a diagnosis of thoracic endometriosis.
Interestingly, the presence of intrathoracic endometrial implants has not fully been explained, but there are a few leading theories. First is the theory of coelomic metaplasia, where pleural and peritoneal structures share a common mesothelial origin as endometrial tissue [1]. However, this theory is unable to explain intrapulmonary endometrial implants or right sided predominance. The second theory describes transplant of endometrial tissue through vascular or lymphatic embolization [1]. Again, this theory cannot describe right sided predominance, but could explain transplant of endometrial tissue to any location outside the pelvis. Finally, there is the theory of retrograde menstruation, which is the favored theory to explain pelvic endometriosis [1]. Spread to thoracic structures would occur through transdiaphragmatic migration from the pelvis to the right subdiaphragmatic area through the right paracolic gutter [1]. This process would be aided by either congenital or acquired diaphragmatic defects and could help explain right sided predominance. It is very likely the full explanation relies on a combination of these theories or a yet to be described mechanism.
In conclusion, this case illustrates the features of thoracic endometriosis: age/sex, cyclical nature, right sided predominance, and recurrence. Using these factors, the radiologist can play a critical role in preventing a delayed diagnosis by suggesting this condition in the appropriate clinical situation and recommending the collection of a detailed history of symptoms relative to the menstrual history. Ultimately, increased awareness can spare these women additional pain and suffering through prompt diagnosis and treatment.
References
- 1.Ailfano M., Trisolini R., Cancellieri A., Regnard J.F. Thoracic endometriosis: current knowledg6e. The Annals of Thoracic Surgery. 2006;81(2):761–769. doi: 10.1016/j.athoracsur.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 2.Bagan P., Berna P., Assouad J., Hupertan V., Barthes F.L.P., Riquet M. Value of cancer antigen 125 for diagnosis of pleural endometriosis in females with recurrent pneumothorax. European Respiratory Journal. 2008;31(1):140–142. doi: 10.1183/09031936.00094206. [DOI] [PubMed] [Google Scholar]
- 3.Bagan P.L.P., Barthes F.L.P., Assouad J.L.P., Souilamas R.L.P., Riquet M.L.P. Catamenial pneumothorax: retrospective study of surgical treatment. The Annals of Thoracic Surgery. 2003;75(2):378–381. doi: 10.1016/s0003-4975(02)04320-5. [DOI] [PubMed] [Google Scholar]
- 4.Channabasavaiah A.D., Joseph J.V. Thoracic Endometriosis. Medicine. 2010;89(3):183–188. doi: 10.1097/md.0b013e3181df67d5. [DOI] [PubMed] [Google Scholar]
- 5.Hagnere P., Deswarte S., Leleu O. [Thoracic endometriosis: a difficult diagnosis] Revue Del Maladies Respiratoires. 2011 doi: 10.1016/j.rmr.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Joseph J., Sahn S.A. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. The American Journal of Medicine. 1996;100(2):164–170. doi: 10.1016/s0002-9343(97)89454-5. [DOI] [PubMed] [Google Scholar]
- 7.Sevinç S., Unsal S., Oztürk T. Thoracic endometriosis syndrome with bloody pleural effusion in a 28 year old woman. J Pak Med Assoc. 2013;63:114. [PubMed] [Google Scholar]